Abstract
Renin-angiotensin-aldosterone system (RAAS) activation mediates increases in reactive oxygen species (ROS) and impaired insulin signaling. The transgenic Ren2 rat manifests increased tissue renin-angiotensin system activity, elevated serum aldosterone, hypertension, and insulin resistance. To explore the role of aldosterone in the pathogenesis of insulin resistance, we investigated the impact of in vivo treatment with a mineralocorticoid receptor (MR) antagonist on insulin sensitivity in Ren2 and aged-matched Sprague-Dawley (SD) control rats. Both groups (age 6–8 wk) were implanted with subcutaneous time-release pellets containing spironolactone (0.24 mg/day) or placebo over 21 days. Systolic blood pressure (SBP) and intraperitoneal glucose tolerance test were determined. Soleus muscle insulin receptor substrate-1 (IRS-1), tyrosine phosphorylated IRS-1, protein kinase B (Akt) phosphorylation, GLUT4 levels, and insulin-stimulated 2-deoxyglucose uptake were evaluated in relation to NADPH subunit expression/oxidase activity and ROS production (chemiluminescence and 4-hydroxy-2-nonenal immunostaining). Along with increased soleus muscle NADPH oxidase activity and ROS, there was systemic insulin resistance and reduced muscle IRS-1 tyrosine phosphorylation, Akt phosphorylation/activation, and GLUT4 expression in the Ren2 group (each P < 0.05). Despite not decreasing blood pressure, low-dose spironolactone treatment improved soleus muscle insulin signaling parameters and systemic insulin sensitivity in concert with reductions in NADPH oxidase subunit expression/activity and ROS production (each P < 0.05). Our findings suggest that aldosterone contributes to insulin resistance in the transgenic Ren2, in part, by increasing NADPH oxidase activity in skeletal muscle tissue.
Keywords: Ren2 rat, mineralocorticoid receptor blockade
the roles of insulin resistance in the pathophysiology of type 2 diabetes mellitus (T2DM) and the metabolic syndrome are well established (33, 38), and exploration of the mechanisms leading to diminished insulin sensitivity is a field of active research. Activation of the tissue renin-angiotensin-system (RAS) has been linked to increased production of reactive oxygen species (ROS) through activation of the NADPH oxidase enzymatic complex in numerous tissues, including skeletal muscle (5, 33). Excessive oxidative stress may result in impairment of intracellular insulin signaling, constituting a potential pathway by which renin-angiotensin-aldosterone system (RAAS) activation induces insulin resistance (5, 14, 31).
There is growing interest in the role of mineralocorticoids in the pathogenesis of insulin resistance. Mineralocorticoids participate in the regulation of blood pressure, water, sodium, and potassium homeostasis through interaction with the mineralocorticoid receptor (MR) located in target epithelial cells, translocation to the nucleus, and triggering of specific genomic actions (10). However, mineralocorticoids also exert acute actions in multiple nonepithelial tissues that appear to be independent of gene transcription (12). These nongenomic actions are particularly relevant in cardiovascular disease (CVD), as they can result in hypertrophy, endothelial dysfunction (4, 17), inflammation (30), fibrosis, apoptosis, and cardiovascular remodeling (27). Recently, aldosterone has been reported to suppress insulin metabolic signaling in vascular smooth muscle cells via an oxidative stress-mediated mechanism (14). This raises the possibility that aldosterone, like angiotensin II (ANG II), can suppress insulin-mediated glucose uptake in various tissues (5, 33).
Available studies in humans have shown, independent of other components of the metabolic syndrome, an association between increased plasma levels of aldosterone and the presence of insulin resistance in the metabolic syndrome (6, 18). In addition, aldosterone excess in patients with primary aldosteronism is related to impaired glucose homeostasis (11) as well as insulin resistance (9). Likewise, euglycemic hyperinsulinemia results in increased aldosterone production in response to ANG II in an animal model (25) and in healthy humans (32). These detrimental effects have been related to proinflammatory effects exerted by mineralocorticoids (18). However, the impact of signaling through the MR on glucose metabolism in ex vivo skeletal muscle has not been investigated.
The transgenic TG(mRen2)27 rat (Ren2), which harbors the mouse renin gene, is an experimental model of excessive tissue local RAS activity that, through paracrine adrenal effects, leads to increased plasma deoxycorticosterone (DOC), 18-hydroxycorticosterone, and aldosterone levels as well as whole body and skeletal muscle insulin resistance (5, 24, 26, 29). Previous studies from our laboratory have demonstrated that AT1R blockade and ROS scavenging improve whole body glucose tolerance and skeletal muscle insulin-stimulated glucose transport (5, 33). Since mineralocorticoids promote oxidative stress in cardiovascular tissue (5), we sought to investigate the impact of in vivo MR blockade with low-dose spironolactone on systemic insulin sensitivity, parameters of skeletal muscle insulin metabolic signaling, and insulin-stimulated glucose uptake in relation to NADPH oxidase activity/ROS in young insulin-resistant Ren2 rats.
METHODS
Animals and Treatments
All animal procedures were approved by the University of Missouri animal care and use committees, and animals were housed in accordance with National Institutes of Health guidelines. Ren2 (6–7 wk of age) and age-matched Sprague-Dawley control (SD) littermates were randomly assigned to untreated (Ren2-C and SD-C, respectively) or spironolactone-treated (Ren2-Sp and SD-Sp) paradigms. Ren2-Sp and SD-Sp animals were implanted with a 5-mg timed-release spironolactone or placebo pellet subcutaneously (Innovative Research of America, Sarasota, FL) for 21 days. Pellets were placed, with animals under anesthesia, via a superscapular incision closed with permanent suture.
Systolic Blood Pressure and Body Weight
Restraint conditioning was initiated before blood pressure measurements. Systolic blood pressure (SBP) was measured in triplicate on separate occasions throughout the day using the tail-cuff method (Harvard Systems, Student Oscillometric Recorder) before initiation of treatment and on day 19 or 20 before death at 21 days (33, 36). Total body weight was obtained before initiation of treatment and at time of death.
Intraperitoneal Glucose Tolerance Test
Animals were fasted overnight before the experiment. On day 21, the rats were weighed and anesthetized with Nembutal (35 mg/kg ip). The femoral artery was carefully dissected and cannulated with a 27-gauge angiocatheter. A 200-μl blood sample was drawn for insulin and glucose measurements. A dose of dextrose (50% solution, 1 g/kg body wt) was injected intraperitoneally, and blood was drawn at 15, 30, 45, and 60 min for insulin and glucose determination. Serum glucose concentrations were determined by means of the glucose oxidase method, and serum was separately analyzed for insulin (ELISA kit; Linco, St. Charles, MO). Insulin resistance index was calculated as the product of areas under the glucose and insulin curves (AUCGlucose × AUCInsulin) as previously described (13).
Oxidative Stress
NADPH oxidase activity.
NADPH oxidase activity was determined in plasma membrane fractions as previously described (5, 33, 35, 36). Aliquots of soleus muscle membrane and cytosolic fractions (12.5–100 mg of proteins) were incubated with NADPH (100 mM) at 37°C. NADPH oxidase activity was determined by measuring the conversion of Radical Detector (Cayman Chemical) in the absence and presence of NADPH inhibitor diphenylene iodonium sulfate (dpi) (500 μM) using spectrophotometric (450 nm) techniques.
NADPH oxidase subunit immunostaining.
Harvested soleus muscle tissue was immersed and fixed in 3% paraformaldehyde and prepared as previously described (34). Blocks were sectioned and incubated with a 1:100 dilution of primary antibodies in 10-fold diluted blocking agent, and third/fourth sections were washed and kept in the blocker. Over the course of 48 h, a fifth, sixth, and seventh section was incubated with 1:100 goat gp91phox (NOX2) (Santa Cruz Biotechnology), 1:100 goat p22phox, and 1:100 and p47phox antibody (Upstate Cell Signaling), respectively, in 10-fold diluted blocker. Other sections were incubated with 1:300 Alexa-fluor rabbit anti-goat 647 (Molecular Probes, Eugene, OR) for 4 h and examined using a laser confocal scanning microscope; images were captured by use of Laser-Sharp software (Bio-Rad), and signal intensities were measured with MetaVue software.
4-Hydroxy-2-nonenal immunostaining.
Anti-4-hydroxy-2-nonenal (anti-4-HNE) antibody was used to detect lipid peroxidation as a marker of ROS generation. Cryostat sections (5 μm) of the soleus muscle samples were microwaved for antigen retrieval. The sections were incubated overnight at 4°C with anti-4-HNE antibody. After a washing, secondary antibody conjugated with Alexa 568 (Molecular Probes) was applied. The images were acquired with a laser-scanning confocal microscope (Olympus IX70). Three sections were examined for each animal studied, with four animals in each group.
ROS formation.
Levels of ROS in skeletal muscle, using isolated soleus tissue sections, were measured by chemiluminescence. Tissue sections were homogenized in sucrose buffer (250 mM sucrose, 0.5 mM EDTA, 50 mM HEPES, protease inhibitor tablet, pH 7.5) using a glass/glass homogenizer. Homogenates were centrifuged: 1,500 rcf × 10 min at 4°C. Supernatants (whole homogenate) were then removed and placed on ice. Whole homogenate (100 μl) was added to 1.4 m of 50 mM phosphate (KH2PO4) buffer (150 mM sucrose, 1 mM EGTA, 5 μM lucigenin, 100 μM NADPH, pH 7.0) in dark-adapt counting vials. After dark adaptation for 1 h, samples were counted every 30 s for 10 min on a scintillation counter, and the last 5 min were averaged. Samples were then normalized to total protein in the whole homogenate. Values are expressed as counts per minute per milligram of protein (cpm/mg).
Glucose Transport
2-Deoxyglucose transport was measured in isolated soleus strips incubated in the presence or absence of a maximally effective dose of insulin (100 nM) as previously described (5, 29). Soleus muscles were dissected out of anesthetized rats that had been fasted overnight. The soleus muscle was carefully dissected into longitudinal strips (<40 mg) and incubated for 45 min in 4 ml of preincubation buffer that had been brought to 37°C and aerated with 95% O2-5% CO2 gas. Preincubation buffer consisted of 8 mM glucose, 32 mM mannitol, and 0.1% BSA in modified Krebs-Henseleit buffer (KHB). After additional incubation for 15 min with or without insulin, muscles were transferred to a rinse buffer (40 mM mannitol and 0.1% BSA in KHB) for 10 min. Thereafter, individual strips were transferred to flasks containing oxygenated incubation buffer (1 mM [3H]-2-deoxyglucose, 39 mM [14C]mannitol, and 0.1% BSA in KHB) with or without insulin for 20 min. Muscle strips were then removed, trimmed of excess tendon, blotted, and snap frozen in liquid nitrogen using aluminum tongs. Weighed samples were placed in 1 ml of 0.1 N NaOH solution, and 1 ml of 0.1 N HCl was added to neutralize the samples before the addition of 15 ml of ScintiVerse SX18-4 (Fisher Scientific). Samples were analyzed with a Beckman scintillation counter set for dual-channel detection (3H and 14C).
Quantification of Insulin Receptor Substrate-1, Akt, and GLUT4
After the animals were euthanized, a strip of soleus muscle was fixed in 3% paraformaldehyde and processed for immunostaining as previously described (35). Then, the 4-μm sections were incubated with rabbit anti-insulin receptor substrate-1 (anti-IRS-1), 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-pIRS-1 Tyr941, 1:50 (phosphatidylinositol 3-kinase binding site; Upstate Biotechnology, Lake Placid, NY); anti-total Akt, 1:75 (Cell Signaling Technology, Charlottesville, VA); anti-Akt (Ser473), 1:75 (Cell Signaling Technology); and mouse anti-GLUT4, 1:100 (Santa Cruz Biotechnology) overnight. The sections were then washed and incubated with 1:300 Alexa-fluor donkey anti-rabbit 647, except for GLUT4, on which donkey anti-mouse was used. After 4 h the sections were mounted with Mowiol, the images were captured, and the signals were analyzed as previously described (35).
Statistics
All results are presented as means ± SE. Analyses of variance (ANOVA) with Fisher's least significant differences (LSD) and Dunnett's multiple post hoc testing were performed as well as unpaired t-test, as appropriate.
RESULTS
SBP
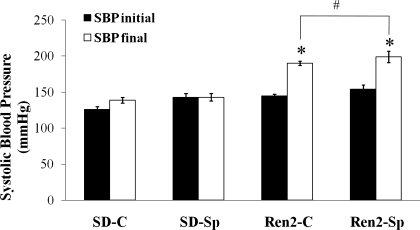
There were no significant differences among the study groups with respect to age or weight. At initiation of treatment (6–7 wk of age), SBP were higher in Ren2-C (145.8 ± 1.7 mmHg) compared with SD controls (129.8 ± 1.1 mmHg, P < 0.05) (Fig. 1). At the end of the treatment period (9–10 wk of age), there was a significant increase in SBP in Ren2-C (192.5 ± 1.2 mmHg) compared with SD-C (142.2 ± 3.3 mmHg, P < 0.05). No reduction in SBP was observed with spironolactone treatment in the Ren2-Sp group (194.3 ± 7.0 mmHg, P > 0.05).
Fig. 1.
Low-dose spironolactone does not reduce systolic blood pressure (SBP) in the transgenic TG(mRen2)27 rat (Ren2). SBP was measured before starting the experimental protocol and at days 19 and 20 before death (day 21). Sprague-Dawley control (SD-C, n = 6), Sprague-Dawley treated with spironolactone (SD-Sp, n = 4), Ren2 control (Ren2-C, n = 5), Ren2 treated with spironolactone (Ren2-Sp, n = 5). Values are means ± SE. *P < 0.05 compared with SD-C; #P > 0.05 compared with Ren2-C.
Whole Body Insulin Sensitivity Studies
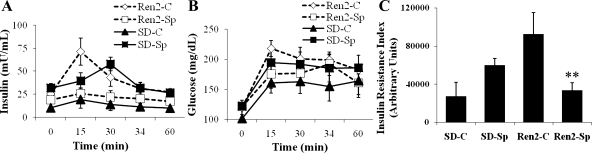
Insulin sensitivity was analyzed during an intraperitoneal glucose tolerance test (IPGTT), in which both serum insulin and glucose concentrations were determined and their respective AUC were calculated. Insulin resistance index (AUCGlucose × AUCInsulin) analysis demonstrated significantly higher insulin resistance in Ren2 control animals compared with SD controls (92.2 ± 22.2 × 103 vs. 27.1 ± 14.7 103 arbitrary units, P < 0.05) (Fig. 2). In vivo treatment with low-dose spironolactone significantly improved whole body insulin sensitivity in Ren2 rats (P < 0.05). Indeed, the insulin resistance index in treated Ren2 animals treated with low-dose spironolactone was not different from SD controls (27.1 ± 14.7 × 103 vs. 33.4 ± 78.5 × 103 arbitrary units, P > 0.05).
Fig. 2.
Low-dose spironolactone improves insulin resistance in Ren2. Insulin sensitivity measured during an intraperitoneal glucose tolerance test (IPGTT) performed after overnight fast on day 21. Samples for serum insulin (A) and glucose (B) were obtained at 0, 15, 30, 45, and 60 min after administering 50% dextrose, 1 g/kg ip. Areas under the curve (AUC) were calculated for insulin and glucose concentrations, and the insulin resistance index (C) was calculated as the product of the AUC for glucose and insulin. Sprague-Dawley control (SD-C, n = 4), Sprague-Dawley treated with spironolactone (SD-Sp, n = 4), Ren2 control (Ren2-C, n = 7), Ren2 treated with spironolactone (Ren2-SP, n = 4). Values are presented as means ± SE. **P < 0.05 compared with Ren2-C.
Soleus Muscle NADPH Oxidase and Oxidative Stress
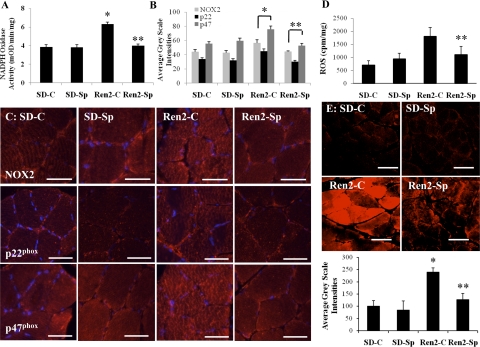
NADPH oxidase activity was elevated in Ren2 (6.32 ± 0.22 mOD·mg−1·min−1) compared with SD controls (3.84 ± 0.24 mOD·mg−1·min−1, P < 0.05) and was normalized in the Ren2-Sp group (3.98 ± 0.17, P < 0.05) (Fig. 3A) to a value comparable with SD-C. There were similar trends in NADPH oxidase subunits NOX2, p22phox, and p47phox with increases in the Ren2 control (56 ± 4.8, 44.9 ± 3.0, and 75.8 ± 4.5 average gray scale intensities, respectively) compared with SD-C (44.0 ± 3.0, 33.6 ± 2.0, and 55.2 ± 2.8 average gray scale intensities, respectively; P < 0.05) (Fig. 3, B and C). There were improvements in treated Ren2-Sp (44.1 ± 1.0, 30.2 ± 1.8, and 52.5 ± 3.6 average gray scale intensities, respectively; P < 0.05).
Fig. 3.
Low-dose spironolactone improves measures of oxidative stress in Ren2. A: NADPH oxidase activity. B and C: NADPH oxidase subunits. D: ROS formation by chemiluminescence. E: 4-hydroxy-2-nonenal (4-HNE) immunostaining was used to detect lipid peroxidation as a marker of reactive oxygen species (ROS). Sprague-Dawley control (SD-C; n = 6 for NADPH oxidase activity, NADPH oxidase subunits and ROS, n = 4 for 4-HNE), Sprague-Dawley treated with spironolactone (SD-Sp; n = 6 for NADPH oxidase activity, NADPH oxidase subunits and ROS, n = 4 for 4-HNE), Ren2 control (Ren2-C; n = 5 for NADPH oxidase, NADPH oxidase subunits and ROS, n = 4 for 4-HNE), and Ren2 treated with spironolactone (Ren2-Sp; n = 5 for NADPH oxidase, NADPH oxidase subunits and ROS, n = 4 for 4-HNE). Values are presented as means ± SE. *P < 0.01 compared with SD-C. **P < 0.05 compared with Ren2-C. Scale bar = 50 μm.
Soleus tissue ROS levels were higher in Ren2 controls (1,805 ± 343 cpm/mg) compared with the placebo-treated SD group (701 ± 175 cpm/mg, P < 0.05). There was a similar trend to lower ROS activity observed in the Ren2-Sp treated rat muscles (1,099 ± 320 cpm/mg, P > 0.05) (Fig. 3D). 4-HNE immunostaining, a surrogate for ROS-mediated membrane lipid peroxidation, was increased in soleus muscle of Ren2-C (240.4 ± 16.0 average gray scale intensities) compared with SD-C (100.0 ± 22.0 average gray scale intensities, P < 0.05) and improved with low-dose spironolactone treatment (126.9 ± 25.0 average gray scale intensities, P < 0.05) (Fig. 3E).
Insulin-Stimulated Glucose Uptake Measurements
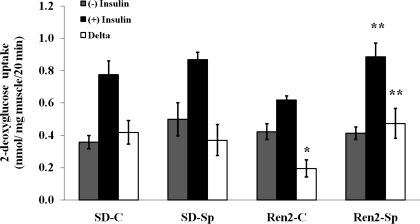
Skeletal muscle 2-deoxyglucose uptake response to insulin was measured in the absence and presence of maximally effective doses of insulin (100 nM) (Fig. 4). There was no significant difference (P > 0.05) in basal (not insulin stimulated) glucose 2-deoxyglucose uptake in SD and treated or untreated Ren2 animals. With the addition of insulin, 2-deoxyglucose uptake was nonsignificantly greater in SD (0.78 ± 0.1 mmol/mg) compared with skeletal muscle from untreated Ren2 rats (0.62 ± 0.03 mmol/mg, P > 0.05). However, in vivo low-dose spironolactone treatment significantly improved insulin-stimulated glucose uptake when compared with placebo-treated Ren2 animals (0.89 ± 0.1 mmol/mg, P < 0.05).
Fig. 4.
Low-dose spironolactone improves glucose transport in Ren2. 2-Deoxyglucose uptake analyzed in ex vivo soleus muscle strips in the absence and presence of a maximally effective dose of insulin. Sprague-Dawley control (SD-C, n = 6), Sprague-Dawley treated with spironolactone (SD-Sp, n = 4), Ren2 control (Ren2-C, n = 5), and Ren2 treated with spironolactone (Ren2-Sp, n = 5). Values are expressed as means ± SE. *P < 0.05 compared with SD-C. **P < 0.05 compared with Ren2-C.
Insulin-stimulated increment (delta) in glucose uptake in untreated Ren2 was nonsignificantly lower compared with untreated SD animals (delta: 0.42 ± 0.1 vs. 0.20 ± 0.1 nmol·mg muscle−1·20 min−1, P = 0.06). Treatment with spironolactone in Ren2 rats increased insulin-stimulated glucose uptake (delta: 0.47 ± 0.1 nmol·mg muscle−1·20 min−1, P < 0.05) compared with untreated Ren2.
IRS-1, Akt, and GLUT4 Immunostaining
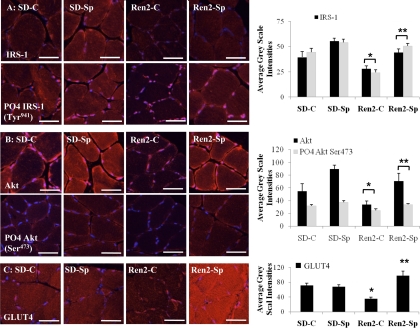
Consistent with glucose uptake studies, there were decreases in IRS-1, Akt, and GLUT4 immunostaining in the soleus muscle of Ren2 rats that improved significantly with low-dose spironolactone treatment. Total and tyrosine (Tyr941) phosphorylated IRS-1 were reduced in the Ren2-C (28.0 ± 2.9 and 24.3 ± 2.9 average gray scale intensities, respectively) compared with SD-C (39.6 ± 5.5 and 44.5 ± 3.9 average gray scale intensities, respectively; P < 0.05) (Fig. 5A). There were improvements in total and Tyr941 phosphorylated IRS-1 in the Ren2-Sp (44.2 ± 3.6 and 50.7 ± 2.5 average gray scale intensities, respectively; P < 0.05). Similarly, there were reductions in total and serine (Ser473) phosphorylated Akt (34.0 ± 5.6 and 24.9 ± 2.2 average gray scale intensities, respectively) compared with SD-C (54.7 ± 11.7 and 32.0 ± 1.9 average gray scale intensities, respectively; P < 0.05). Following spironolactone treatment, Akt Ser473 phosphorylation was increased compared with placebo treatment in Ren2-Sp (70.6 ± 12.3 and 34.1 ± 1.5 average gray scale intensities, respectively; P < 0.05) (Fig. 5B). There were reductions in skeletal muscle GLUT4 in Ren2-C (34.6 ± 4.5 average gray scale intensities) compared with SD-C (70.8 ± 6.4 average gray scale intensities, P < 0.05), and GLUT4 levels increased in Ren2-Sp (96.9 ± 13.3 average gray scale intensities, P < 0.05) (Fig. 5C).
Fig. 5.
Low-dose spironolactone improves insulin receptor substrate (IRS), GLUT4, and Akt in Ren2. A: representative fluorescent images of total IRS-1 and tyrosine (Tyr941) phosphorylated (PO4) IRS-1 and quantification of converted signal intensities in average gray scale intensities to the right. B: representative fluorescent images of total and serine (Ser473) phosphorylated (PO4) Akt and quantification of converted signal intensities in average gray scale intensities to the right. C: representative fluorescent images of GLUT4 and quantification of converted signal intensities in average gray scale intensities to the right. Sprague-Dawley control (SD-C, n = 6), Sprague-Dawley treated with spironolactone (SD-Sp, n = 4), Ren2 control (Ren2-C, n = 5), and Ren2 treated with spironolactone (Ren2-Sp, n = 5). *P < 0.05 compared with SD-C. **P < 0.05 compared with Ren2-C. Scale bar = 50 μm.
DISCUSSION
The present investigation explored the effect of a very low non-blood pressure-lowering dose of the MR blocker spironolactone on oxidative stress and insulin-stimulated glucose transport in skeletal muscle from a rodent model of increased tissue RAS activation and elevated aldosterone levels. In vivo treatment of young Ren2 rats with low dose of spironolactone for 3 wk improved systemic insulin sensitivity, reduced soleus muscle NADPH oxidase activity/ROS production, and improved skeletal muscle parameters of insulin metabolic signaling as well as insulin-stimulated glucose transport. That these beneficial effects were independent of changes in SBP suggests direct MR-mediated effects on skeletal tissue. Thus this is the first study that demonstrates that MR antagonism directly affects skeletal muscle insulin metabolic signaling in conjunction with reductions in oxidative stress.
As previously observed (5), oxidative stress as measured by skeletal muscle NADPH oxidase activity, ROS production, and membrane lipid peroxidation were increased in Ren2 compared with control SD rats. These MR-mediated effects on skeletal muscle oxidative stress and insulin metabolic signaling are similar to previously observed effects of AT1R activation (5, 33). Our laboratory has previously observed that in vivo treatment of young Ren2 rats with a ROS scavenger or AT1R blocker results in decreases in skeletal muscle NADPH oxidase activity and ROS generation, associated with improved systemic and skeletal muscle insulin sensitivity (5, 33). Collectively, these observations suggest that both ANG II and aldosterone reduce skeletal muscle insulin metabolic signaling, in part, through increases in oxidative stress.
NADPH oxidase is a highly regulated membrane-bound enzyme complex that catalyzes the production of superoxide anion (O2−). Complex components include membrane-bound subunits p22phox and Nox2 as well as cytosolic regulatory subunits p47phox, p67phox, and p40phox and the small GTP binding protein Rac1/Rac2, all of which are expressed in skeletal muscle tissue (5, 15, 33). Activation of the complex involves the interaction between cytosolic subunits p47phox and p67phox, followed by their translocation to the plasma membrane along with COOH-terminal-prenylated Rac1 (28), where they interact with plasma membrane-bound subunits (2). There is an emerging body of evidence demonstrating that mineralocorticoids, like ANG II, may activate NADPH oxidase in various tissues (14, 16, 20). Results from the present investigation indicate that MR activation increases skeletal muscle NADPH oxidase activity, in part via activation of membrane-bound Nox2 and p22phox as well as the cytosolic p47phox subunits. The resulting generation of ROS appears to be associated with reduced IRS-1 levels, reduced Tyr phosphorylation of IRS-1 and Akt phosphorylation/activation as well as insulin-stimulated glucose transport.
The results of this investigation complement observational studies in humans demonstrating an association among increased levels of aldosterone, impaired glucose homeostasis, and insulin resistance. For example, patients with primary aldosteronism, either tumor induced or idiopathic, manifest a greater insulin response to an oral glucose load as well as reduced systemic insulin sensitivity compared with age-, sex-, and body mass index-matched normotensive patients (9). Surgical removal of aldosterone-producing adenoma or medical therapy with spironolactone restored insulin sensitivity, further supporting a direct role of aldosterone in mediating systemic insulin resistance.
There are several potential mechanisms by which MR activation may reduce insulin metabolic signaling in skeletal muscle tissue. One potential mechanism for aldosterone-induced insulin resistance appears to be the transcriptional downregulation of the insulin receptor in addition to impairment of the intracellular insulin signaling (8). Glucocorticoid response elements (GRE) have been identified in the promoter of the insulin receptor gene (19), and interactions between activated MR and GRE may result in a negative transcriptional effect of the aldosterone over the insulin receptor gene (8). Aldosterone also activates glucocorticoid receptors in extrarenal tissue (37), which has direct negative effects on insulin metabolic signaling (23). A recent report suggests that aldosterone suppresses insulin metabolic signaling via proteosomal degradation of the IRS-1 docking protein in vascular smooth muscle cells (14). Commensurate with our results, in vitro treatment with an MR antagonist abolished the reduction in IRS-1 expression, Akt phosphorylation, and glucose transport in vascular smooth muscle cells. It was also observed that treatment with antioxidants such as N-acetylcysteine or an inhibitor of the ubiquitin proteosomal pathway prevented aldosterone-induced reductions of IRS-1 (14). Reductions in Tyr phosphorylated IRS-1, as observed in the present investigation, would in turn result in less engagement of IRS-1 docking protein with phosphoinositol kinase (PI3-K) and decreased downstream Akt phosphorylation/activation, as was observed in this study. Collectively, these observations suggest that aldosterone likely acts through MR-mediated stimulation of redox-sensitive serine kinase signaling pathways (3, 14) to promote proteosomal degradation of IRS-1 with consequent reductions in insulin metabolic signaling through the PI3-K/Akt and GLUT4 facilitated glucose uptake.
A limitation of our study is the absence of measurements of plasma spironolactone concentrations, which would have assessed the absorption from the pellets. However, use of spironolactone pellets is supported in the available literature, and it is estimated that the absorption is adequate to elicit biological activity (1, 7). Also, previous studies in our laboratory have demonstrated that, in cultured myocytes (L6 cells), ANG II is linked to increased activity of NADPH oxidase and production of ROS as well as impairment of intracellular insulin signaling mediated through IRS-1 and Akt pathways (33), which were reversed by blocking AT1R and ROS scavenging. However, this study did not specifically include measurements of mineralocorticoids, and thus we cannot draw conclusions about the impact of these strategies on mineralocorticoid activity in cultured myocytes.
Our data support the hypothesis that MR antagonism can reduce oxidative stress and improve insulin sensitivity in a rodent model of activated RAAS activity and insulin resistance. These effects were obtained in skeletal muscle, a classical target tissue of insulin action, using low-dose spironolactone and were independent of changes in SBP. These data add to previous observations that increased oxidative stress leads to impaired insulin sensitivity in skeletal muscle and that RAAS interruption using AT1R blockade and/or ANG-converting enzyme inhibition attenuates these changes (5, 33). The specific mechanisms of the beneficial effects of MR blockade remain to be fully elucidated and potentially include local actions in skeletal muscle tissue in addition to reduced NADPH oxidase-mediated oxidative stress. Furthermore, in addition to direct effects on skeletal muscle tissue, systemic MR antagonism and consequent effects on other mineralocorticoid target tissues implicated in energy homeostasis, such as adipose and brain tissues, may be important in mediating improvements in systemic insulin sensitivity.
Interruption of MR signaling is particularly attractive, as increased activation of these receptors is linked to multiple pathologic mechanisms that lead to CVD, including hypertension, oxidative stress, inflammation, apoptosis and fibrosis in cardiovascular and renal tissue. Moreover, MR antagonism has provided solid benefits in terms of cardiovascular morbidity and mortality, as demonstrated in the RALES and EPHESUS trials (21, 22). A better understanding of the mechanisms underlying the participation of MR activation in oxidative stress and insulin resistance could thus identify new therapeutic targets and potentially reduce the disease burden imposed by insulin resistance that frequently accompanies hypertension (38).
GRANTS
This research was support by National Institutes of Health (NIH) Grant R01-HL-73101-01A1 (J. R. Sowers), Veterans Affairs Merit System 0018 (J. R. Sowers) awards and VISN 15 (A. Whaley-Connell), NIH Grant P01-HL-51952 (C. Ferrario), and the Missouri Kidney Program (A. Whaley-Connell). Male transgenic Ren2 rats and male Sprague-Dawley controls were kindly provided by C. Ferrario, Wake Forest University School of Medicine, Winston-Salem, NC, through the Transgenic Core Facility supported in part by NIH Grant P-01-HL-51952.
Acknowledgments
Special thanks to Grace Uptergrove and Suzanne Clark for technical assistance in completion of the studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na+/Cl−-cotransporter abundance: role of aldosterone. J Am Soc Nephrol 12: 1335–1341, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Abo A, Pick A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxides involves the small GTP binding protein p21rac1. Nature 353: 668–670, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Marquez-Rodas I, Salaices M, Xavier FE, Ferrer M, Balfagon G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive hypertensive rats. Hypertension 46: 107–112, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab 288: E353–E359, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bochud M, Nussberger J, Bovet P, M, Shamlaye C, Burnier M. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension 48: 239–245, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brown NJ, Nakamura S, Ma LJ, Donnert E, Freeman M, Vaughan DE, Fogo AB. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 58: 1219–1227, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Calle C, Campion J, Garcia-Arencibia M, Maestro B, Davila N. Transcriptional inhibition of the human insulin receptor gene by aldosterone. J Steroid Biochem Mol Biol 84: 543–553, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, Favret G, Melis A, Cavarape A, Sechi LA. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 91: 3457–3463, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol 186: 1–20, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Fallo F, Veglio F, Bertello C, Sonino N, Mea PD, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 91: 454–459, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Funder JW The nongenomic actions of aldosterone. Endocr Rev 26: 313–321, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension 38: 884–890, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension 50: 750–755, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilator muscles. Am J Respir Crit Care Med 165: 412–418, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Johnson FK, Johnson RA, Durante W. Aldosterone promotes endothelial dysfunction via prostacyclin independent of hypertension. Hypertension 46: 29–30, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kraus D, Jager J, Meier B, Fasshauer M, Klein J. Aldosterone inhibits uncoupling protein-1, induces insulin resistance, and stimulates proinflammatory adipokines in adipocytes. Horm Metab Res 37: 455–459, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Tsai SY. Multiple hormone response elements can confer glucocorticoid regulation on the human insulin receptor gene. Mol Endocrinol 8: 625–634, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimiura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16: 2906–2912, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Remme WJ, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab 292: E654–E667, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Rebuffat P, Rocco S, Andreris PG. Morphology and function of the adrenal zona glomerulosa of transgenic rats TGR[mREN-2] 27: effects of prolonged sodium restriction. J Steroid Biochem Mol Biol 54: 155–162, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Rocchini AP, Moorehead C, De Remer S, Goodfriend TL, Ball DL. Hyperinsulinemia and the aldosterone pressor responses to angiotensin II. Hypertension 15: 861–866, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Sander M, Bader M, Djavidani B, Masanten D, Peters J. The role of the adrenal gland in hypertensive transgenic rat TGR(mREN-2) 27. Endocrinology 131: 807–814, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Schiffrin EL Effects of aldosterone on the vasculature. Hypertension 47: 312–218, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Sigal N, Gorzalczany Y, Sarfstein R, Weinbaum C, Zheng Y, Pick E. The guanine exchange factor trio activates the phagocyte NADPH oxidase in the absence of GDP to GTP exchange on Rac. J Biol Chem 7: 4854–4861, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Sloniger JA, Saengsiriswan V, Diehl CJ, Henriksen EJ. Selective angiotensin II receptor antagonism enhances whole-body insulin sensitivity and muscle glucose transport in hypertensive TG(mREN2)27 rats. Metabolism 54: 1659–1668, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the heart: role of oxidative stress. Am J Pathol 161: 1773–1781, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urukawa H, Katsuki A, Sumida Y, Gabazza EC, Shuichi M, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatami K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab 88: 4673–4676, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Vierhapper H, Waldhausl W, Nowotny P. The effect of insulin on the rise in blood pressure and plasma aldosterone after angiotensin II in normal man. Clin Sci (Lond) 64: 383–386, 1983. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137–35146, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Whaley-Connell A, Chen K, Habibi J, Uptergrove G, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance and remodeling in the transgenic (mRen2) rat. Hypertension 50: 384–391, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi P, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of angiotensin II-mediated NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita R, Kikuchi T, Mori Y, Aoki K, Kaburagi Y, Yasuda K, Sekihara H. Aldosterone stimulates gene expression of hepatic gluconeogenic enzymes through the glucocorticoid receptor in a manner independent of the protein kinase B cascade. Endocr J 51: 243–251, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Zimmet P, Boyko EJ, Collier GR, De Courten M. Etiology of the metabolic syndrome: potential role of insulin resistance, leptin resistance and other players. Ann NY Acad Sci 892: 25–43, 1999. [DOI] [PubMed] [Google Scholar]