Abstract
This review identifies the various pathways responsible for modulating hepatic protein synthesis following acute and chronic alcohol intoxication and describes the mechanism(s) responsible for these changes. Alcohol intoxication induces a defect in global protein synthetic rates that is localized to impaired translation of mRNA at the level of peptide-chain initiation. Translation initiation is regulated at two steps: formation of the 43S preinitiation complex [controlled by eukaryotic initiation factors 2 (eIF2) and 2B (eIF2B)] and the binding of mRNA to the 40S ribosome (controlled by the eIF4F complex). To date, alcohol-induced alterations in eIF2 and eIF2B content and activity are best investigated. Ethanol decreases eIF2B activity when ingested either acutely or chronically. The reduced eIF2B activity most likely is a consequence of twofold increased phosphorylation of the α-subunit of eIF2 on Ser51 following acute intoxication. The increase in eIF2α phosphorylation after chronic alcohol consumption is the same as that induced by acute ethanol intoxication, and protein synthesis is not further reduced by long-term alcohol ingestion despite additional reduced expression of initiation factors and elongation factors. eIF2α phosphorylation alone appears sufficient to maximally inhibit hepatic protein synthesis. Indeed, pretreatment with Salubrinal, an inhibitor of eIF2α(P) phosphatase, before ethanol treatment does not further inhibit protein synthesis or increase eIF2α phosphorylation, suggesting that acute ethanol intoxication causes maximal eIF2α phosphorylation elevation and hepatic protein synthesis inhibition. Ethanol-induced inhibition of hepatic protein synthesis is not rapidly reversed by cessation of ethanol consumption. In conclusion, sustained eIF2α phosphorylation is a hallmark of excessive alcohol intake leading to inhibition of protein synthesis. Enhanced phosphorylation of eIF2α represents a unique response of liver to alcohol intoxication, because the ethanol-induced elevation of eIF2α(P) is not observed in skeletal muscle or heart.
Keywords: mRNA translation initiation, alcohol, eukaryotic initiation factor 2B, eukaryotic initiation factor 2
in western industrialized countries, approximately two-thirds of the entire population above the age of 18 yr consumes alcohol. Chronic alcoholic beverage abuse is the leading cause of end-stage liver disease and subsequent mortality in the United States (46). The liver is particularly at risk for alcohol-related damage because it receives portal blood directly from the intestinal tract and thus experiences the highest concentration of alcohol presented to any organ. In addition, ethanol metabolism in the liver produces potentially harmful toxic metabolites such as acetaldehyde, acetate, and reactive oxygen species (24). Clinically, the syndrome leading to end-stage hepatic failure in alcoholics is referred to as alcoholic liver disease. Liver pathologies, including steatosis, alcoholic hepatitis, and cirrhosis of varying degrees, typify the alcoholic liver disease process (44) and result from both direct and indirect effects of ethanol. Furthermore, decompensated alcoholic liver disease requiring admission to intensive care units is associated with high hospital mortality rates (26). Indeed, the 4-yr mortality is >60% in patients with cirrhosis and superimposed alcoholic hepatitis. Alcoholic liver disease has a known etiology but possesses a complex and incompletely known pathogenesis. In addition to the cumulative amount of alcohol intake and alcohol consumption patterns, factors such as sex and ethnicity, genetic background, nutrition, energy metabolism abnormalities, oxidative stress, immunological mechanisms, and hepatic comorbidity conditions play a key role in the genesis and progression of alcoholic liver injury (66).
Animal models of acute alcohol intoxication and chronic alcohol consumption have been developed to approximate human “binge drinking” and long-term alcohol abuse. In acute models, animals receive ethanol by oral gavage, intraperitoneal injection, or intragastric infusion, and livers are usually examined within several hours of ethanol administration. Blood alcohol concentrations in these animals reach ∼250–450 mg/dl, similar to concentrations observed in humans after binge drinking. Variations of two different animal models of chronic ethanol consumption are commonly employed: 1) an all-liquid diet, fed ad libitum or intragastrically infused (13, 25), and 2) a mixed solid-liquid diet, where ethanol is supplied in the drinking water (10–12%, vol/vol) with solid chow ad libitum (48) or solid chow ad libitum supplemented with agar containing 30–40% ethanol (3, 23, 60). Blood alcohol concentrations fluctuate widely but range from 50 to 150 mg/dl, a level sufficient to cause impaired motor activity in humans. Controls of all models are pairfed to the ethanol-consuming groups so that nutritional intake is approximately equal among groups. The liquid and mixed solid-liquid diets differ in that animals on the liquid diet rapidly develop steatosis (enlarged fatty liver) (25), whereas animals on the mixed diet fail to develop overt steatosis (41).
Besides its central role in intermediary nutrient metabolism, the liver synthesizes plasma proteins essential for maintenance of whole body homeostasis and host defense. Alcohol affects the turnover of both resident and secreted liver proteins (35, 42). Hepatic protein turnover can be altered by changes in the rate of protein synthesis and/or the rate of proteolysis, but additional factors also determine liver weight and total protein content. Synthesis and storage of lipid in steatosis increase liver weight, and the associated increase in fatty acid binding protein (34) contributes to the protein content. Alcohol-induced inhibition of secretion leads to accumulation of exported proteins such as albumin and transferrin (2, 52, 63). Decreased plasma concentrations of these two proteins would be expected to alter homeostatic mechanisms. In addition, alcohol intoxication inhibits degradation of both secreted and resident hepatic proteins (9, 12, 27, 36, 37).
Protein synthesis is also inhibited by ethanol. Ethanol inhibits protein synthesis in isolated hepatocytes in culture (14, 29, 64) and decreases protein (albumin) synthesis in isolated perfused rabbit (31) and rat liver (17) and in rat liver slices (52). Furthermore, both acute alcohol intoxication (39, 51) and chronic feeding of an alcohol-containing diet (19, 38, 47, 48) depress hepatic protein synthesis in vivo. Even moderate social drinking in humans (a bottle of wine with a mixed meal over a 3-h period, inducing blood alcohol of 75 mg/dl) inhibits hepatic protein synthesis (7). Therefore, understanding the molecular mechanisms of alcohol action on protein synthesis may have implications for prevention or treatment of alcohol-related liver disease.
Within the last decade, identification of the molecular mechanisms underlying the inhibitory effect of alcohol on protein synthesis has begun. This review presents the current understanding of the means by which acute and chronic alcohol consumption alter steps in the complex scheme of molecular reactions that comprise the translation pathway of mRNA into proteins. To date, most studies have focused on alcohol-induced alterations in content and activity of the translation initiation factors eIF2 and eIF2B with less emphasis on the eIF4 system.
Pathway of Protein Synthesis
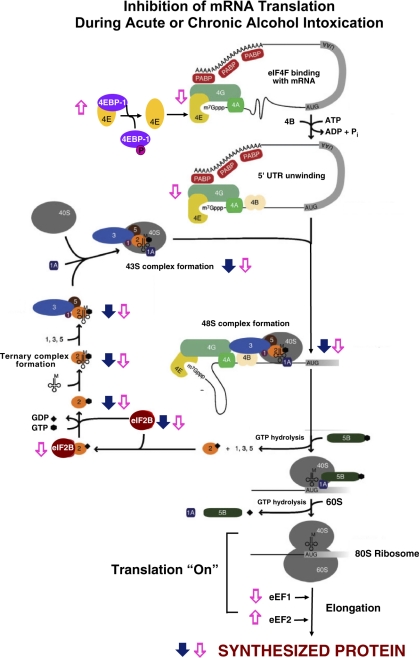
Translation of mRNA into protein is a multistep process that involves the association of the 40S and 60S ribosomal subunits, mRNA, initiator methionyl-tRNA (met-tRNA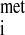 ), other amino acyl-tRNAs, cofactors (i.e., GTP, ATP), and protein factors, collectively known as eukaryotic initiation factors (eIFs), elongation factors (eEFs), and releasing factors (RFs) (Fig. 1). The total abundance of RNA is not reduced by acute ethanol intoxication, indicating that the number of ribosomes available for protein synthesis is unaffected (19). Chronic alcohol consumption does not change (23, 60) or slightly reduces (38) hepatic RNA content. The efficiency of translation, calculated by dividing protein synthesis rate by total RNA content, provides an index of how rapidly existing ribosomes synthesize protein. In livers from animals acutely consuming ethanol, the translational efficiency is reduced by ∼24% compared with fed control animals (19). Likewise, translational efficiency is depressed by chronic ethanol ingestion (23, 38). Thus the decrease in hepatic protein synthesis following alcohol intoxication results from decreased translational efficiency. Reduced translation efficiency can occur in any one or more of the three phases of mRNA translation: 1) initiation, whereby met-tRNA
), other amino acyl-tRNAs, cofactors (i.e., GTP, ATP), and protein factors, collectively known as eukaryotic initiation factors (eIFs), elongation factors (eEFs), and releasing factors (RFs) (Fig. 1). The total abundance of RNA is not reduced by acute ethanol intoxication, indicating that the number of ribosomes available for protein synthesis is unaffected (19). Chronic alcohol consumption does not change (23, 60) or slightly reduces (38) hepatic RNA content. The efficiency of translation, calculated by dividing protein synthesis rate by total RNA content, provides an index of how rapidly existing ribosomes synthesize protein. In livers from animals acutely consuming ethanol, the translational efficiency is reduced by ∼24% compared with fed control animals (19). Likewise, translational efficiency is depressed by chronic ethanol ingestion (23, 38). Thus the decrease in hepatic protein synthesis following alcohol intoxication results from decreased translational efficiency. Reduced translation efficiency can occur in any one or more of the three phases of mRNA translation: 1) initiation, whereby met-tRNA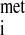 and mRNA bind to 40S ribosomal subunits and subsequent binding of the 40S ribosomal subunit to the 60S subunit forms a ribosome complex capable of translation (Fig. 1); 2) elongation, during which tRNA-bound amino acids are incorporated into growing polypeptide chains according to the mRNA template, regulated by eEF1 and eEF2; and 3) termination, where the ribosomal subunits and completed polypeptides are released.
and mRNA bind to 40S ribosomal subunits and subsequent binding of the 40S ribosomal subunit to the 60S subunit forms a ribosome complex capable of translation (Fig. 1); 2) elongation, during which tRNA-bound amino acids are incorporated into growing polypeptide chains according to the mRNA template, regulated by eEF1 and eEF2; and 3) termination, where the ribosomal subunits and completed polypeptides are released.
Fig. 1.
Inhibition of mRNA translation during acute or chronic alcohol intoxication. Filled arrows indicate location and direction of changes during acute intoxication; open arrows indicate location and direction of changes during chronic alcohol consumption (see text for details). Filled diamond, GDP; filled hexagon, GTP. PABP, polyA binding protein; 4EBP-1, eukaryotic initiation factor 4E (eIF4E) binding protein 1; 1A, eIF1A; 2, eIF2; 3, eIF3; 4A, eIF4A; 4B, eIF4B; 4E, eIF4E; 4G, eIF4G; 5B, eIF5B; AUG, translation start site; UAA, translation stop site; 40S, 40S ribosomal subunit; 60S, 60S ribosomal subunit.
Decreased translational efficiency may result from inhibition of peptide-chain initiation and/or elongation/termination. Relative rates of peptide-chain initiation and elongation can be assessed by the measurement of protein synthetic rates coupled with analysis of the distribution of ribosomal subunits between free subunits and polysomes. The amount of RNA in free ribosomal subunits reflects the balance between the rates of peptide-chain initiation and elongation/termination. Alcohol causes disaggregation of polysomes into free ribosomes (23, 31, 42). An increase in the relative abundance of free ribosomal subunits in conjunction with a decreased rate of protein synthesis in livers from rats consuming alcohol indicates that ethanol reduces the rate of peptide-chain initiation relative to elongation/termination. Therefore, the ethanol-induced reduction in hepatic translational efficiency results from a greater inhibition of peptide-chain initiation. The alcohol-induced inhibition of initiation of hepatic protein synthesis is not related to diminished amino acid concentrations (23, 45), high-energy phosphates (23), abundance of ribosomes (19, 23, 39), or aminoacyl-tRNA formation (15). Instead, the rate of peptide-chain initiation is modulated by changes in the amount and/or activity of various initiation factors.
Alcohol-Induced Changes in Translation Initiation
Two major regulatory sites in the pathway responsible for peptide-chain initiation are catalyzed by 1) the eIF2/eIF2B system, which controls formation of the 43S preinitiation complex (Fig. 2) ; and 2) the eIF4F system, which regulates association of the 43S complex with the 5′-cap of mRNA, forming the 48S preinitiation complex (Fig. 1).
Fig. 2.
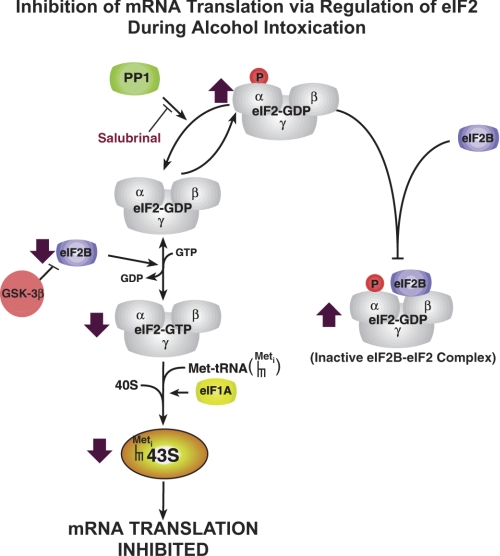
Inhibition of mRNA translation via regulation of eIF2 during alcohol intoxication. Filled arrows indicate location and direction of changes during both acute intoxication and chronic alcohol consumption (see text for details). PP1, protein phosphatase 1; GSK-3β, glycogen synthase kinase-3β.
Alterations in the eIF2/eIF2B system.
Formation of the 43S preinitiation complex, the first regulatory step in initiation, involves the attachment of the met-tRNAimet to the 40S ribosomal subunit, a reaction mediated by eIF2 (Figs. 1 and 2). eIF2 is a GTP-binding protein and cycles between GTP- and GDP-bound forms during initiation. eIF2·GDP is released with the other initiation factors when the 48S preinitiation complex combines with the 60S to form an 80S initiation complex capable of allowing elongation of the growing polypeptide chain to proceed. In this process, formation of the eIF2·GTP·met-tRNAimet ternary complex is required to activate the initiator met-tRNA and 40S ribosome to initiate another round of translation. At the end of each cycle of initiation, eIF2 is released in complex with GDP. Because the GTP-bound form of eIF2 is required for ternary complex formation, eIF2-bound GDP must be exchanged for GTP. The exchange of GDP for GTP on eIF2 is catalyzed by the nucleotide exchange factor eIF2B. Both acute alcohol intoxication and chronic alcohol consumption primarily disrupt the eIF2/eIF2B system (19, 23). eIF2B activity is inhibited through changes in its cellular content and phosphorylation of eIF2B on Ser535 of its ε-subunit (eIF2Bε) by a signaling pathway involving glycogen synthase kinase-3 (GSK-3) (65). It is not known, however, whether alcohol modulates GSK-3 activity in the liver.
In addition, the exchange activity of eIF2B is inhibited by phosphorylation of eIF2 on its α-subunit (eIF2α), which results in formation of a stable, inactive eIF2·eIF2B complex (Fig. 2). Phosphorylation of the α-subunit of eIF2 is increased by both acute intoxication and chronic ethanol intake with consequent reduction of eIF2B nucleotide exchange activity and inhibition of protein synthesis (19, 23). Acute administration of ethanol does not alter the cellular content of eIF2 or eIF2B (19). With continued alcohol abuse, eIF2α phosphorylation remains elevated (23). In addition, long-term alcohol ingestion reduces the cellular content of eIF2 (Karinch AM and Vary TC, unpublished observation) and of eIF2B (20% decrease) (23). Decreased eIF2B content may contribute to the decreased eIF2B exchange activity. Thus other changes in the eIF2/eIF2B system develop with chronic alcohol abuse, in addition to eIF2α phosphorylation.
Sustained eIF2α phosphorylation is a hallmark of excessive alcohol intake whether intoxication occurs acutely or chronically. eIF2α phosphorylation increased approximately twofold after both chronic alcohol consumption (23) and acute ethanol intoxication (19). Interestingly, protein synthesis was inhibited 24% by acute ethanol intoxication (19) and was not further reduced by long-term alcohol ingestion (25% decrease) (38) (Karinch AM and Vary TC, unpublished observation), suggesting that eIF2α phosphorylation alone is sufficient to maximally inhibit hepatic protein synthesis. There are at least two possible explanations for the failure of decreased eIF2 and eIF2B content to further depress protein synthesis. First, eIF2B is present in liver at a two- to threefold lower molar concentration than eIF2 (30). An effect of decreased eIF2B content on protein synthesis during chronic alcohol consumption may be negated by sequestration of the majority of eIF2B by phosphorylated eIF2α into inactive eIF2B·eIF2 complex (Fig. 2). Second, decreased eIF2B activity would be expected to increase the proportion of inactive eIF2·GDP complex compared with active eIF2·GTP complex, perhaps minimizing the effect of decreased eIF2 content. The ability of alcohol to enhance the phosphorylation of eIF2α represents a unique response of liver to alcohol intoxication, because ethanol-induced elevation of eIF2α(P) is not observed in skeletal muscle or heart (19, 20, 22).
Alterations in the eIF4F system.
The second regulatory step in mRNA translation initiation, which involves binding the 43S preinitiation complex to the m7GTP cap of mRNA, is mediated by the eIF4F complex (Fig. 1). The eIF4F complex consists of the cap-binding protein eIF4E, eIF4A (RNA helicase), and eIF4G (a scaffold protein). During initiation, eIF4E binds to mRNA via the m7GTP cap, and the eIF4E·mRNA complex binds with eIF4A and eIF4G to form a functional eIF4F cap-binding complex. eIF4F complex formation may be modulated by availability of eIF4E, which can be sequestered in an inactive complex with an eIF4E-binding protein (4E-BP1). Phosphorylation of 4E-BP1 releases eIF4E from 4E-BP1, making eIF4E available for interaction with eIF4G and assembly of a functional eIF4F complex.
Acute alcohol administration does not affect the hepatic content or phosphorylation of initiation factors that comprise the eIF4F system (19). However, chronic alcohol consumption decreases the content of active eIF4E·eIF4G complex as a result of sequestration of eIF4E by 4E-BP1 in the inactive eIF4E·4E-BP1 complex (23). Reduced eIF4E·eIF4G complex assembly did not result from altered content or phosphorylation of 4E-BP1 or eIF4E, suggesting that the upstream effector kinase mTOR (mammalian target of rapamycin) that phosphorylates 4E-BP1 is not involved. In contrast to liver, alcohol-induced protein synthesis inhibition in skeletal and cardiac muscle is accomplished by disruption of the eIF4F system by decreased 4E-BP1 phosphorylation secondary to impaired mTOR (Ser2448) phosphorylation (54) without affecting eIF2/eIF2B (19, 59). Therefore, alcohol inhibits peptide-chain initiation, and thus protein synthesis, via different mechanisms in liver and striated muscles.
Sex Differences Influence Protein Metabolism in Liver
Women who consume large amounts of ethanol tend to exhibit symptoms of alcoholic liver disease and develop cirrhosis more quickly than their male counterparts (53). In addition, they have a higher relative risk than men of developing liver disease for a given alcohol consumption (4). Many studies have identified ways in which females and males differ in their response to alcohol that could render females more susceptible than males to alcohol-induced liver injury and disease. These include, but are not restricted to, lower first-pass metabolism of ethanol taken up by the gut, resulting in higher blood alcohol concentrations in females with accompanying enhanced oxidative stress and lipid peroxidation (1, 11), and activation of Kupffer cells in females followed by the subsequent cascade of events that accompany elevation of cytokines, chemokines, and adhesion molecules (50).
In addition, sex-dependent changes in hepatic protein metabolism appear to play a role in development of ethanol-induced hepatic dysfunction. In this regard, females show a greater leakage of lysosomal proteolytic enzymes (cathepsin B) into the cytosol for a given intake of ethanol (8). The effect of alcohol consumption on cardiac and skeletal muscle structure and function has been compared in male and female rats (21, 33, 58). In these tissues, protein synthesis is inhibited to a greater extent by ethanol intake in males compared with females, and although both males and females show echocardiographic evidence of cardiomyopathy, the male group is more significantly affected by alcohol than the female group. These differences do not relate to alcohol-induced alterations in plasma estradiol concentration (18, 21).
The basal rate of hepatic protein synthesis is higher in females than in males (10, 48), but little is known about sex differences in hepatic protein synthesis in response to alcohol. Incorporation of valine into plasma proteins is unchanged in male rats consuming an alcohol-supplemented diet for 6–7 wk but is decreased 36% in female rats (48). Preliminary observations from our laboratory suggest that hepatic protein synthesis is inhibited to a greater extent in female rats compared with males following chronic consumption of ethanol. Additional detailed comparative studies of the activity and content of initiation factors of the eIF2/eIF2B and eIF4F systems in males and females under control conditions and during alcohol intoxication are required to elucidate molecular mechanisms that underlie sex differences in hepatic protein synthesis that may contribute to the increased susceptibility of females to alcohol-induced liver disease.
Effect of Acute Withdrawal of Alcohol
Liver disease secondary to alcohol abuse ranges from alcoholic fatty liver disease to acute hepatitis to cirrhotic liver disease. It is imperative that alcohol be discontinued to allow for any potential improvement in liver function, with most benefit being seen in the early stages of the disease process (28, 32, 49). Maintaining abstinence can lead to significant regression of fibrosis and possibly early cirrhosis. Alcohol-induced inhibition of hepatic protein synthesis in rats is not reversed when alcohol is withdrawn for 3 days after long-term consumption of an alcohol-containing diet (61). In contrast, alcohol-induced inhibition of protein synthesis in skeletal and cardiac muscle is completely reversed within 3 days after return of animals to an alcohol-free diet (61). The reason for the tissue-specific difference may reside in the response of eIF2 in the liver. eIF2α phosphorylation returns to normal, but the hepatic cellular content of eIF2 remains below control levels after return to an alcohol-free diet, consistent with the reported correlation between eIF2 content and protein synthesis (6, 16, 56).
Regulation of Peptide-Chain Elongation by Chronic Alcohol Consumption
Although mRNA translation initiation is the principal site of regulation by alcohol, changes in elongation may also modulate protein synthesis. Inhibition of elongation may not play a major role in the response of liver protein synthesis to acute intoxication. Indeed, elongation factors eEF1A and eEF2 are not affected by acute intoxication (60). In contrast, alterations in eEF1A and eEF2 occur with chronic ethanol consumption. The major role of eEF1A is to mediate the transfer of aminoacyl-tRNA to the A site of the ribosome, whereas eEF2 catalyzes translocation of the deacylated tRNA in the P site and peptidyl tRNA in the A site into the E and P sites, respectively (Fig. 1). Long-term consumption decreases the content of eEF1A, whereas eEF2 content is unaffected. In contrast, chronic alcohol abuse decreases eEF2 phosphorylation (60). Reduced phosphorylation of eEF2 would be expected to enhance the rate of peptide-chain elongation (43). Therefore, the overall effect of ethanol on elongation involves the balance between the divergent effects on eEF1 and eEF2 in liver. The result may be no net effect on the rate of elongation. The effect of alcohol consumption on peptide-chain elongation has not been investigated in detail.
Inhibitor Studies
Inhibitors of alcohol metabolism have been used to determine whether effects of ethanol on cellular processes are ascribed to alcohol metabolites or to ethanol, per se. The principal pathway of ethanol metabolism in liver is oxidation to acetaldehyde, mediated by alcohol dehydrogenase (ADH) and subsequent rapid oxidation of acetaldehyde to acetate by aldehyde dehydrogenase (ALDH). 4-Methyl pyrazole (4-MP) is a specific competitive inhibitor of ADH. Administration of 4-MP inhibits both the ADH pathway and the microsomal oxidation of ethanol in the liver, preventing the production of acetaldehyde and alterations in redox potential. In one series of experiments using perfused rabbit liver, ethanol, acetaldehyde, and 4-MP were added singly or in various combinations to the perfusate (31). Ethanol inhibited protein synthesis and caused polysome disaggregation, but the effects of 4-MP and acetaldehyde were dependent on the nutritional state (fed or fasted) of the liver donor, complicating interpretation of the results. Addition of 4-MP partially prevents the inhibition of protein synthesis and completely restores the polysome distribution in rat hepatocytes in culture, suggesting that ethanol inhibition of protein synthesis is partly linked to ethanol metabolism (14). In heart and skeletal muscle, the effect of pretreatment with 4-MP or cyanamide, an inhibitor of ALDH, before ethanol administration suggests that both alcohol itself and its metabolite acetaldehyde are inhibitory agents (40, 55).
Salubrinal is a specific inhibitor of protein phosphatase 1 (PP1), the cellular complex that dephosphorylates eIF2α, resulting in sustained, elevated eIF2α phosphorylation (5) (Fig. 2). eIF2α phosphorylation was measured by immunoblot techniques using eIF2α and eIF2α(P) antibodies, giving a relative rather than absolute degree of phosphorylation. The magnitude of the elevation in relative eIF2α phosphorylation and inhibition of hepatic protein synthesis following Salubrinal treatment is not different from that observed following acute ethanol treatment (Table 1). Furthermore, pretreatment with Salubrinal before ethanol treatment does not further increase eIF2α phosphorylation or reduce protein synthesis, suggesting that acute ethanol intoxication causes maximal increase in eIF2α phosphorylation and hepatic protein synthesis inhibition. These data are consistent with ethanol enhancing eIF2α phosphorylation by activation of an eIF2α kinase or by inactivation of eIF2α phosphatase PP1, the phosphatase inhibited by Salubrinal.
Table 1.
Effect of inhibition of eIF2α phosphatase activity on ethanol-induced eIF2 α phosphorylation and hepatic protein synthesis inhibition
| Relative eIF2α Phosphorylation, arbitrary units/mg protein | Hepatic Protein Synthesis, nmol·mg protein−1·h−1 | |
|---|---|---|
| Control | 1.00±0.08 | 18.3±1.9 |
| Salubrinal | 1.85±0.21* | 12.3±0.7* |
| Ethanol | 2.99±0.71* | 12.0±1.0* |
| Ethanol + Salubrinal | 2.74±0.50* | 10.7±1.1* |
Mice were pretreated with DMSO vehicle (controls) or Salubrinal (1 mg/kg body wt ip) for 30 min before injection with ethanol (75 mmol/kg) or saline. Hepatic tissue was sampled 60 min later. Protein synthesis was measured by the flooding dose method (57) and relative eIF2α phosphorylation by immunoblot analysis using anti-eIF2α and anti-eIF2α(P) antibodies (62); n = 8–10 mice per group. eIF2α(P) ANOVA, P < 0.0005, Kruskal-Wallis statistic (KW) = 18.4; protein synthesis ANOVA, P < 0.05, KW = 11.0.
P < 0.05 vs. control.
Summary and Future Directions
Alcoholic liver disease presents numerous challenges to clinicians. Screening for alcohol abuse and alcoholism should be routine with close attention to signs and symptoms of liver disease, because hepatic damage is reversible in the early stages of the disease. In patients with evidence of liver dysfunction or injury, consideration should be given to performance of liver biopsy for diagnosis, staging, and prognosis. Recent research, which has shed light on the mechanisms of alcohol-induced liver injury, offers the prospect of advances in the management of alcoholic liver disease, including inhibition of protein synthesis.
It will be important to delineate both binge drinking (acute models) and long-term alcohol consumption (chronic models) because of differences in the molecular mechanism of protein synthesis inhibition in each scenario. Chronic studies using the mixed solid-liquid diet suggest that reduced hepatic protein synthesis is an early manifestation of alcohol abuse that develops before significant lipid accumulation (47). Our recent elucidation of the mechanisms underlying alcohol-induced inhibition of hepatic protein synthesis suggests that the principal site of regulation of translation is ternary complex formation, regulated by the initiation factors eIF2 and eIF2B. eIF2α is maximally phosphorylated and protein synthesis is maximally inhibited by acute alcohol intoxication, and these changes persist following long-term ethanol abuse. However, long-term consumption of alcohol causes additional changes including alterations in the eIF4F system, and possibly in the elongation phase of translation, that may act to limit return of protein synthesis to pre-alcohol states after abstention from alcohol ingestion. Although significant progress has been made in recent years in identifying a number of the key molecules and complexes affected by alcohol consumption, many questions remain. Because of the key role of eIF2 and eIF2B in regulating hepatic protein synthesis in response to alcohol and the clinical importance of sex differences and abstinence, it will be important to examine the activity and content of components of the eIF2/eIF2B system 1) in liver of male and female rats under normal conditions and during consumption of alcohol and 2) during abstinence following withdrawal from chronic alcohol consumption. Finally, proteomic analysis will be a useful tool to identify specific proteins that are differentially expressed during acute intoxication or chronic alcohol consumption.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant 5R21 AA-15349-2 (T. C. Vary).
Acknowledgments
Part of this work was completed by J. H. Martin in fulfillment of a Medical Student Research Project at the Penn State University College of Medicine.
REFERENCES
- 1.Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schafer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res 25: 502–507, 2001. [PubMed] [Google Scholar]
- 2.Baraona E, Leo MA, Borowsky S, Lieber CS. Pathogenesis of alcohol-induced accumulation of protein in the liver. J Clin Invest 60: 546–554, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bautista AP Chronic alcohol intoxication induced hepatic injury through enhanced macrophage inflammation protein-2 production and intracellular adhesion molecule-1 expression in the liver. Hepatology 25: 335–342, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 23: 1025–1029, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307: 935–939, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Cooney RN, Kimball SR, Maish G 3rd, Shumate M, Vary TC. Effects of tumor necrosis factor-binding protein on hepatic protein synthesis during chronic sepsis. J Surg Res 93: 257–264, 2000. [DOI] [PubMed] [Google Scholar]
- 7.De Feo P, Volpi E, Lucidi P, Cruciani G, Maonacchia F, Reboldi G, Santeusanio F, Bolli G, Brunetti P. Ethanol impairs post-prandial protein metabolism. J Clin Invest 95: 1472–1479, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue TM, Curry-McCoy TV, Nanji AA, Kharbanda KK, Osna NA, Radio SJ, Todero SL, White RL, Casey CA. Lysosomal leakage and lack of adaptation of hepatoprotective enzyme contribute to enhanced susceptibility to ethanol-induced liver injury in female rats. Alcohol Clin Exp Res 31: 1944–1952, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Donohue TM, McVicker DL, Kharbanda KK, Chaisson ML, Zetterman RK. Ethanol administration alters the proteolytic activity of hepatic lysosomes. Alcohol Clin Exp Res 18: 536–541, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Farber E, Corban MS. Sex difference in ethionine inhibition of hepatic protein synthesis. J Biol Chem 233: 625–630, 1958. [PubMed] [Google Scholar]
- 11.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 322: 95–99, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Gouillon Z, Lucas D, Li J, Hagbjork A, French B, Fu P, Fang C, Ingleman-Sundberg M, Donohue TJ, French S. Inhibition of ethanol-induced liver disease in the intragastric feeding model by chlormethiazole. Proc Soc Exp Biol Med 224: 302–308, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hall PM, Lieber CS, DeCarli LM, French SW, Lindros KO, Jarvelainen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res 25: 254S–261S, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Harbitz I, Wallin B, Hauge JG, Morland J. Effect of ethanol metabolism on initiation of protein synthesis in rat hepatocytes. Biochem Pharmacol 33: 3465–3470, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi K, Watanabe Y, Nagayama C, Hirose S. Effect of alcohols on polypeptide elongation and aminoacyl-tRNA formation. J Biochem (Tokyo) 78: 981–987, 1975. [DOI] [PubMed] [Google Scholar]
- 16.Kimball SR, Jefferson LS, Vary TC. Age-dependent decrease in the amount of eukaryotic initiation factor 2 in various rat tissues. Biochem J 286: 263–268, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsch RE, Frith LO, Stead RH, Saunders SJ. Effect of alcohol on albumin synthesis by the isolated perfused rat liver. Am J Clin Nutr 26: 1191–1194, 1973. [DOI] [PubMed] [Google Scholar]
- 18.Kono H, Wheeler MD, Rsuyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-κB, and TNF-α. Am J Physiol Gastrointest Liver Physiol 278: G652–G661, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Lang CH, Frost RA, Kumar V, Wu D, Vary TC. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res 24: 322–331, 2000. [PubMed] [Google Scholar]
- 20.Lang CH, Frost RA, Sumner AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol 37: 2180–2195, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab 292: E1497–E1506, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Lang CH, Wu D, Frost R, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Lang CH, Wu D, Frost RA, Jefferson LS, Vary TC, Kimball SR. Chronic alcohol feeding impairs hepatic translation initiation by modulating eIF2 and eIF4E. Am J Physiol Endocrinol Metab 277: E805–E814, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Lieber C Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta 257: 59–84, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res 6: 523–531, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Mackle I, Swann D, Cook B. One year outcome of intensive care patients with decompensated alcoholic liver disease. Br J Anaesth 97: 496–498, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Mezey E, Potter JJ, Slusser RJ, Brandes D, Romero J, Tamura T, Halsted CH. Effect of ethanol feeding on hepatic lysosomes in the monkey. Lab Invest 43: 88–93, 1980. [PubMed] [Google Scholar]
- 28.Morgan MY The prognosis and outcome of alcoholic liver disease. Alcohol Alcohol Suppl 2: 335–343, 1994. [PubMed] [Google Scholar]
- 29.Morland J, Bessesen A, Svendsen L. The role of alcohol metabolism in the effect of ethanol on protein synthesis in isolated hepatocytes. Alcohol Clin Exp Res 4: 313–321, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Oldfield S, Jones BL, Tanton D, Proud CG. Use of monoclonal antibodies to study the structure and function of eukaryotic protein synthesis initiation factor eIF2B. Eur J Biochem 221: 399–410, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Oratz M, Rothschild MA, Schreiber SS. Alcohol, amino acids, and albumin synthesis. Gastroenterology 74: 672–676, 1978. [PubMed] [Google Scholar]
- 32.Palencia G, Teixeira F, Ortiz A, Perez R, Sotelo J. Reversibility of alterations induced by chronic alcoholism and malnutrition in rats after alcohol withdrawal and proper nutrition. J Stud Alcohol 56: 140–146, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Piano MR, Geenen DL, Schwertz DW, Chowdhury SA, Yuzhakova M. Long-term effects of alcohol consumption in male and female rats. Cardiovasc Toxicol 7: 247–254, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Pignon J, Bailey N, Baraona E, Lieber CS. Fatty acid binding protein: a major contributor to the ethanol-induced increase in liver cytosolic proteins in the rat. Hepatology 7: 856–871, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Poso A Ethanol and hepatic protein turnover. Alcohol Alcohol Suppl 1: 83–90, 1987. [PubMed] [Google Scholar]
- 36.Poso AR, Hirsimaki P. Inhibition of proteolysis in the liver by chronic ethanol feeding. Biochem J 273: 149–152, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poso AR, Surmacz CA, Mortimore GE. Inhibition of intracellular protein degradation by ethanol in perfused rat liver. Biochem Med 242: 459–464, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preedy VR, Peters TJ. An investigation into the effects of chronic ethanol feeding on hepatic mixed protein synthesis in immature and mature rats. Alcohol Alcohol 25: 489–498, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Preedy VR, Duane P, Peters TJ. Comparison of the acute effects of ethanol on liver and skeletal muscle protein synthesis in the rat. Alcohol Alcohol 23: 155–162, 1988. [PubMed] [Google Scholar]
- 40.Preedy VR, Keating JW, Peters TJ. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles in the rat. Alcohol Alcohol 27: 241–251, 1992. [PubMed] [Google Scholar]
- 41.Rao GA, Larkin EC. Nutritional factors required for alcoholic liver disease in rats. J Nutr 127: 896S–898S, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Rothschild MA, Oratz M, Morland J, Schreiber SS, Burks A, Martin B. Effects of ethanol on protein synthesis and secretion. Pharmacol Biochem Behav 13: 31–36, 1980. [DOI] [PubMed] [Google Scholar]
- 43.Ryazanov A, Shestakova E, Natapov P. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature 334: 170–173, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Savolainen V, Liesto K, Mannikko A, Penttila A, Karhunen P. Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res 17: 1112–1117, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Shoemaker J, Visek W. Growth, liver lipid and amino acids in rats fed ethanol with an adequate diet. Drug Alcohol Depend 22: 49–54, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Singh GK, Hoyert DL. Social epidemiology of chronic liver disease and cirrhosis mortality in the United States, 1935–1997: trends and differentials by ethnicity, socioeconomic status, and alcohol consumption. Hum Biol 72: 801–820, 2000. [PubMed] [Google Scholar]
- 47.Smith-Kielland A, Blom G, Svendsen L, Bessesen A, Morland J. A study of hepatic protein synthesis, three subcellular enzymes, and liver morphology in chronically ethanol fed rats. Acta Pharmacol Toxicol 53: 113–120, 1983. [DOI] [PubMed] [Google Scholar]
- 48.Smith-Kielland A, Svendsen L, Bessesen A, Morland J. Effect of chronic ethanol consumption on in vivo protein synthesis in livers from female and male rats fed two different diet regimens. Alcohol Alcohol 18: 285–292, 1983. [Google Scholar]
- 49.Sorensen T, Orholm M, Bentsen K, Hoybye G, Eghoje K, Christofersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet 2: 241–244, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Thurman R Mechanisms of hepatic toxicity. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol Gastrointest Liver Physiol 275: G605–G611, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Tiernan JM, Ward LC. Acute effects of ethanol on protein synthesis in the rat. Alcohol Alcohol 21: 171–179, 1986. [PubMed] [Google Scholar]
- 52.Tuma D, Jennett R, Sorrel M. Effect of ethanol on the synthesis and secretion of hepatic secretory glycoproteins and albumin. Hepatology 1: 590–598, 1981. [DOI] [PubMed] [Google Scholar]
- 53.Tuyns A, Pequignot G. Greater risk of ascitic cirrhosis in females in relation to alcohol consumption. Int J Epidemiol 13: 53–57, 1984. [DOI] [PubMed] [Google Scholar]
- 54.Vary TC, Deiter GM, Lantry RL. Chronic alcohol feeding impairs mTOR(Ser 2448) phosphorylation in rat hearts. Alcohol Clin Exp Res 32: 43–51, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Vary TC, Lang CH. Differential phosphorylation of translation initiation regulators 4EBP1, S6K1, and ERK 1/2 following inhibition of alcohol metabolism in mouse hearts. Cardiovasc Toxicol 8: 23–32, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Vary TC, Jurasinski C, Karinch AM, Kimball SR. Regulation of eukaryotic initiation factor 2 expression during sepsis. Am J Physiol Endocrinol Metab 266: E193–E200, 1994. [DOI] [PubMed] [Google Scholar]
- 57.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch fibers. Am J Physiol Cell Physiol 262: C1513–C1519, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Vary TC, Kimball SR, Sumner A. Sex-dependent differences in the regulation of myocardial protein synthesis following long-term ethanol consumption. Am J Physiol Regul Integr Comp Physiol 292: R778–R787, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Vary TC, Lynch CJ, Lang CH. Effects of chronic alcohol consumption on regulation of myocardial protein synthesis. Am J Physiol Heart Circ Physiol 281: H1242–H1251, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Vary TC, Nairn AC, Deiter GM, Lang CH. Differential effects of alcohol consumption on eukaryotic elongation factors in heart, skeletal muscle, and liver. Alcohol Clin Exp Res 26: 1794–1802, 2002. [PubMed] [Google Scholar]
- 61.Vary TC, Nairn AC, Lang CH. Restoration of protein synthesis in heart and skeletal muscle after withdrawal of alcohol. Alcohol Clin Exp Res 28: 517–525, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Voisin L, Gray K, Flowers KM, Kimball SR, Jefferson LS, Vary TC. Altered expression of eukaryotic initiation factor 2B in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab 270: E43–E50, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Volentine GD, Tuma DJ, Sorrell MF. Subcellular location of secretory proteins retained in the liver during the ethanol-induced inhibition of hepatic protein secretion in the rat. Gastroenterology 90: 158–165, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Wallin B, Morland J. The role of glucose and insulin in the effect of ethanol on protein synthesis in isolated rat hepatocytes. Alcohol Alcohol 22: 219–226, 1987. [PubMed] [Google Scholar]
- 65.Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett 421: 125–130, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology 46: 2032–2039, 2007. [DOI] [PubMed] [Google Scholar]