Abstract
At concentrations around 10−9 M or higher, glucagon increases cardiac contractility by activating adenylate cyclase/cyclic adenosine monophosphate (AC/cAMP). However, blood levels in vivo, in rats or humans, rarely exceed 10−10 M. We investigated whether physiological concentrations of glucagon, not sufficient to increase contractility or ventricular cAMP levels, can influence fuel metabolism in perfused working rat hearts. Two distinct glucagon dose-response curves emerged. One was an expected increase in left ventricular pressure (LVP) occurring between 10−9.5 and 10−8 M. The elevations in both LVP and ventricular cAMP levels produced by the maximal concentration (10−8 M) were blocked by the AC inhibitor NKY80 (20 μM). The other curve, generated at much lower glucagon concentrations and overlapping normal blood levels (10−11 to 10−10 M), consisted of a dose-dependent and marked stimulation of glycolysis with no change in LVP. In addition to stimulating glycolysis, glucagon (10−10 M) also increased glucose oxidation and suppressed palmitate oxidation, mimicking known effects of insulin, without altering ventricular cAMP levels. Elevations in glycolytic flux produced by either glucagon (10−10 M) or insulin (4 × 10−10 M) were abolished by the phosphoinositide 3-kinase (PI3K) inhibitor LY-294002 (10 μM) but not significantly affected by NKY80. Glucagon also, like insulin, enhanced the phosphorylation of Akt/PKB, a downstream target of PI3K, and these effects were also abolished by LY-294002. The results are consistent with the hypothesis that physiological levels of glucagon produce insulin-like increases in cardiac glucose utilization in vivo through activation of PI3K and not AC/cAMP.
Keywords: insulin, glycolysis, phosphoinositide 3-kinase, cyclic adenosine monophosphate
glucagon is secreted from pancreatic islet α-cells in response to reduced insulin, variations in extracellular fluid glucose concentrations, and other influences (12). Prominent metabolic actions include stimulation of hepatic glycogenolysis and gluconeogenesis and promotion of lipolysis in adipose tissue (19). In heart, glucagon produces epinephrine-like cardiotonic responses, manifested by pronounced increases in both contractility and frequency of contraction (27, 35).
The hormone can elicit all of these responses by activating adenylate cyclase (AC) and stimulating the intracellular production of cyclic adenosine monophosphate (cAMP). This nucleotide was revealed as the first known intracellular signal, or second messenger, by the groundbreaking work of Sutherland, Rall, and coworkers (32) a half-century ago. Since then, a wealth of compelling evidence has all but established cAMP as glucagon's predominant, if not exclusive, signal mediating metabolic responses in liver and adipose tissue as well as contractile effects in heart (6, 11).
This conclusion is based largely on information gathered from perfused organs, isolated cells, or cell-free systems in vitro. Although exogenous glucagon is clearly active in vivo at sufficiently high doses, the extent to which endogenous glucagon contributes to the regulation of glycemia remains controversial (31). A persistent, unresolved issue is a significant disparity, of at least 10-fold, between fasting (maximal) blood levels (1, 24) and the substantially higher concentrations required to activate AC in target tissues (26, 35). Alternative signals have been proposed, but their physiological relevance has not been established (42).
The purpose of this study was to determine whether physiological levels of glucagon, below the threshold required to increase contractile force (inotropy), can influence metabolic fuel use in heart tissue. The results show, presumably for the first time, that glucagon produces rapid insulin-like metabolic responses in perfused working rat hearts at concentrations within the normal physiological blood range. Stimulation of glycolysis by the hormone appears to be mediated by phosphoinositide 3-kinase (PI3K) and not by AC/cAMP.
MATERIALS AND METHODS
Working heart preparation.
Male Sprague-Dawley rats (200–400 g; Harlan Teklad, Indianapolis, IN) were housed communally, given food and water ad libitum, and handled in accordance with the Institutional Animal Care and Use Committee at the University of Rhode Island, which approved our study. Animals were given intraperitoneal heparin (1,000 IU/kg) injections 15 min prior to heart excision. Hearts were initially perfused in the Langendorff mode with Krebs-Henseleit (KH) solution containing the following solutes (in mM): NaCl, 120; CaCl2·2H20, 1.8; NaHCO3, 25; NaH2PO4·H20, 1.2; MgS04·7H20, 0.65; and KCl, 5.6, pH 7.4, when gassed with 95% O2-5% CO2 at 37°C. Working hearts were perfused as described previously (34). Hearts were paced (260 beats/min) if the spontaneous rate was <240 beats/min. Glucagon concentrations <10−9 M had no effect on spontaneous frequency of contraction. Left atrial filling pressure was maintained at 10 cm of H2O, and resistance to left ventricular ejection was kept at a constant 1 kPa·ml−1·min−1. Inotropic responses to glucagon were quantified as the change in peak left ventricular pressure (LVP) in millimeters of mercury. The working heart perfusate (100 ml) consisted of KH solution containing 3% low-endotoxin bovine serum albumin (BSA) (A3675; Sigma) prepared with tissue culture water (W3500; Sigma) and dialyzed (6–8,000 MW; Spectra/Por) in 4 L KH solution at 4°C overnight. Palmitate (0.5 mM), glucose (10 mM), and sodium bicarbonate (25 mM) were added on the day of the experiment.
Preparation of hormone stock solutions.
Preliminary studies established that the procedure for preparing glucagon stock solutions is critical for preserving full biological activity, particularly at low hormone concentrations. If glucagon is dissolved in a protein-free aqueous medium, activity declines rapidly with storage. This is presumably because the peptide adheres to the walls of any container used, even if it is silated. The problem can be minimized or eliminated by dissolving the glucagon in a protein-containing solution such as plasma (7). Therefore, stock solutions were prepared by dissolving powdered synthetic glucagon (G-1774; Sigma) in the perfusate containing 3% BSA without glucose, sodium bicarbonate, or palmitate but with 1% citric acid added (pH 2.0). The stock solution was then aliquoted and stored at −80°C. On the day of the experiment, the stock solution was thawed and diluted in a small volume of the working heart perfusate just prior to heart perfusion. This protocol preserved full activation of glycolysis for ≥8 wk. Insulin stock solutions (I-5500; Sigma) were prepared similarly and stored at −20°C.
Measurements of glycolysis, glucose oxidation, and palmitate oxidation.
Rates of glycolysis, glucose oxidation, and palmitate oxidation were quantified as described previously (34). Glycolysis and glucose oxidation were measured as the rate of production of 3H2O from [5-3H]glucose (specific activity 15,000 counts·min−1·μmol−1) and 14CO2 from [U-14C]glucose (specific activity 15,000 counts·min−1·μmol−1), respectively. Palmitate oxidation was measured as the rate of 3H2O produced from [9,10-3H]palmitate (specific activity 150,000 counts·min−1·μmol−1). Rates were calculated from regressions of 5-min samples and expressed as the change in rate induced by the hormone in micromoles per gram dry weight per minute (using a wet/dry weight ratio of 5.02). After 20 min of sampling, either glucagon or insulin was added at the final concentrations indicated. When appropriate, either the PI3K inhibitor LY-294002 (10 μM; Biomol International, Plymouth Meeting, PA) or the AC inhibitor NKY80 (20 μM; Calbiochem, San Diego, CA) was added to the perfusate prior to heart perfusion. LY-294002 was selected over the more widely used wortmannin as the PI3K inhibitor because our preliminary studies indicated that wortmannin alone had significant, and rather pronounced, intrinsic stimulatory activity on glycolysis. Neither LY-294002 nor NKY80 significantly affected basal glycolytic rates or LVP in untreated hearts (Table 1).
Table 1.
Basal rates of glycolysis and LVP in untreated perfused working rat hearts and hearts treated with the PI3K inhibitor LY-294002 (10 μM) or the AC inhibitor NKY80 (20 μM)
| LVP, mmHg | Glycolytic Rate, μmol·g−1·min−1 | |
|---|---|---|
| Untreated | 109±7 | 3.19±0.40 |
| LY-294002 (10 μM) | 116±7 | 3.16±0.37 |
| NKY80 (20 μM) | 95±5 | 3.24±0.42 |
Values are means ± SE; n = 9–31/group. LVP, left ventricular pressure; PI3K, phosphoinositide 3-kinase; AC, adenylate cyclase. See materials and methods for calculations.
Quantification of tissue levels of total Akt, phospho-Akt, and cAMP.
Ventricular tissue was quickly frozen with flat steel tongs precooled in liquid nitrogen for subsequent measurement of tissue levels of Akt/phospho-Akt and cAMP. Samples were stored at −80°C until use. For immunoblotting of Akt, hearts were frozen after 10 min of exposure to glucagon, insulin, or vehicle. Tissues were homogenized in a Tris·HCl-based homogenization buffer (pH 7.4) containing protease and phosphatase inhibitor cocktails (1:100) and 150 mM phenylmethanesulfonyl fluoride and centrifuged at 1,000 g for 10 min. The supernatant was spun at 100,000 g for 60 min at 4°C, and the pellet was resuspended in Triton buffer and centrifuged at 15,000 g for 30 min at 4°C. The supernatants were combined and assayed for protein content using the Pierce BCA Protein Assay (Pierce, Rockford, IL). Samples were loaded onto 10% Tris·HCl resolving gels (3450009; Bio-Rad, Hercules, CA), at a final concentration of 2.5 μg/μl, in Laemmli sample buffer. Gels were were electrophoresed at 200 V for 1 h in 25 mM Tris, 192 mM glycine, and 0.1% (wt/vol) SDS running buffer and transferred to nitrocellulose paper for 35 min at 100 V in 25 mM Tris, 192 mM glycine, and 25% (vol/vol) methanol transfer buffer. The blots were blocked [5% (wt/vol) dry milk + TBS + 0.1% Tween-20] for 1 h at room temperature. Both total Akt (9272) and phospho-Akt (9271) antibodies were purchased from Cell Signaling (Danvers, MA) and were used according to manufacturer's protocol. The phospho-Akt antibody recognizes serine473-phosphorylated Akt1, -2, or -3. Enhanced Chemiluminescent Reagent (Pierce Biotechnology, Rockford, IL) was used to detect bands, which were then imaged and quantified using the Kodak Image Station 2000MM.
For cAMP levels, ventricles were frozen after ∼3 min of glucagon or vehicle exposure, corresponding to the time of peak inotropic responses to 10−8 M, and assayed for cAMP as described previously (35) using a colorimetric assay kit (Pierce Biotechnology) according to the manufacturer's instructions. Results were expressed as picomoles per milligram protein (Pierce BCA protein assay).
Statistics.
All data are reported as means ± SE. Changes relative to basal values induced by the hormones within groups were analyzed by the one-tailed (paired) Student's t-test. Differences in mean values between groups were compared using ANOVA followed by the Neuman-Keuls comparison test. P values of <0.05 were considered to be significant; lower values were not reported.
RESULTS
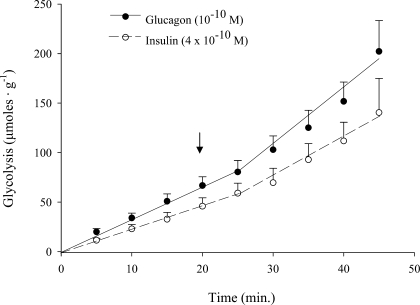
In exploratory studies, we investigated whether glucagon can influence glucose metabolism in perfused rat hearts at a concentration that is representative of normal blood levels (Fig. 1). We found that glucagon at 10−10 M, a high physiological concentration (16), produced a marked stimulation of glycolysis. The response was quantitatively indistinguishable from the effect of a high physiological concentration of insulin. The same concentration of glucagon also substantially stimulated glucose oxidation and moderately inhibited palmitate oxidation (Table 2), nearly duplicating analogous effects of insulin that we had reported previously (34).
Fig. 1.
Effects of high physiological concentrations of glucagon or insulin on glycolytic flux in perfused working rat hearts. Hormones were administered at the arrow. Glycolysis was quantified as the production of 3H20 from [5-3H]glucose (see materials and methods). Values at each 5-min time point are means ± SE; n = 5–9. Basal glycolytic rates prior to administration of glucagon and insulin were 2.56 ± 0.39 and 2.25 ± 0.43, respectively. The increases in rate (slope) in response to glucagon and insulin were significant (P < 0.05).
Table 2.
Effects of glucagon (10−10 M) on the rates of oxidation of glucose and palmitate in perfused working rat hearts
|
Oxidation Rate, μmol·g−1·min−1 |
||||||
|---|---|---|---|---|---|---|
|
Glucose |
Palmitate
|
|||||
| − | + | Δ | − | + | Δ | |
| Glucagon (10−10 M) | 1.40±0.47 | 2.36±0.74 | 0.96±0.35* | 0.518±0.131 | 0.428±0.149 | −0.090±0.037* |
Values are means ± SE; n = 4–6/group. See materials and methods for calculations.
Significant change (P < 0.05).
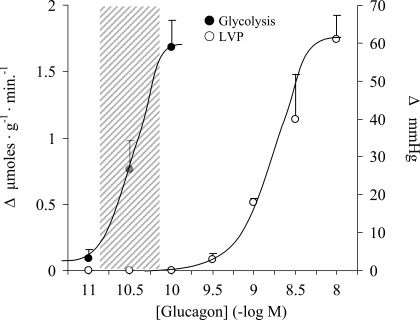
Subsequently, we focused on glycolysis to determine the range of glucagon concentrations necessary to generate metabolic responses and whether they are different from those required to enhance contractility. Inotropic responses are well established, sensitive, and reliable overt markers for AC activation by glucagon in the myocardium (6, 27, 35). We found that when glucagon was administered over a broad concentration range, two distinct dose-response curves emerged (Fig. 2). The right-hand curve depicts inotropic responses as quantified by dose-dependent increases in peak LVP. The threshold concentration of 3 × 10−10 M and the estimated EC50 of 1.6 × 10−9 M are representative of previously reported values for increasing both inotropy and ventricular cAMP levels in isolated rat myocardial preparations (33, 35). In contrast, the left-hand curve reveals a dose-dependent stimulation of glycolysis that occurred over a much lower concentration range, between 10−11 and 10−10 M with an estimated EC50 value of 2.8 × 10−11 M, in the absence of any observable changes in LVP. The position of the curve overlapped the normal blood concentration range, which is nearly identical in rodents and humans (8, 36). The wide separation of the two curves suggested strongly that the metabolic response did not involve stimulation of AC/cAMP.
Fig. 2.
Concentration-effect curves for glucagon showing stimulation of glycolysis (left) and left ventricular pressure (LVP; right) in perfused working rat hearts. Responses represent increases over basal values. The shaded area denotes the blood glucagon concentration range in either rats or humans (1, 16, 17, 24). Each point represents the mean ± SE; n = 3–11.
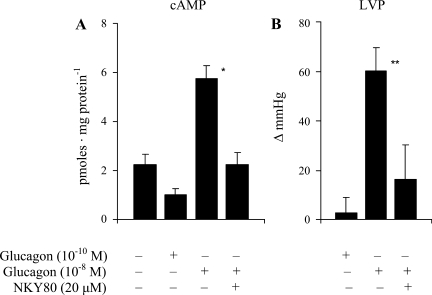
We investigated this possibility further by measuring changes in tissue levels of cAMP produced by glucagon in the absence and presence of NKY80, an inhibitor of AC (Figs. 3 and 4). As we had observed previously (35), the substantial increase in LVP produced by the high pharmacological concentration of 10−8 M was associated with an elevation of ventricular cAMP levels of about threefold at or near the time of the peak inotropic response (Fig. 3). By contrast, the 100-fold lower concentration of glucagon that was sufficient to generate a substantial increase in glycolysis did not affect either cAMP (Fig. 3A) or LVP (Fig. 3B) levels at the equivalent time point. As expected, insulin also had no effect on LVP (data not shown). The presence of NKY80 (20 μM) in the perfusate suppressed the increases in both LVP and cAMP in response to 10−8 M glucagon but had no significant influence on the glycolytic responses to the lower physiological concentration of 10−10 M (Fig. 4A). In the positive control group, the AC inhibitor did not significantly alter the glycolytic response to insulin either (Fig. 4B). These results were not consistent with the hypothesis that AC/cAMP mediates the glycolytic effect of glucagon.
Fig. 3.
Effects of physiological (10−10 M) and pharmacological (10−8 M) concentrations of glucagon on ventricular cAMP (A) and LVP (B) levels in the absence and presence of the adenylate cyclase (AC) inhibitor NKY80 (20 μM). For determination of ventricular cAMP levels, hearts were frozen at 3 min, corresponding to the average time of the peak pressure response after administration of the hormone. *Significant difference in tissue cAMP levels compared with the untreated group (ANOVA/Newman-Keuls); **significant change in LVP above basal levels (paired Student's t-test; P < 0.05, n = 3–7).
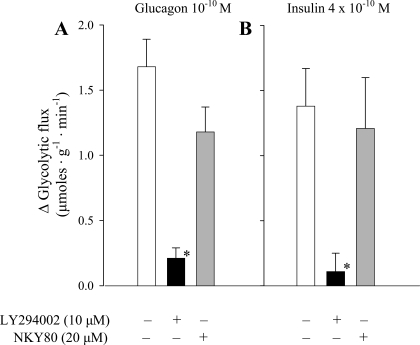
Fig. 4.
Change in glycolytic rate above basal rates in response to physiological concentrations of glucagon or insulin in the absence and presence of the phosphoinositde 3-kinase (PI3K) inhibitor LY-294002 (10 μM) or the AC inhibitor NYK80 (20 μM). *Significant difference from the response generated in the absence of inhibitor (P < 0.05, n = 3–7).
We then searched for an alternative signal that might mediate the stimulation of glycolysis. The nearly identical metabolic response profiles produced by glucagon and insulin (Table 2 and Ref. 34) suggested that a good candidate would be PI3K, which has been established as a mediator of rapid metabolic effects of insulin, including the stimulation of glycolysis in heart (21). We approached this question using the PI3K inhibitor LY-294002 (Fig. 4). As a control, we confirmed that pretreatment with 10 μM LY-294002 was sufficient to completely block the stimulation of glycolysis produced by insulin. The same concentration of the inhibitor also abolished the equivalent glycolytic response to glucagon, implicating PI3K as an important metabolic signal for that hormone as well.
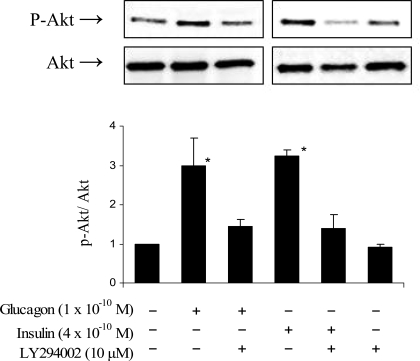
That conclusion was further supported by more direct measurements of PI3K activation (Fig. 5). Western blots of ventricular tissue treated with glucagon (10−10 M) or insulin (4 × 10−10 M) revealed significant and similar increases in the phosphorylation of Akt (PKB), a downstream target of PI3K activation. Consistent with its blockade of glycolytic responses to the two hormones, LY-294002 also suppressed both glucagon- and insulin-induced Akt phosphorylation. These results confirmed that stimulation of the PI3K signal is necessary for the rapid and marked activation of glycolysis in heart by physiological concentrations of either glucagon or insulin.
Fig. 5.
Western analysis of the effects of physiological concentrations of glucagon or insulin on the ratio of phosphorylated Akt (p-Akt) to total Akt in the absence and presence of the PI3K inhibitor LY-294002 (10 μM) in perfused working rat hearts. Bars depict means ± SE of 3 determinations. *Significant difference from a normalized ratio of 1.0 in the untreated group (P < 0.05).
DISCUSSION
The central finding of this study is the observation that glucagon produces robust and rapid metabolic effects in the myocardium without increasing tissue levels of cAMP or affecting contractility (Figs. 2 and 3 and Table 2). Previously, it had been widely held that cAMP is the predominant, if not the exclusive, mediator of glucagon's cellular actions in vivo (6). The hormone figured prominently as a model agonist in the discovery of the cyclic nucleotide as the first known intracellular signal by Sutherland and coworkers (32, 39). All four of “Sutherland's criteria” (39) for establishing whether a substance acts as a second messenger, or an intracellular mediator, of hormonal effects have been fulfilled repeatedly for glucagon and cAMP in liver, heart, and adipose (2, 6, 9, 11).
Of course, an implicit but critical assumption is that both the activation of the cAMP/AC signal and the generation of the expected response, manifested in heart by increased contractility, should occur at physiologically relevant concentrations of the hormone (20). With regard to glucagon specifically, there is a substantial disparity between blood levels on the one hand and the potency of the hormone to generate cAMP and related responses in target tissues on the other. The concentration range in portal or systemic blood of mammals, including rats and humans (1, 16, 17, 24), is consistently between 20 and 80 pmol (10−10.7 to 10−10.1 M). By contrast, threshold concentrations required to activate AC in heart and liver are reported to be in the nanomolar to low-micromolar range (23, 27, 33, 35).
This discrepancy is illustrated by the two distinct dose-response curves depicted in Fig. 2. The position of the right-hand curve is consistent with previous reports of glucagon concentrations required to generate AC-linked inotropic responses in the myocardium (2, 33, 35). The curve clearly lies well outside the normal blood concentration range. Its dependence on activation of AC is verified by the effectiveness of the AC inhibitor NKY80 to suppress both the inotropic response and the associated increase in ventricular tissue cAMP produced by the highest concentration (10−8 M; Fig. 3). The left-hand curve in Fig. 2, by contrast, is a new finding and depicts a dose-dependent stimulation of glycolysis in the absence of any change in contractility. Significantly, the position of the glycolysis curve generated here in the perfused heart ex vivo overlaps the consistently observed blood concentration range in vivo. The lack of involvement of AC/cAMP is supported by two findings. 1) The maximal increase in glycolysis produced by 10−10 M glucagon was not associated with an increase in tissue cAMP levels (Fig. 3), and 2) NKY80 did not inhibit the response (Fig. 4). In the control group, the AC inhibitor also had no effect on the same response insulin. It has been fairly well established that the AC/cAMP signal does not mediate rapid metabolic actions of insulin in heart (38).
Another potentially important finding here is the observation that glucagon, rather than opposing effects of insulin as it does on liver (31), instead mimics rapid metabolic actions of insulin on the myocardium (Figs. 1 and 3, Table 2, and Ref. 34). Each hormone stimulates both glycolysis and glucose oxidation and suppresses palmitate oxidation to similar degrees (Table 2 and Ref. 34). Significantly, glucagon stimulates glycolysis by activating the same signal, PI3K/Akt (Figs. 4 and 5), that mediates the glycolytic response to insulin in heart (21). We cannot identify the specific isoforms of PI3K or Akt activated by glucagon in this study because LY-294002 is not selective for individual PI3K isoforms and because we used a polyclonal antibody against phospho-Akt (Ser473) in the Western analysis. However, the nearly identical responses to glucagon and insulin would suggest that a good candidate might be PI3K Class IA (p110α/β), a predominant PI3K isoform in heart tissue that is activated by insulin (29). Alternatively, Class IB (PI3Kγ) is a possibility because it has been shown to be activated by G protein-coupled receptor (GPCR) signaling (5, 40).
The distinct metabolic and contractile responses observed here in heart might be explained by the stimulation of the two isoforms of the well-characterized GPCR for glucagon (18) that has been reported for liver. Houslay and coworkers (28, 42) have proposed the existence of two hepatic GPCRs, GR1 and GR2, associated with different G proteins. The GR1 receptor has a higher affinity for glucagon than the better-characterized, AC-linked GR2 isoform. Binding studies (15) indicate that the Kd of the high-affinity site in liver is ∼9 × 10−11 M, on the upper end of blood concentrations, and that of the low-affinity AC-linked receptor is ∼1.3 × 10−9 M, near the estimated EC50 for the inotropic dose-response curve depicted in Fig. 2 of this study (1.6 × 10−9 M). Consistent with our findings in heart, two distinct dose-response curves can be generated by glucagon in liver as well (13, 15). The hormone is capable of stimulating glycogenolysis and glucose output in hepatic tissue preparations ex vivo at concentrations that are below those required to measurably increase tissue cAMP levels. Interestingly, the estimated EC50 values for the cAMP-independent hepatic effect, 4–6 × 10−11 M (13, 15), are very close to the estimated EC50 value of 2.8 × 10−11 M for stimulating glycolysis in heart reported here.
Despite these similarities, however, the signal associated with GR1 activation in liver does not appear to be the same one that is linked to the glycolytic response to glucagon reported in this study for heart. Stimulation of hepatic GR1 receptors at low hormone concentrations does not activate PI3K but instead activates phospholipase C, increasing the generation of inositol phosphates, diacylglycerol, and intracellular calcium transients (14, 42). The latter signal has been associated with rapid desensitization of GR2-mediated effects of higher glucagon concentrations in liver (14) but is not itself associated with acute control of hepatic glucose metabolism by the hormone (41). In preliminary studies, we found that 1 μM GF109203X, a nonselective inhibitor of PKC (a downstream target of phospholipase C), did not alter the stimulation of myocardial glucose oxidation by 10−10 M glucagon (data not shown). Even at pharmacological levels, glucagon alone only weakly activates hepatic PI3K and PKB/Akt; it does, however, enhance the activation of PI3K by insulin or leptin (43). The interaction appears to be an example of cross-talk between the AC/cAMP and PI3K-signaling pathways. Glucagon can also activate p38 mitogen-activated protein kinase in liver (3), but again, pharmacological concentrations of the hormone are required. The signal seems to be involved in cAMP-dependent regulation of gene expression but not in acute regulation of glucose metabolism. Other reported AC-independent signals also require high hormone concentrations and are of questionable physiological significance (22). In any case, the cardiac receptor responsible for the glycolytic response to glucagon in this study, perhaps a GPCR receptor associated with PI3K (5, 40) or an unidentified tyrosine kinase receptor (25, 29), remains to be characterized.
Another implication of the results reported here is that the role of glucagon in regulating glycemia in vivo by targeting extrahepatic tissues may be more important, and more insulin like, than previously recognized. This hypothesis is indirectly supported by recent studies. For example, glucagon receptor knockout mice (4, 10, 30, 37) display most or all of the following characteristics depending on the specific model: hypoinsulinemia, moderate hypoglycemia, and profound hyperglucagonemia but surprisingly normal or near-normal hepatic glycogen levels, body weight, food intake, and energy expenditure. The satisfactory maintenance of glycemia in these models was partially explained by evidence of enhanced sensitivity of peripheral tissues to insulin and possibly diminished hepatic glucose production. However, our results suggest that, in addition, endogenous glucagon under these conditions may act to offset the hypoinsulinemia by stimulating unidentified cognate receptors to sustain glucose utilization in extrahepatic tissues. Similarly, in normal animals, glucagon may serve to maintain support of glucose utilization in heart, and perhaps other extrahepatic tissues, during periods of fasting when insulin levels are low.
In summary, the two major new findings of this study are that 1) glucagon stimulates myocardial glycolysis and glucose oxidation and inhibits palmitate oxidation at concentrations that are physiologically relevant and do not influence contractility and 2) the stimulation of glycolysis by glucagon is mediated by a signaling pathway or pathways that involve the activation of PI3K but do not require activation of the AC/cAMP pathway. Significantly, all of these actions of glucagon mimic well-established and prominent cardiac effects of insulin. The results are consistent with the view that control of fuel metabolism and stimulation of contractility represent physiological and pharmacological effects, respectively, of glucagon on the myocardium.
GRANTS
J. Harney received a fellowship through Rhode Island IDeA Network of Biomedical Research Excellence (RI-INBRE) Grant No. P20-RR-016457 from the National Center for Research Resources, National Institutes of Health (NIH). The research was also supported by the use of the RI-INBRE Centralized Research Core Facility that was supported by the same grant. The views expressed in this article are those of the authors and do not necessarily represent the views of NIH.
Acknowledgments
We are grateful to Dr. Joseph Beavo for helpful comments during the preparation of the manuscript. A portion of this work was presented in abstract form at the 19th World Congress of the International Society for Heart Research, Bologna, Italy, June 22–25, 2007.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Balks HJ, Jungermann K. Regulation of peripheral insulin/glucagon levels by rat liver. Eur J Biochem 141: 645–650, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum M, Fain JN. Activation of protein kinase and glycogen phosphorylase in isolated rat liver cells by glucagon and catecholamines. J Biol Chem 252: 528–535, 1977. [PubMed] [Google Scholar]
- 3.Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG, Xiong Y, Daniel KW, Floering L, Collins S. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem 280: 42731–42737, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Conarello S, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50: 142–150, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oiveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110: 737–749, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Davis S Glucagon. In: Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 2006, p. 1641–1643.
- 7.Delinsky D, Hill KT, White CA, Bartlett MG. Quantitation of the large polypeptide glucagon by protein precipitation and LC/MS. Biomed Chromatogr 18: 700–705, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Evans M, Hopkins D, Macdonald IA, Amiel SA. Alanine infusion during hypoglycaemia partly supports cognitive performance in healthy human subjects. Diabet Med 21: 440–446, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Exton J, Robison GA, Sutherland EW, Park CR. Studies on the role of adenosine 3′,5′-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem 246: 6166–6177, 1971. [PubMed] [Google Scholar]
- 10.Gelling R, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 100: 1438–1443, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerich J Physiology of glucagon. Int Rev Physiol 24: 243–275, 1981. [PubMed] [Google Scholar]
- 12.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hermsdorf T, Dettmer D, Hofmann E. Age-dependent effects of phorbol ester on adenylate cyclase stimulation by glucagon in liver of female rats. Biomed Biochim Acta 48: 255–260, 1989. [PubMed] [Google Scholar]
- 14.Houslay M Compartmentalization of cyclic AMP phosphodiesterases, signalling ‘crosstalk’, desensitization and the phosphorylation of Gi-2 add cell specific personalization to the control of the levels of the second messenger cyclic AMP. Adv Enzyme Regul 35: 303–338, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Ikezawa Y, Yamatani K, Ogawa A, Ohnuma H, Igarashi M, Daimon M, Manaka H, Sasaki H. Effects of glucagon on glycogenolysis and gluconeogenesis are region-specific in periportal and perivenous hepatocytes. J Lab Clin Med 132: 547–555, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Inouye K, Shum K, Chan O, Mathoo J, Matthews SG, Vranic M. Effects of recurrent hyperinsulinemia with and without hypoglycemia on counterregulation in diabetic rats. Am J Physiol Endocrinol Metab 282: E1369–E1379, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Jaspan J, Ruddick J, Rayfield E. Transhepatic glucagon gradients in man: evidence for glucagon extraction by human liver. J Clin Endocrinol Metab 58: 287–292, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Jelinek L, Lok S, Rosenberg GB, Smith RA, Grant FJ, Biggs S, Bensch PA, Kuijper JL, Sheppard PO, Sprecher CA, O'Hara PJ, Foster D, Walker KM, Chen LHJ, McKernan PA, Kindsvogel W. Expression cloning and signaling properties of the rat glucagon receptor. Science 259: 1614–1616, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284: E671–E678, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kreisberg R, Williamson JR. Metabolic effects of glucagon in the perfused rat heart. Am J Physiol 207: 721–727, 1964. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre V, Mechin MC, Louckx MP, Rider MH, Hue L. Signaling pathway involved in the activation of heart 6-phosphofructo-2-kinase by insulin. J Biol Chem 271: 22289–22292, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Carretero OA, Shao Y, Zhuo JL. Glucagon receptor-mediated extracellular signal-regulated kinase 1/2 phosphorylation in rat mesangial cells: role of protein kinase A and phospholipase C. Hypertension 47: 580–585, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston J, Einarsson K, Backman L, Ewerth S, Arner P. Glucagon receptor of human liver. Studies of its molecular weight and binding properties, and its ability to activate hepatic adenylyl cyclase of non-obese and obese subjects. J Clin Invest 75: 397–403, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marliss E, Aoki TT, Unger RH, Soeldner JS, Cahill GF Jr. Glucagon levels and metabolic effects in fasting man. J Clin Invest 49: 2256–2270, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol 38: 63–71, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Méry P, Brechler V, Pavoine C, Pecke F. Glucagon stimulates the cardiac Ca2+ current by activation of adenylyl cyclase and inhibition of phosphodiesterase. Nature 345: 158–161, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Murad F, Vaughan M. Effect of glucagon on rat heart adenyl cyclase. Biochem Pharmacol 18: 1053–1059, 1969. [DOI] [PubMed] [Google Scholar]
- 28.Murphy G, Hruby VJ, Trivedi D, Wakelam MJ, Houslay MD. The rapid desensitization of glucagon-stimulated adenylate cyclase is a cyclic AMP-independent process that can be mimicked by hormones which stimulate inositol phospholipid metabolism. Biochem J 243: 39–46, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oudit G, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol 37: 449–471, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Parker J, Andrews KM, Allen MR, Stock JL, McNeish JD. Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun 290: 839–843, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Raju B, Cryer PE. Maintenance of the postabsorptive plasma glucose concentration: insulin or insulin plus glucagon? Am J Physiol Endocrinol Metab 289: E181–E186, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Rall T, Sutherland EW, Berthet J. The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J Biol Chem 224: 463–475, 1957. [PubMed] [Google Scholar]
- 33.Ramachandran C, Angelos KL, Walsh DA. Cyclic AMP-dependent and cyclic AMP-independent antagonism of insulin activation of cardiac glycogen synthase. J Biol Chem 257: 1448–1457, 1982. [PubMed] [Google Scholar]
- 34.Rodgers R, Christe ME, Tremblay GC, Babson JR, Daniels T. Insulin-like effects of a physiologic concentration of carnitine on cardiac metabolism. Mol Cell Biochem 226: 97–105, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers R, MacLeod KM, McNeill JH. Responses of rat and guinea pig hearts to glucagon. Lack of evidence for a dissociation between changes in myocardial cyclic 3′5′-adenosine monophosphate and contractility. Circ Res 49: 216–225, 1981. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval D, Ping L, Neill RA, Morrey S, Davis SN. The effects of dehydroepiandrosterone sulfate on counterregulatory responses during repeated hypoglycemia in conscious normal rats. Diabetes 53: 679–686, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Sørensen H, Winzell MS, Brand CL, Fosgerau K, Gelling RW, Nishimura E, Ahren B. Glucagon receptor knockout mice display increased insulin sensitivity and impaired beta-cell function. Diabetes 55: 3463–3469, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Sugden M, Orfali KA, Fryer LG, Holness MJ, Priestman DA. Molecular mechanisms underlying the long-term impact of dietary fat to increase cardiac pyruvate dehydrogenase kinase: regulation by insulin, cyclic AMP and pyruvate. J Mol Cell Cardiol 29: 1867–1875, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland E Studies on the mechanism of hormone action. Science 177: 401–408, 1972. [DOI] [PubMed] [Google Scholar]
- 40.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387: 673–676, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Unson CG, Gurzenda EM, Merrifield RB. Biological activities of des-His1[Glu9]glucagon amide, a glucagon antagonist. Peptides 10: 1171–1177, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Wakelam M, Murphy GJ, Hruby VJ, Houslay MD. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature 323: 68–71, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Zhao A, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem 275: 11348–11354, 2000. [DOI] [PubMed] [Google Scholar]