Abstract
Many studies have demonstrated a biphasic effect of peroxynitrite in the myocardium, but few studies have investigated this biphasic effect on β-adrenergic responsiveness and its dependence on contractile state. We have previously shown that high SIN-1 (source of peroxynitrite, 200 μmol/L) produced significant anti-adrenergic effects during maximal β-adrenergic stimulation in cardiomyocytes. In the current study, we hypothesize that the negative effects of high SIN-1 will be greatest during high contractile states, while the positive effects of low SIN-1 (10 μmol/L) will predominate during low contractility. Isolated murine cardiomyocytes were field stimulated at 1 Hz and [Ca2+]i transients and shortening were recorded. Following submaximal ISO (β-adrenergic agonist, 0.01 μmol/L) stimulation, 200 μmol/L SIN-1 induced two distinct phenomena. Cardiomyocytes undergoing a large response to ISO showed a significant reduction in contractility, while cardiomyocytes exhibiting a modest response to ISO showed a further increase in contractility. Additionally, 10 μmol/L SIN-1 always increased contractility during low ISO stimulation, but had no effect during maximal ISO (1 μmol/L) stimulation. 10 μmol/L SIN-1 also increased basal contractility. Interestingly, SIN-1 only produced a contractile effect under one condition in phospholamban knockout cardiomyocytes, providing a potential mechanism for the biphasic effect of peroxynitrite. These results provide clear evidence for a biphasic effect of peroxynitrite, with high peroxynitrite modulating high levels of β-adrenergic responsiveness and low peroxynitrite regulating basal function and low levels of β-adrenergic stimulation.
Keywords: β-adrenergic stimulation, Excitation-contraction coupling, Peroxynitrite, Phospholamban, Reactive nitrogen species
INTRODUCTION
The process of excitation-contraction coupling is responsible for contraction in the cardiomyocyte [1]. Following the cardiac action potential, Ca2+ enters the cell via L-type Ca2+ channels, triggering the opening of sarcoplasmic reticulum (SR) Ca2+ release channels, or ryanodine receptors, and the efflux of additional Ca2+ from the SR. This Ca2+ subsequently activates the myofilaments, resulting in myocyte contraction. Relaxation is primarily mediated by the SR Ca-ATPase/phospholamban complex (SERCA/PLB), which serves to reuptake Ca2+ into the SR. PLB plays a critical role in this process by regulating SERCA uptake of Ca2+ into the SR, and thus is important in determining SR Ca2+ load, a critical determinant of myocyte contractility.
Within the cardiomyocyte, nitric oxide (NO.) is produced by three distinct isoforms of nitric oxide synthase (NOS) [2]. Neuronal NOS (nNOS, NOS1) and endothelial NOS (eNOS, NOS3) are constitutively expressed, while inducible NOS (iNOS, NOS2) is only expressed during immune responses and many pathophysiological conditions of the myocardium, such as heart failure [3]. NOS1 co-localizes with xanthine oxidase [4], a superoxide (O2.-) producing enzyme, and may potentially lead to the production of low levels of peroxynitrite (ONOO−), as nitric oxide and superoxide react with a very high rate constant [5]. When expressed, NOS2 is capable of producing large amounts of both nitric oxide and superoxide [6, 7]. Additionally, NADPH oxidase and xanthine oxidoreductase increase superoxide production during these pathophysiological conditions of the myocardium [8, 9], thus leading to the formation of high levels of peroxynitrite. Consequently, it has been hypothesized that NOS1 may lead to the endogenous production of low levels of peroxynitrite, while the expression of NOS2 may lead to the production high levels of peroxynitrite.
Many reactive nitrogen species, including peroxynitrite, have been shown to have biphasic effects on myocardial contractility. For instance, numerous studies have shown high concentrations of peroxynitrite to be detrimental to myocardial contractility [10-15], while at low concentrations peroxynitrite appears to act as a positive inotropic agent [16-18]. However, the majority of these studies examined effects on basal contractility and did not address the effects of peroxynitrite as they relate to β-adrenergic responsiveness. In a previous study, we demonstrated that the positive and negative effects of spermine NONOate, a nitric oxide donor, were dependent upon the level of β-adrenergic stimulation in the cardiomyocyte [19]. Thus, nitric oxide appears capable of modulating β-adrenergic responsiveness in a biphasic manner. However, given that nitric oxide and peroxynitrite are distinct species with chemistries that are very different, the ability of peroxynitrite to modulate β-adrenergic responsiveness in a biphasic manner warrants further investigation.
Therefore, the objective of this study is to examine the biphasic effect of the nitric oxide/superoxide donor SIN-1 in myocytes under varying levels of β-adrenergic stimulation. We hypothesize that the effects of SIN-1 are via peroxynitrite and that the negative effects of high SIN-1 (200 μmol/L) will be greatest during high contractile states (maximal β-adrenergic stimulation), while the positive effects of low SIN-1 (10 μmol/L) will be greater during low contractile states (basal, submaximal β-adrenergic stimulation).
MATERIALS AND METHODS
Cardiomyocyte Isolation
Ventricular myocytes were isolated from PLB knockout (PLB−/−) and their corresponding wildtype (WT, CF1) as previously described [13]. Briefly, hearts were excised from mice anesthetized with sodium pentobarbital. Using a Langendorff apparatus, hearts were perfused with nominally Ca2+-free Joklik Modified MEM (Sigma, St. Louis, MO) for 5 minutes at 37° C. Perfusion was then switched to the same solution, but now containing Liberase Blendzyme 4 (Roche Diagnostics, Indianapolis, IN). Hearts were digested until the drip rate reached one per second. Following digestion, the heart was taken down and the tissue minced, triturated, and filtered. The cell suspension was then rinsed and stored in Joklik Modified MEM containing 200 μmol/L Ca2+. Cells were used within 6 hours of isolation. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85−23, revised 1996) and was approved by the Institutional Laboratory Animal Care and Use Committee.
Measurement of Peroxynitrite Release Rate
Electron paramagnetic resonance (EPR) spectroscopy with 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride (CP-H; Alexis, Lausen, Switzerland) was used to measure the rate of peroxynitrite release from SIN-1 under our experimental conditions as previously described [13, 20]. Briefly, EPR spectra were recorded using a quartz flat cell at room temperature with a Bruker ESP 300E spectrometer (Billerica, MA) operating at X-band with 100-KHz modulation frequency and a TM110 cavity. EPR instrument parameters used were as follows: microwave frequency, 9.775 GHz; scan width, 100 G; modulation amplitude, 1 G; microwave power, 20 mW; number of scans, 1; scan time, 30 s; and time constant, 82 ms.
CP-H reacts with peroxynitrite to form 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxy (CP) [20]. EPR spectra were recorded for the reaction mixture which contained CP-H (1 mmol/L) and SIN-1 (10 μmol/L; Alexis) in normal Tyrode solution, pH 7.4. In order to inhibit reactions of CP-H catalyzed by transition metal ion impurities in the buffer, the transition metal chelators diethylenetriaminepentaacetic acid (DTPA, 1 mmol/L; Sigma) and sodium diethyldithiocarbamate trihydrate (DETC, 10 μmol/L; Sigma) were added to the normal Tyrode solution. EPR spectra were collected for 15 minutes. Quantitation of the observed CP radical signals was performed by computer simulation of the spectra and comparison of the double integral of the observed signal with that of a 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, 1 μmol/L; Sigma) standard measured under identical conditions [21].
Simultaneous Measurement of Cellular [Ca2+]i Transient and Shortening
[Ca2+]i transients and shortening were measured in isolated myocytes as previously described [13]. Briefly, isolated myocytes were loaded at 22° C with 10 μmol/L Fluo-4 AM (Molecular Probes, Eugene, OR) for 30 minutes. Excess dye was removed by washout with 200 μmol/L Ca2+ normal Tyrode solution. Myocytes were then de-esterfied for an additional 30 minutes. Following loading, cells were stimulated at 1 Hz via platinum electrodes connected to a Grass Telefactor S48 stimulator (West Warwick, RI). Fluo-4 was excited with 480±20 nm light, and the fluorescent emission of a single cell was collected at 530±25 nm using an epifluorescence system (Cairn Research Limited, Faversham, UK). The illumination field was restricted to collect the emission of a single cell. The ratio of F/F0 (R), where F0 was the fluorescence intensity and F the intensity at rest, was then converted to nmol/L Ca2+ using the equation [Ca2+]i = KdR/[(Kd/[Ca2+]i − rest+1)-R] [22], and assuming Kd − Fuo-4 = 1100 nmol/L [23] and [Ca2+]i − rest = 125 nmol/L. Simultaneous measurement of shortening was performed using an edge detection system (Crescent Electronics, Sandy, UT). Cardiomyocyte shortening amplitude was normalized to resting cell length (% RCL). All measurements were recorded at room temperature (22° C). Additionally, as each myocyte was perfused with both control (normal Tyrode) and various experimental solutions (ISO, ISO+SIN-1, etc.) until steady-state was reached, [Ca2+]i transient amplitude and myocyte shortening amplitude were used to determine the % Δ from control and the % Δ from ISO (where applicable) for each cell. This measure allows each myocyte to serve as its own control and also normalizes each data set.
Solutions and Drugs
Normal Tyrode control solution consisted of (in mmol/L): NaCl (140), KCl (4), MgCl2 (1), CaCl2 (1), Glucose (10), and HEPES (5); pH = 7.4 adjusted with NaOH and/or HCl. Isoproterenol was used as a non-specific β-adrenergic agonist (ISO; Sigma, St. Louis, MO). 3-Morpholinosydnonimine (SIN-1; Alexis) was used as a nitric oxide/superoxide donor and a source of peroxynitrite. 5,10,15,20-tetrakis-[4-sulfonatophenyl]-porphyrinato-iron[III] (FeTPPS; Calbiochem, La Jolla, CA) was used as a specific peroxynitrite decomposition catalyst. The L-arginine analog L-N(G)-nitroarginine methyl ester (L-NAME) was used as a non-specific inhibitor of nitric oxide synthase. Forskolin (Sigma) was used as an adenylate cyclase activator. All solutions were made fresh on the day of experimentation.
Statistics
Data are presented as the mean±S.E.M. Statistical significance (p<0.05) was determined between groups using an ANOVA (followed by Neuman-Keuls test) for multiple groups or a Student's paired t-test for two groups.
RESULTS
Peroxynitrite Production Resulting from SIN-1
Using EPR spectroscopy with CP-H, we determined the rate of peroxynitrite release by 10 μmol/L SIN-1 under our experimental conditions (normal Tyrode control solution, 22° C). At this concentration, SIN-1 released 3 nmol L−1min−1 of peroxynitrite over the same time course as our functional experiments. We previously determined that 200 μmol/L SIN-1 released 18 nmol L−1min−1 of peroxynitrite [13].
Effect of Low SIN-1 (10 μmol/L) on WT Myocyte Function
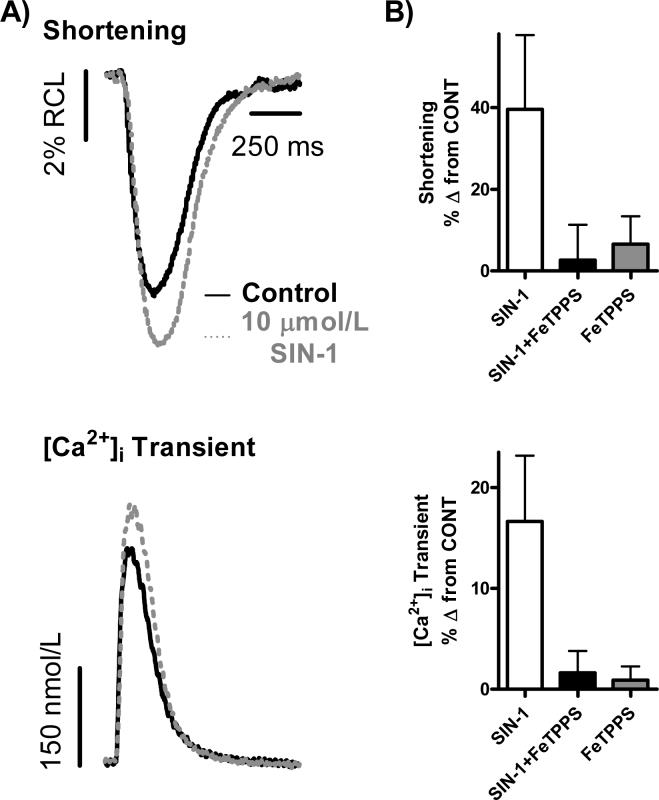
We first investigated the effect of 10 μmol/L SIN-1 on basal contractility in isolated WT myocytes and examined basal [Ca2+]i transients and myocyte shortening (Fig. 1). After reaching steady-state in normal Tyrode control solution, perfusion with 10 μmol/L SIN-1 increased basal myocyte contractility, with a significant increase in [Ca2+]i transient amplitude and a trend towards significantly increased myocyte shortening (n = 22 myocytes/9 hearts; [Ca2+]i Transient: 17±7%*, Shortening: 40±18% Δ from CONT, *p<0.05 vs. CONT). This effect can be seen in the representative traces shown in Fig. 1A and in the summary data found in Fig. 1B. However, upon simultaneous perfusion of 10 μmol/L SIN-1 and 10 μmol/L FeTPPS (Fig. 1B), a peroxynitrite decomposition catalyst, the positive effect of 10 μmol/L SIN-1 on basal myocyte function was attenuated (n = 13 myocytes/4 hearts; [Ca2+]i Transient: 2±2%, Shortening: 3±8% Δ from CONT). Perfusion with 10 μmol/L FeTPPS alone had no effect on basal myocyte function (n = 16 myocytes/4 hearts; [Ca2+]i Transient: 1±1%, Shortening: 6±7% Δ from CONT), as seen in Fig. 1B.
Figure 1. 10 μmol/L SIN-1 increases basal WT myocyte contractility.
A.) Individual, steady-state shortening (top) and [Ca2+]i transient (bottom) traces representing the positive effect of 10 μmol/L SIN-1 on basal WT myocyte contractility. B.) Pooled data (mean±S.E.M.) demonstrating the effect SIN-1 (10 μmol/L, clear bar), SIN-1+FeTPPS (10 μmol/L, filled bar) and FeTPPS alone (10 μmol/L, gray bar) on shortening (top) and [Ca2+]i transients (bottom) in WT myocytes.
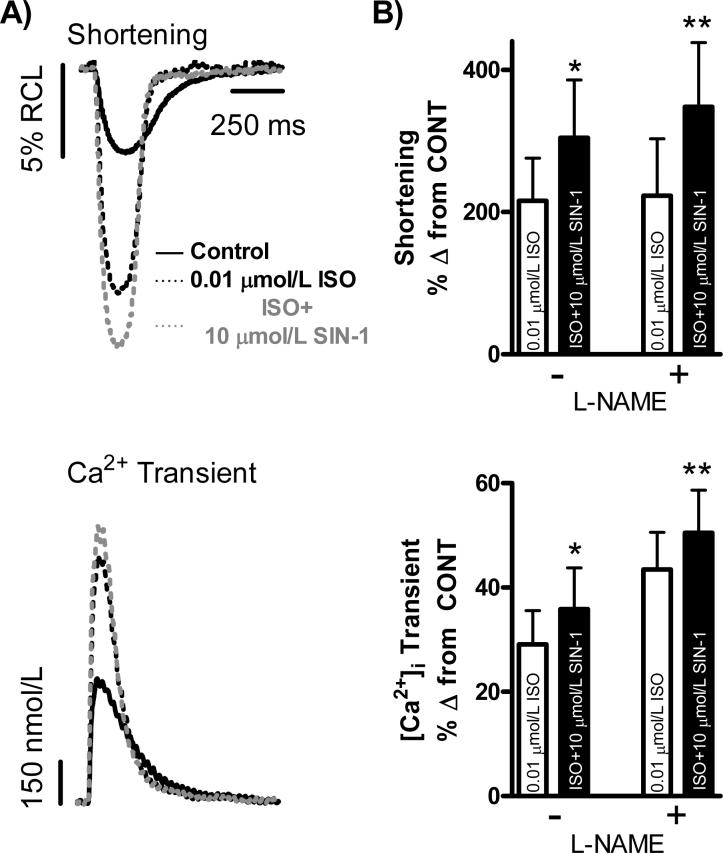
We next examined the effect of 10 μmol/L SIN-1 on WT myocyte function under a submaximal level of β-adrenergic stimulation (Fig. 2). After reaching steady-state in normal Tyrode control solution and a steady-state response to 0.01 μmol/L ISO, superfusion with 0.01 μmol/L ISO+10 μmol/L SIN-1 significantly increased myocyte [Ca2+]i transient amplitude and shortening (n = 15 myocytes/3 hearts; [Ca2+]i Transient: 29±6% vs. 36±8%, Shortening: 216±60% vs. 305±81% Δ from CONT, p<0.05 vs. ISO). This effect can be seen in the representative traces shown in Fig. 2A and the summary data found in Fig. 2B. However, upon repetition of the above experimental protocol with the addition of 10 μmol/L FeTPPS, the positive effect of 10 μmol/L SIN-1 on submaximal β-adrenergic stimulation was no longer observed (data not shown). Additionally, since endogenous nitric oxide production has been shown to change during acute β-adrenergic stimulation [24, 25], we chose to inhibit endogenous nitric oxide production in another series of functional experiments using the L-arginine analog L-NAME, a non-specific inhibitor of nitric oxide synthase. After reaching steady-state in normal Tyrode control solution and a steady-state response to 0.01 μmol/L ISO, superfusion with 0.01 μmol/L ISO+10 μmol/L SIN-1 in the presence of 100 μmol/L L-NAME still resulted in a significant increase in myocyte [Ca2+]i transient amplitude and shortening (n = 14 myocytes /5 hearts; [Ca2+]i Transient: 43±7% vs. 50±8%, Shortening: 223±80% vs. 348±90% Δ from CONT, p<0.05 vs. ISO). This effect is shown in Fig. 2B.
Figure 2. 10 μmol/L SIN-1 increases WT myocyte submaximal β-adrenergic responsiveness.
A.) Individual, steady-state shortening (top) and [Ca2+]i transient (bottom) traces representing the positive effect of 10 μmol/L SIN-1 on WT myocyte submaximal β-adrenergic responsiveness. B.) Pooled data (mean±S.E.M.) demonstrating the effect of ISO (0.01 μmol/L, clear bar) and ISO+SIN-1 (10 μmol/L, filled bar) on shortening (top) and [Ca2+]i transients (bottom) in WT myocytes with and without L-NAME. *p<0.05 vs. ISO without L-NAME, **p<0.05 vs. ISO with L-NAME.
Finally, the effect of 10 μmol/L SIN-1 on maximal β-adrenergic responsiveness was examined in isolated WT myocytes. After reaching steady-state in normal Tyrode control solution and a steady-state response to 1 μmol/L ISO, superfusion with 10 μmol/L SIN-1 did not have an effect on maximal β-adrenergic stimulated [Ca2+]i transients and shortening in WT myocytes (n = 22 myocytes/9 hearts; [Ca2+]i Transient: 105±19% vs. 97±16%, Shortening: 374±97% vs. 242±115% Δ from CONT).
Effect of High SIN-1 (200 μmol/L) on WT Myocyte Function
In a previous study, we reported that 200 μmol/L SIN-1 had no effect on basal [Ca2+]i transients or myocyte shortening in WT myocytes compared to WT myocyte function in normal Tyrode control solution alone [13].
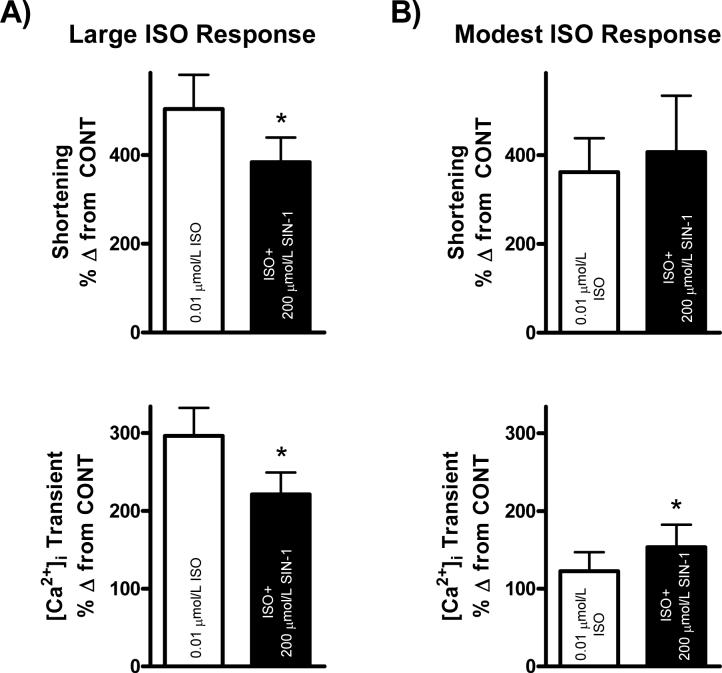
We further examined the effect of 200 μmol/L SIN-1 on WT myocyte function during submaximal β-adrenergic stimulation (Fig. 3). After reaching steady-state in normal Tyrode control solution and in response to 0.01 μmol/L ISO, we observed two distinct phenomena upon simultaneous perfusion with 0.01 μmol/L ISO and 200 μmol/L SIN-1. Namely, one population of myocytes displayed a positive response to 200 μmol/L SIN-1, whereas the other displayed a negative response. Upon further examination of these effects, we observed that the population of myocytes which underwent a negative response to 200 μmol/L SIN-1 also exhibited large responses on average to submaximal ISO ([Ca2+]i Transient: 296±36%, Shortening: 504±77% Δ from CONT), while the population of myocytes that underwent a positive response to 200 μmol/L SIN-1 showed on average only modest responses to submaximal ISO ([Ca2+]i Transient: 123±24%*, Shortening: 362±76% Δ from CONT, *p<0.05 vs. large response to ISO). Thus, in myocytes undergoing a large response to submaximal ISO, 200 μmol/L SIN-1 induced a significant reduction in [Ca2+]i transient amplitude and myocyte shortening (n = 18 myocytes/4 hearts; [Ca2+]i Transient: 296±36% vs. 221±28%, Shortening: 504±77% vs. 384±56% Δ from CONT, p<0.05 vs. ISO). This effect was demonstrated in our previous publication [13], and can be seen in Fig. 3A. However, in myocytes undergoing a modest response to submaximal ISO, SIN-1 further increased [Ca2+]i transient amplitude and myocyte shortening (n = 11 myocytes/4 hearts; [Ca2+]i Transient: 123±24% vs. 153±29%*, Shortening: 362±76% vs. 407±127% Δ from CONT, *p<0.05 vs. ISO). This effect can be seen in Fig. 3B. Additionally, 200 μmol/L SIN-1 produced a further increase in [Ca2+]i transient amplitude and myocyte shortening during stimulation with a low level of forskolin, a direct activator of adenylate cyclase (data not shown).
Figure 3. Effect of 200 μmol/L SIN-1 is dependent upon contractile state in WT myocytes.
A.) Large response to ISO: Pooled data (mean±S.E.M.) expressed as a % Δ from control demonstrating the effect of ISO (0.01 μmol/L, clear bar) and ISO+SIN-1 (200 μmol/L, filled bar) on shortening (top) and [Ca2+]i transients (bottom) in WT myocytes undergoing a large response to ISO. *p<0.05. B.) Modest response to ISO: Pooled data (mean±S.E.M.) expressed as a % Δ from c ontrol demonstrating the effect of ISO (0.01 μmol/L, clear bar) and ISO+SIN-1 (200 μmol/L, filled bar) on shortening (top) and [Ca2+]i transients (bottom) in WT myocytes undergoing a modest response to ISO. *p<0.05 vs. ISO. NOTE: 200 μmol/L SIN-1 data displayed in panel A (left) have been published previously in a different form [13].
We previously reported that 200 μmol/L SIN-1 always reduced [Ca2+]i transient amplitude and shortening in WT myocytes under maximal β-adrenergic stimulation (ISO, 1 μmol/L) and to a greater degree than that observed with submaximal β-adrenergic stimulation [13]. Additionally, upon inhibition of endogenous nitric oxide synthase production with 100 μmol/L L-NAME, a significant negative effect of 200 μmol/L SIN-1 remained (data not shown).
Effect of SIN-1 on PLB−/− Myocyte Function
As previous studies have implicated PLB as a potential target of SIN-1 and peroxynitrite signaling [13, 26], we sought to examine the effect of SIN-1 in PLB−/− myocytes. We first examined the effect of SIN-1 on basal contractility. After reaching steady-state in normal Tyrode control solution, perfusion with 10 μmol/L SIN-1 did not alter basal contractility in PLB−/− myocytes (n = 8 myocytes/3 hearts; [Ca2+]i Transient: −1±2%, Shortening: −3±4% Δ from CONT). In our previous study, we reported that 200 μmol/L SIN-1 also had no effect on basal contractility in PLB−/− myocytes compared to PLB−/− myocyte function in normal Tyrode control solution alone [13].
We next examined the effect of 10 μmol/L SIN-1 during submaximal β-adrenergic stimulation. PLB−/− myocytes showed the typical enhanced basal contractility, and thus exhibited reduced β-adrenergic responsiveness upon perfusion with 0.01 μmol/L ISO compared to WT myocytes, as has been previously demonstrated [27]. However, superfusion with 0.01 μmol/L ISO+10 μmol/L SIN-1 had no effect on the [Ca2+]i transient amplitude or on myocyte shortening (n = 22 myocytes/6 hearts; [Ca2+]i Transient: 23±4% vs.20±7%, Shortening: 62±20% vs. 64±21% Δ from CONT).
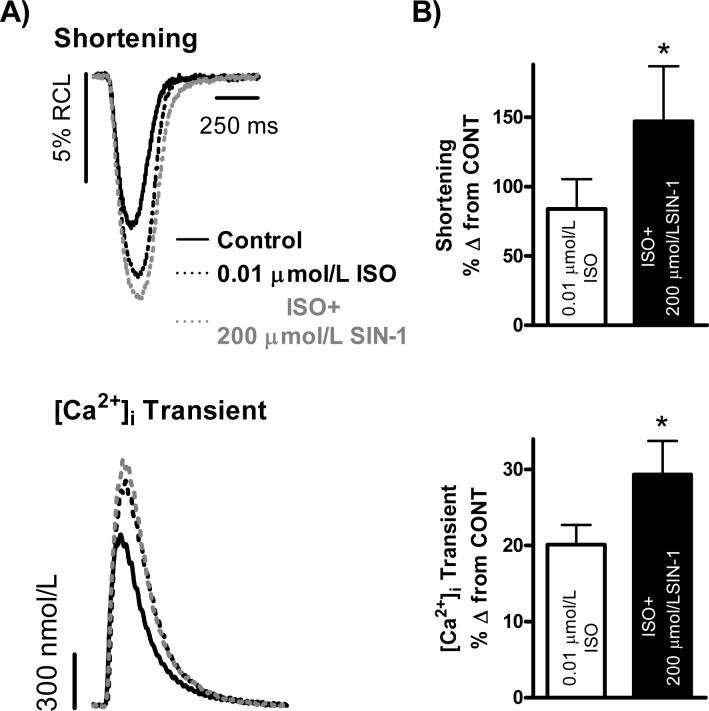
The effect of 200 μmol/L SIN-1 during submaximal β-adrenergic stimulation was examined next (Fig. 4). Again, the PLB−/− myocytes showed the typical enhanced basal contractility as seen in the representative traces shown in Fig. 4A, and thus exhibited reduced β-adrenergic responsiveness to 0.01 μmol/L ISO compared to WT. After a steady-state response to 0.01 μmol/L ISO, superfusion with 0.01 μmol/L ISO+200 μmol/L SIN-1 significantly increased [Ca2+]i transient amplitude and myocyte shortening (n = 32 myocytes/7 hearts; [Ca2+]i Transient: 20±3% vs. 30±4%, Shortening: 84±21% vs.147±40% Δ from CONT, p<0.05 vs. ISO). This effect can be seen in Fig. 4B.
Figure 4. 200 μmol/L SIN-1 increases PLB−/− myocyte submaximal β-adrenergic responsiveness.
A.) Individual, steady-state shortening (top) and [Ca2+]i transient (bottom) traces representing the positive effect of 200 μmol/L SIN-1 on PLB−/− myocyte submaximal β-adrenergic responsiveness. B.) Pooled data (mean±S.E.M.) demonstrating the effect of ISO (0.01 μmol/L, clear bar) and ISO+SIN-1 (200 μmol/L, filled bar) on shortening (top) and [Ca2+]i transients (bottom) in PLB−/− myocytes. *p<0.05 vs. ISO.
Finally, we examined the effect of 10 μmol/L SIN-1 on maximal β-adrenergic responsiveness in isolated PLB−/− myocytes. After reaching steady-state in normal Tyrode control solution and in response to 1 μmol/L ISO, superfusion with 1 μmol/L ISO+10 μmol/L SIN-1 had no effect on [Ca2+]i transient amplitude or myocyte shortening (n = 23 myocytes/7 hearts; [Ca2+]i Transient: 40±8% vs. 36±10%, Shortening: 100±27% vs. 130±44% Δ from CONT). We previously reported that 200 μmol/L SIN-1 had no effect on maximal β-adrenergic responsiveness in the PLB−/− myocytes [13]. Thus, SIN-1 only produced a contractile effect under one condition in PLB−/− cardiomyocytes (0.01 μmol/L ISO+200 μmol/L SIN-1). This is in direct contrast to the effects of SIN-1 observed in WT cardiomyocytes, which can be seen in Fig. 5.
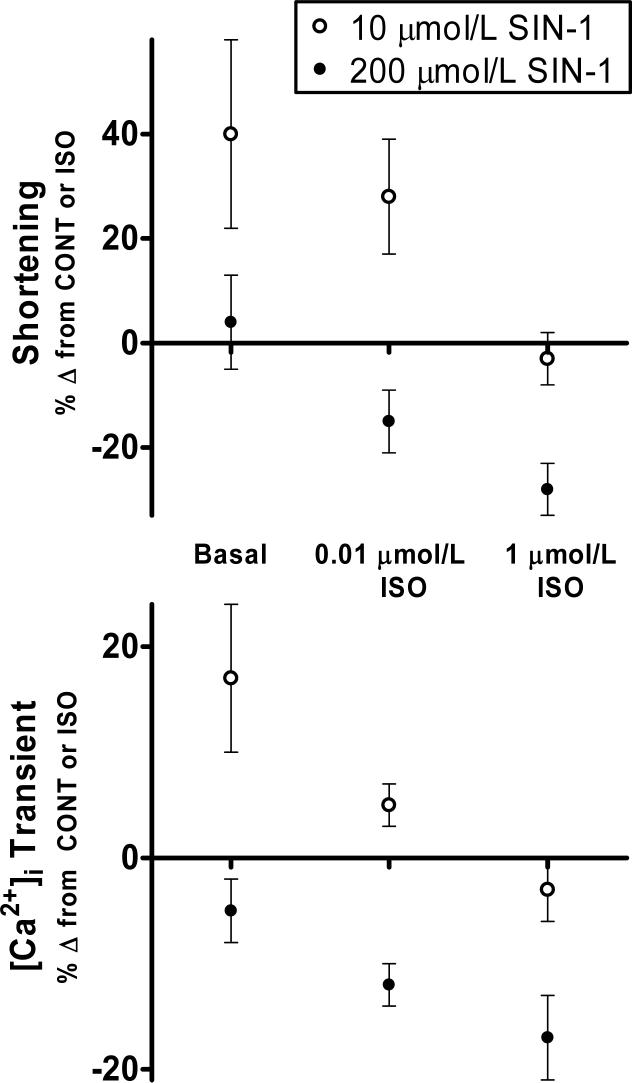
Figure 5. PLB-dependent effects of 10 μmol/L SIN-1 and 200 μmol/L SIN-1 on β-adrenergic stimulated myocyte contractility.
Pooled data (mean±S.E.M.) expressed as a % Δ from control (Basal) or a % Δ from ISO (0.01 μmol/L ISO, 1 μmol/L ISO) demonstrating the positive effect of 10 μmol/L SIN-1 (open circles) and the negative effect of 200 μmol/L SIN-1 (closed circles) during varying levels of β-adrenergic stimulation for both myocyte shortening (top panel) and [Ca2+]i transients (bottom panel). NOTE: 200 μmol/L SIN-1 data (closed circles) have been published previously in a different form [13].
DISCUSSION
Few studies have examined the biphasic effect of peroxynitrite during β-adrenergic stimulation in the mammalian myocardium or the mechanism underlying this effect(s). There are many studies which demonstrate a concentration-dependent biphasic effect of peroxynitrite on basal myocardial contractility, but the majority of these studies did not address these effects on β-adrenergic responsiveness [10-18, 26, 28]. In our study, SIN-1 (source of peroxynitrite) had a biphasic effect on murine cardiomyocyte contractility that was not only dependent on concentration, but also on the contractile state of the cardiomyocyte. Our results demonstrated that during maximal β-adrenergic stimulation, high SIN-1 (200 μmol/L) always produced anti-adrenergic effects, as previously shown [13]. During submaximal β-adrenergic stimulation, 200 μmol/L SIN-1 also produced significant anti-adrenergic effects during vigorous responses to ISO, but further increased β-adrenergic responsiveness during modest responses. Conversely, low SIN-1 (10 μmol/L) increased basal myocyte contractility and the response to submaximal β-adrenergic stimulation, but had no effect during maximal β-adrenergic stimulation. Further, SIN-1 had no effect at either concentration on the contractile state in PLB−/− myocytes apart from one condition (0.01 μmol/L ISO+200 μmol/L SIN-1), thus providing a potential mechanism for the actions of peroxynitrite. These results provide clear evidence for a biphasic effect of peroxynitrite, with high peroxynitrite modulating high levels of β-adrenergic responsiveness and low peroxynitrite modulating basal function and low levels of β-adrenergic stimulation.
Peroxynitrite Production Resulting from SIN-1
SIN-1 breaks down to release both nitric oxide and superoxide, which could potentially react leading to the formation of peroxynitrite. Thus, we determined the peroxynitrite production resulting from SIN-1. The low concentration of SIN-1 (10 μmol/L) used in this study was determined to release peroxynitrite at a rate of 3 nmol L−1min−1 under our experimental conditions. As expected, the concentration of peroxynitrite produced by 10 μmol/L SIN-1 was much lower than that produced by the high concentration of SIN-1 (200 μmol/L), which was previously determined to produce 18 nmol L−1min−1 [13].
Low SIN-1 (10 μmol/L) and Myocyte Contractility
Many studies have demonstrated the positive effect of low concentrations of the nitric oxide/superoxide donor SIN-1 and of peroxynitrite on basal myocardial contractility [16-18]. We confirmed these results in isolated murine cardiomyocytes, in that 10 μmol/L SIN-1 induced an increase in basal [Ca2+]i transient amplitude and myocyte shortening (Fig. 1). These positive effects on basal contractility were attributed to peroxynitrite, as coinfusion with the peroxynitrite decomposition catalyst, FeTPPS, attenuated the positive effect of 10 μmol/L SIN-1 (Fig. 1B).
During submaximal β-adrenergic stimulation we observed the typical increase in myocyte contractility. Subsequent perfusion with 10 μmol/L SIN-1 during submaximal β-adrenergic stimulation always produced a further increase in contractility (Fig. 2A and Fig. 2B). As with the myocytes under basal conditions, FeTPPS was able to alleviate the positive effect of 10 μmol/L SIN-1 on submaximal β-adrenergic stimulation, thus implicating peroxynitrite as the causal species. In our previous publication, FeTPPS (10 μmol/L) alone was shown to have no effect on the cardiomyocyte response to ISO [13]. Additionally, studies have shown that acute β-adrenergic stimulation could alter endogenous nitric oxide production [24, 25]. This change in nitric oxide levels could shift the equilibrium of nitric oxide and superoxide produced by SIN-1, potentially changing the level of peroxynitrite production. Thus, we chose to inhibit endogenous nitric oxide production using L-NAME, a non-specific inhibitor of nitric oxide synthase. The positive effect of 10 μmol/L SIN-1 remained with the inhibition of endogenous nitric oxide production. Although we previously demonstrated in this study that SIN-1 does indeed produce peroxynitrite, SIN-1 has a complex chemistry and other reactive nitrogen species can be formed (i.e., nitrosoperoxycarbonate). However, the formation of nitrosoperoxycarbonate tends to result in irreversible protein nitration [29], while the effects we observed with SIN-1 during our functional experiments were entirely reversible and could be washed out.
Upon maximal β-adrenergic stimulation, however, 10 μmol/L SIN-1 no longer had an effect on myocyte contractility. Although we did not observe an additional positive effect of 10 μmol/L SIN-1 during maximal β-adrenergic stimulation, any additional positive effect may have been masked by the large positive effect of maximal β-adrenergic stimulation. Taken together, these data indicate that at a low concentration, SIN-1, and thus peroxynitrite, solely modulates basal myocyte function and low levels of β-adrenergic stimulation (Fig. 5). To our knowledge, this study is the first to reveal that peroxynitrite is responsible for the SIN-1-induced increase in basal contractility. Moreover, we are the first to demonstrate that a low concentration peroxynitrite is able to further increase contractility during submaximal β-adrenergic stimulation, independent of endogenous nitric oxide production.
High SIN-1 (200 μmol/L) and Myocyte Contractility
In contrast to the positive effects observed on myocyte contractility with 10 μmol/L SIN-1, 200 μmol/L SIN-1 had no effect on basal contractility in WT myocytes. This lack of effect was demonstrated in our previous study [13].
During submaximal β-adrenergic stimulation, 200 μmol/L SIN-1 produced anti-adrenergic effects only when the response to submaximal β-adrenergic stimulation was vigorous (Fig. 3A), as previously shown [13]. However, when the response to submaximal β-adrenergic stimulation was modest, 200 μmol/L SIN-1 induced a further increase in myocyte contractility (Fig. 3B). This result is not unexpected as another one of our previous studies demonstrated that the positive and negative effects of spermine NONOate, a nitric oxide donor, were dependent upon the degree of β-adrenergic stimulation [19]. During high states of β-adrenergic stimulation, spermine NONOate decreased Ca2+ spark frequency, while during low levels of β-adrenergic stimulation, the same concentration of spermine NONOate produced a large increase in Ca2+ spark frequency. It is important to realize, however, that nitric oxide and peroxynitrite are distinct species with chemistries that are very different. In fact, one study demonstrated that spermine NONOate produce effects during β-adrenergic stimulation in papillary muscles isolated from PLB−/− mice [30], while our current and previous study demonstrated a lack of effect of either 10 μmol/L or 200 μmol/L SIN-1 in PLB−/− myocytes, aside from one condition. Additionally, the positive effect of 200 μmol/L SIN-1 during a low level of forskolin stimulation indicate that the positive effects of 200 μmol/L SIN-1 are likely mediated via targets downstream of adenylate cyclase.
Finally, the effect of 200 μmol/L SIN-1 was always negative during maximal β-adrenergic stimulation, as previously published [13]. This negative effect was also greater than that observed with submaximal β-adrenergic stimulation (Fig. 3A). Further, inhibition of endogenous nitric oxide production did not alter the cardiomyocyte response to 200 μmol/L SIN-1. These data indicate that 200 μmol/L SIN-1 solely modulates β-adrenergic responsiveness and, aside from the positive effect during modest response to submaximal β-adrenergic stimulation, is able to more effectively produce anti-adrenergic effects with increasing levels of β-adrenergic stimulation as seen in Fig. 5.
SIN-1 and PLB−/− Myocyte Function
Although we observed a biphasic effect of SIN-1 in WT myocytes, we saw no effect at either concentration (10 μmol/L or 200 μmol/L) in PLB−/− myocytes under basal conditions. This is in direct contrast to the effect observed in WT myocytes where 10 μmol/L SIN-1 elicited a positive increase in contractility (Fig. 1). The lack of effect in PLB−/− myocytes may therefore indicate that the positive effect of 10 μmol/L SIN-1 may be mediated via PLB. This result is not unexpected, as our previous publication demonstrated that 200 μmol/L SIN-1 produced anti-adrenergic effects by triggering the dephosphorylation of PLB via increased protein phosphatase activity, thus decreasing SR Ca2+ uptake, SR Ca2+ load, and myocyte contractility [13]. In the case of 10 μmol/L SIN-1, however, these positive effects may result from an increase PLB phosphorylation. Studies have shown that low concentrations of peroxynitrite are capable of decreasing protein phosphatase activity [31]. Other post-translational modifications to PLB, including s-nitrosylation and nitration, may also be responsible for the positive effect of 10 μmol/L SIN-1. PLB has an exposed tyrosine residue (Tyr6) that is susceptible to nitration [32].
During submaximal β-adrenergic stimulation, 10 μmol/L SIN-1 appeared to have no effect in PLB−/− myocytes. Therefore, this result indicates that the positive effects of 10 μmol/L SIN-1 during submaximal β-adrenergic stimulation in WT myocytes (Fig. 2) may occur through a PLB-dependent mechanism. 200 μmol/L SIN-1, however, further increased contractility in PLB−/− myocytes (Fig. 4). These results coincide with those observed in WT myocytes, where myocytes exhibiting a modest response to submaximal β-adrenergic stimulation showed a further increase in contractility in response to 200 μmol/L SIN-1 (Fig. 3B). This increase is possible because the PLB−/− myocytes also exhibited a modest response to submaximal β-adrenergic stimulation. As the effect of 200 μmol/L SIN-1 was observed in WT and PLB−/− myocytes, SIN-1 may be signaling independent of PLB by targeting other excitation-contraction coupling proteins, including the L-type Ca2+ channel. SIN-1 has been shown to increase the L-type Ca2+ current in cardiomyocytes [33]. SIN-1 has also been shown to exert an activating effect on the ryanodine receptor, thus increasing Ca2+ release from the SR [34]. Additionally, SIN-1 has been shown to alter cyclic AMP levels in cardiomyocytes [26].
Finally, in PLB−/− myocytes under maximal β-adrenergic stimulation, we observed no effect of either 10 μmol/L or 200 μmol/L SIN-1. In our previous publication, we showed that the negative effects of 200 μmol/L SIN-1 were dependent upon PLB, as decreased contractility resulted from a reduction in PLB serine 16 phosphorylation [13]. The lack of effect of 10 μmol/L SIN-1 in PLB−/− myocytes during maximal β-adrenergic stimulation coincided with the lack of effect in WT myocytes, and further demonstrates that 10 μmol/L SIN-1 exclusively modulates basal stimulation and low levels of β-adrenergic stimulation. The PLB-dependent effects of both 10 μmol/L and 200 μmol/L SIN-1 on WT cardiomyocyte function can be seen in Fig. 5.
In conclusion, the nitric oxide/superoxide donor SIN-1 exudes a biphasic effect on cardiomyocyte contractility that is not only dependent on the concentration of SIN-1, but also on the contractile state of the cardiomyocyte. In the case of high SIN-1 (200 μmol/L), an increasing anti-adrenergic effect was observed with increasing levels of β-adrenergic stimulation (Fig. 5), such that high SIN-1 actually produced a positive effect during low responses to β-adrenergic stimulation, but large negative effects during high responses to β-adrenergic stimulation. Further, as high SIN-1 had no effect on basal contractility, it stands to reason that high SIN-1 exclusively modulates β-adrenergic responsiveness. It also appears that the negative effect of high SIN-1 is mediated through a PLB-dependent signaling pathway leading to alterations in Ca2+-handling as we and others have previously demonstrated [13, 15]. However, the positive effect of high SIN-1 is mediated via PLB-independent mechanisms.
In the case of low SIN-1 (10 μmol/L), positive effects were observed during basal stimulation and during submaximal β-adrenergic stimulation. No effects of low SIN-1 were observed during maximal β-adrenergic stimulation. Thus, it appears that low SIN-1 exclusively modulates basal stimulation and low levels of β-adrenergic stimulation, parameters that can be considered physiologic. Although we did not observe an additional positive effect of 10 μmol/L SIN-1 during maximal β-adrenergic stimulation, any additional positive effect may have been masked by the large positive effect of maximal β-adrenergic stimulation. Additionally, the lack of effect of low SIN-1 in PLB−/− myocytes may indicate that these positive effects are mediated via PLB-dependent mechanisms. However, the effects of low SIN-1 on other excitation-contraction coupling proteins cannot be completely ruled out, as peroxynitrite has been shown to have effects on other excitation-contraction coupling proteins [35-39]. Further, SIN-1 has a complex chemistry that could potentially lead to the formation of other reactive nitrogen species. However, all of our effects were reversible and could be inhibited with the peroxynitrite decomposition catalyst, FeTPPS, thus implicating peroxynitrite as the causal species.
Overall, the opposing effects of both high and low SIN-1 lend insight into the physiological and pathophysiological regulation of myocardial contractility by peroxynitrite. The biphasic nature of SIN-1 may relate to the physiological regulation of basal and submaximal β-adrenergic-stimulated contractility with low peroxynitrite production (i.e., NOS1) [40], and the pathophysiological regulation of chronic β-adrenergic stimulation with high peroxynitrite production (i.e., NOS2), as occurs in heart failure [3]. Thus, it appears that low SIN-1 may potentially mimic the peroxynitrite production of NOS1, which is hypothesized to produce low concentrations of peroxynitrite due to a co-localization with xanthine oxidase [4]. NOS1 is considered a physiological regulator of cardiac function and is also thought to increase myocardial contractility [41]. Conversely, high SIN-1 may be mimicking the peroxynitrite production of NOS2, which is considered to be a pathophysiological regulator of cardiac function by reducing β-adrenergic responsiveness [3, 36]. During many pathophysiological conditions of the myocardium, nitric oxide production is increased with NOS2 expression [3, 42] and superoxide production is increased through NADPH oxidase and/or xanthine oxidoreductase [8, 9], leading to supraphysiological peroxynitrite production and cardiac dysfunction. Additionally, this physiological and pathophysiological regulation of cardiac contractility by peroxynitrite likely relies on signaling pathways that target the excitation-contraction coupling protein PLB as well as other targets, thus resulting in the biphasic effect of SIN-1.
ACKNOWLEDGEMENTS
Supported by the American Heart Association (Pre-doctoral Fellowship 0715159B, MJK; Post-doctoral Fellowship, 0725560B HW) and the National Institutes of Health (R01HL079283, MTZ; R01HL063744, JLZ; P01HL065608, JLZ). We would also like to thank Dr. Evangelia G Kranias (University of Cincinnati) for providing the PLB−/− mice.
LIST OF ABBREVIATIONS
- CONT
Control
- CP-H
1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride
- EPR
Electron paramagnetic resonance
- FeTPPS
5,10,15,20-tetrakis-[4-sulfonatophenyl]-porphyrinato-iron[III]
- ISO
Isoproterenol
- L-NAME
L-N(G)-nitroarginine methyl ester
- NADPH
Nicotinamide adenine dinucleotide phosphate-oxidase
- NO.
Nitric oxide
- NOS
Nitric oxide synthase
- NOS1
Neuronal nitric oxide synthase
- NOS2
Inducible nitric oxide synthase
- NOS3
Endothelial nitric oxide synthase
- NS
Not significant
- O2.-
Superoxide
- ONOO−
Peroxynitrite
- PLB
Phospholamban
- PLB−/−
Phospholamban knockout
- RCL
Resting cell length
- SIN-1
3-Morpholinosydnonimine
- SERCA
Sarco/endoplasmic reticulum Ca2+ ATPase
- SR
Sarcoplasmic reticulum
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Ziolo MT, Bers DM. The real estate of NOS signaling: location, location, location. Circ Res. 2003;92:1279–1281. doi: 10.1161/01.RES.0000080783.34092.AF. [DOI] [PubMed] [Google Scholar]
- 3.Ziolo MT, Maier LS, Piacentino V, 3rd, Bossuyt J, Houser SR, Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation. 2004;109:1886–1891. doi: 10.1161/01.CIR.0000124231.98250.A8. [DOI] [PubMed] [Google Scholar]
- 4.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 6.Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 8.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 9.Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Barouch LA, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 10.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 11.Ferdinandy P, Panas D, Schulz R. Peroxynitrite contributes to spontaneous loss of cardiac efficiency in isolated working rat hearts. Am J Physiol. 1999;276:H1861–1867. doi: 10.1152/ajpheart.1999.276.6.H1861. [DOI] [PubMed] [Google Scholar]
- 12.Yin X, Shan Q, Deng C, Bourreau JP. Effect of SIN-1 in rat ventricular myocytes: interference with beta-adrenergic stimulation. Life Sci. 2002;71:287–297. doi: 10.1016/s0024-3205(02)01625-9. [DOI] [PubMed] [Google Scholar]
- 13.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77:353–361. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma XL, Lopez BL, Liu GL, Christopher TA, Ischiropoulos H. Peroxynitrite aggravates myocardial reperfusion injury in the isolated perfused rat heart. Cardiovasc Res. 1997;36:195–204. doi: 10.1016/s0008-6363(97)00179-x. [DOI] [PubMed] [Google Scholar]
- 15.Katori T, Donzelli S, Tocchetti CG, Miranda KM, Cormaci G, Thomas DD, Ketner EA, Lee MJ, Mancardi D, Wink DA, Kass DA, Paolocci N. Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic Biol Med. 2006;41:1606–1618. doi: 10.1016/j.freeradbiomed.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Chesnais JM, Fischmeister R, Mery PF. Peroxynitrite is a positive inotropic agent in atrial and ventricular fibres of the frog heart. J Physiol. 1999;521:375–388. doi: 10.1111/j.1469-7793.1999.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesnais JM, Fischmeister R, Mery PF. Positive and negative inotropic effects of NO donors in atrial and ventricular fibres of the frog heart. J Physiol. 1999;518:449–461. doi: 10.1111/j.1469-7793.1999.0449p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paolocci N, Ekelund UE, Isoda T, Ozaki M, Vandegaer K, Georgakopoulos D, Harrison RW, Kass DA, Hare JM. cGMP-independent inotropic effects of nitric oxide and peroxynitrite donors: potential role for nitrosylation. Am J Physiol Heart Circ Physiol. 2000;279:H1982–1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- 19.Ziolo MT, Katoh H, Bers DM. Positive and negative effects of nitric oxide on Ca(2+) sparks: influence of beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2295–2303. doi: 10.1152/ajpheart.2001.281.6.H2295. [DOI] [PubMed] [Google Scholar]
- 20.Dikalov S, Skatchkov M, Bassenge E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6, 6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem Biophys Res Commun. 1997;231:701–704. doi: 10.1006/bbrc.1997.6174. [DOI] [PubMed] [Google Scholar]
- 21.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 22.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 23.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 24.Dedkova EN, Wang YG, Blatter LA, Lipsius SL. Nitric oxide signalling by selective beta(2)-adrenoceptor stimulation prevents ACh-induced inhibition of beta(2)-stimulated Ca(2+) current in cat atrial myocytes. J Physiol. 2002;542:711–723. doi: 10.1113/jphysiol.2002.023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanai AJ, Mesaros S, Finkel MS, Oddis CV, Birder LA, Malinski T. Beta-adrenergic regulation of constitutive nitric oxide synthase in cardiac myocytes. Am J Physiol. 1997;273:C1371–1377. doi: 10.1152/ajpcell.1997.273.4.C1371. [DOI] [PubMed] [Google Scholar]
- 26.Stojanovic MO, Ziolo MT, Wahler GM, Wolska BM. Anti-adrenergic effects of nitric oxide donor SIN-1 in rat cardiac myocytes. Am J Physiol Cell Physiol. 2001;281:C342–349. doi: 10.1152/ajpcell.2001.281.1.C342. [DOI] [PubMed] [Google Scholar]
- 27.Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+. Am J Physiol. 1996;271:C391–397. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- 28.Lopez BL, Liu GL, Christopher TA, Ma XL. Peroxynitrite, the product of nitric oxide and superoxide, causes myocardial injury in the isolated perfused rat heart. Coron Artery Dis. 1997;8:149–153. doi: 10.1097/00019501-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Uppu RM, Pryor WA. Carbon dioxide catalysis of the reaction of peroxynitrite with ethyl acetoacetate: an example of aliphatic nitration by peroxynitrite. Biochem Biophys Res Commun. 1996;229:764–769. doi: 10.1006/bbrc.1996.1878. [DOI] [PubMed] [Google Scholar]
- 30.Dias FA, Ribeiro CD, Szkudlarek AC, Pena JR, Wolska BM. Cardiac TnI and phospholamban are not major targets for NO-mediated attenuation of beta-adrenergic inotropic effect. Biophysical Journal. 2008;94:1458. (Abstract) [Google Scholar]
- 31.Sommer D, Coleman S, Swanson SA, Stemmer PM. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch Biochem Biophys. 2002;404:271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Bigelow DJ, Squier TC. Phosphorylation by cAMP-dependent protein kinase modulates the structural coupling between the transmembrane and cytosolic domains of phospholamban. Biochemistry. 2003;42:10674–10682. doi: 10.1021/bi034708c. [DOI] [PubMed] [Google Scholar]
- 33.Malan D, Levi RC, Alloatti G, Marcantoni A, Bedendi I, Gallo MP. Cyclic AMP and cyclic GMP independent stimulation of ventricular calcium current by peroxynitrite donors in guinea pig myocytes. J Cell Physiol. 2003;197:284–296. doi: 10.1002/jcp.10368. [DOI] [PubMed] [Google Scholar]
- 34.Harmatina O, Azarov VI, Moibenko OO. [Effects of nitric oxide donor SIN-1 on calcium transport in rat cardiac sarcoplasmic reticulum]. Fiziol Zh. 2001;47:10–14. [PubMed] [Google Scholar]
- 35.Ishida H, Genka C, Hirota Y, Hamasaki Y, Nakazawa H. Distinct roles of peroxynitrite and hydroxyl radical in triggering stunned myocardium-like impairment of cardiac myocytes in vitro. Mol Cell Biochem. 1999;198:31–38. doi: 10.1023/a:1006989826711. [DOI] [PubMed] [Google Scholar]
- 36.Ziolo MT, Katoh H, Bers DM. Expression of inducible nitric oxide synthase depresses beta-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation. 2001;104:2961–2966. doi: 10.1161/hc4901.100379. [DOI] [PubMed] [Google Scholar]
- 37.Rork TH, Hadzimichalis NM, Kappil MA, Merrill GF. Acetaminophen attenuates peroxynitrite-activated matrix metalloproteinase-2-mediated troponin I cleavage in the isolated guinea pig myocardium. J Mol Cell Cardiol. 2006;40:553–561. doi: 10.1016/j.yjmcc.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 39.Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, Haworth RA. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005;111:988–995. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Kohr MJ, Wheeler DG, Ziolo MT. Neuronal nitric oxide synthase enhances cardiac contractility by modulation of phospholamban phosphorylation. Circulation. 2006;114:II–74. (Abstract) [Google Scholar]
- 41.Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fradley M, Shoukas AA, Berkowitz DE, Hare JM. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 42.Drexler H, Kastner S, Strobel A, Studer R, Brodde OE, Hasenfuss G. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol. 1998;32:955–963. doi: 10.1016/s0735-1097(98)00336-2. [DOI] [PubMed] [Google Scholar]