Abstract
Previous studies have indicated that methamphetamine (MA) potentiates neurodegeneration induced by ischemia in brain. We, and others, have reported that bone morphogenetic protein 7 (BMP7) is protective against MA and ischemic brain injury. The purpose of this study is to examine whether BMP7 reduces synergistic injury induced by both MA and cerebral ischemia. Adult CD-1 mice were treated with MA (10 mg/kg × 4, each dose two hours apart) or saline. Using the quantitative real time polymerase chain reaction, we found that MA suppressed the expression of BMP7 mRNA in the cerebral cortex one day after injection. Ischemic and reperfusional injuries were introduced by ligation of the right middle cerebral artery for 90 min after MA injection. Animals were sacrificed for caspase 3/7 activity assay and tri-phenyl-tetrazolium chloride staining at 1 hour and 2 days after reperfusion, respectively. Cerebral infarction and caspase-3/7 activity were enhanced in the stroke animals pretreated with MA; both responses were attenuated by pretreatment with BMP7. In conclusion, our data suggest that MA facilitates cerebral infarction after ischemia possibly mediated, in part, through the suppression of BMP7.
Methamphetamine (MA) has been reported to be associated with ischemic injury. MA facilitates ischemic damage in rodent brain [1]. Clinical studies have indicated that patients with an acute or chronic MA abuse history develop cerebral hemorrhages and infarction in the central nervous system [2-5]. Similarly, some patients using the anorexiant phentermine, an analog of amphetamine, developed ischemic stroke [6]. These data suggest that the use of amphetamine analogs can potentiate ischemic injury in brain.
Bone morphogenetic protein-7 (BMP7) is a trophic factor in the transforming growth factor (TGF)-β superfamily. BMP7 and receptors for BMP (BMPR) are both expressed in the central nervous system [7] and can be upregulated after transient cerebral ischemia [8;9]. Intraperitoneal injection of BMP-7 prior to general hypoxia reduces brain infarction volume and mortality in neonatal rats [10]. Intracerebral administration of BMP-7, given before middle cerebral artery (MCA) ligation, reduced stroke insults in adult rats [11]. Similarly, intracerebral transplantation of fetal kidney tissue, which contains high levels of BMP7 protein, reduced caspase-3 activity and cerebral infarction. The protective effect of fetal kidney transplants was antagonized by the BMP antagonist noggin in stroke rats [12]. Taken together, these data suggest that BMP-7 has protective effects against ischemic injury in the CNS.
The protective effect of BMP7 is not limited to ischemic injury. Acute administration of high doses of MA activates caspase-3 and produces DNA fragmentation and cell death in dopaminergic neurons [13]. These responses were all antagonized by BMP7 [14]. Furthermore, reduced BMP7 expression in BMP7 -/+ mice or defective BMPRII receptors in BMPRIIDN mice increase vulnerability to MA insults [14;15]. These data suggest that BMP7 is protective against MA -mediated neurotoxicity in central dopaminergic neurons. Their interaction in the non-dopaminergic neurons, such as in cerebral cortex, is still not clear.
In this study, we first examined if MA suppresses BMP7 expression in the cerebral cortex. A total of 18 adult male CD1 (Charles River Laboratories) were injected subcutaneously with 4 doses of MA (10 mg/kg × 4, each dose two hours apart, n=9) or saline (0.01 ml/10 g × 4, n=9). During the injection period, animals were housed individually without bedding. At 1 day after MA or saline injection, mice were euthanized and the brains were immediately harvested and chilled on ice. The cerebral cortex was dissected out and total RNA was extracted following instruction from the kit (RNAqueous, Ambion). Total RNA (1 μg) was treated with RQ-1 Rnase-free Dnase I and reverse transcribed into cDNA using random hexamers by AMV reverse transcriptase (Roche). cDNA levels for HPRT, PGK1, and BMP7 were determined by the quantitative real time polymerase chain reaction (qRT-PCR). Primers and FAM-labeled probes used in the quantitative RT-PCR for each gene are as follows:
HPRT: forward primer (5′ -tgatagatccattcctatgactgtaga); reverse primer (5′ -aagacattctttccagttaaagttgag); probe (5′ -tggtggag, mouse universal probe Library #22, Roche). PGK1: forward primer (5′ - tacctgctggctggatgg); reverse primer (5′- cacagcctcggcatatttct); probe (5′-gagagcag, mouse universal probe Library #108, Roche); BMP7: forward primer (5′ - cgagaccttccagatcacagt); reverse primer (5′- cagcaagaagaggtccgact); probe (5′-gctccagg, mouse universal probe Library #1, Roche).
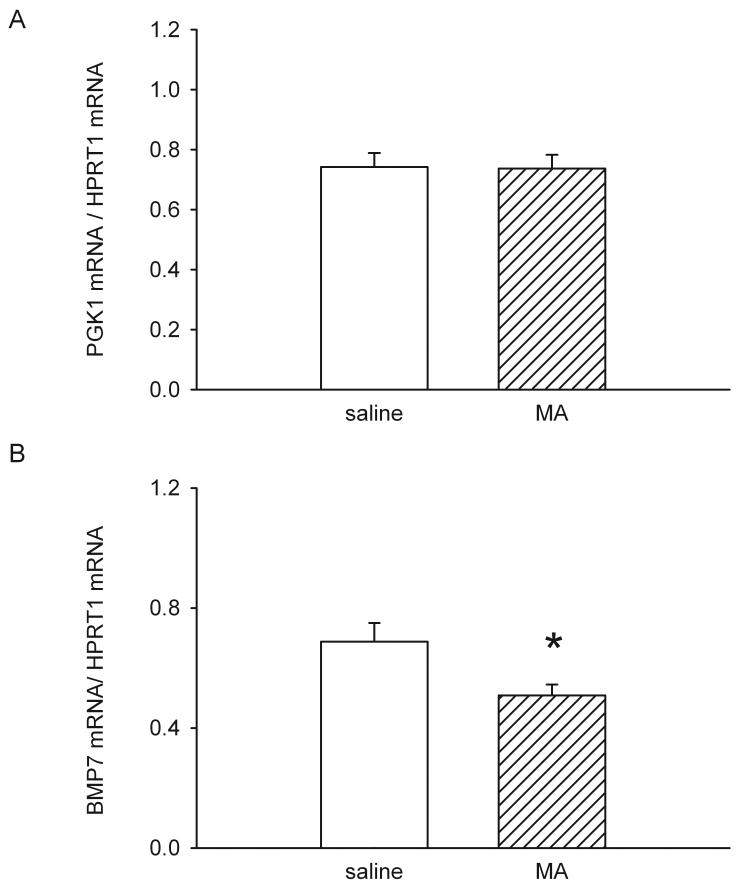
For each sample, gene expression was normalized to the housekeeping gene HPRT1 (hypoxanthine guanine phosphoribosyl transferase 1). We found that the expression level of another housekeeping gene PGK1 (phosphoglycerate kinase 1) was not altered by MA injection (Fig 1A). MA significantly decreased BMP7 mRNA levels, compared to animals receiving saline (p<0.05, t test, Fig 1B). These data suggest that pre-exposure to MA reduced BMP7 mRNA expression in the cerebral cortex.
Fig 1.
MA suppresses BMP7 mRNA levels in mouse cortex. mRNA levels for BMP7, PGK1, and HPRT were measured by qRT-PCR 1 day after injection of either MA or saline in CD1 mice. mRNA levels for the specific gene were normalized to that of housekeeping gene HPRT. The housekeeping gene PGK1 mRNA levels did not differ between saline and MA groups (A). BMP7 mRNA levels were significantly decreased in MA -treated mice, compared with the levels in the saline -treated group (B, * P<0.05, Student’s t-test).
We, and others, have previously reported that BMP7 has a protective effect against stroke. Pretreatment with BMP7 or BMP7 -enriched tissue decreased cerebral infarction and caspase-3 activation in the ischemic cortex [11;16]. Since ischemia activates the expression of BMP7 mRNA [8], the upregulation of BMP7 may be a result of activating endogenous neuroprotective processes during insults. In the present study, we found that MA decreases BMP7 expression in cortex. It is thus possible that there is less neuronal protection induced by endogenous BMP7 after MA treatment, which increases vulnerability to ischemic insults.
Previous studies have indicated that MA, at dose of 10mg/kg × 4, given immediately before middle cerebral artery occlusion (MCAo) enhances cerebral infarction in mice [1]. We next examined if pre-exposure to MA at an earlier time had similar synergistic neurodegenerative effects. A total of 14 adult male CD1 were injected subcutaneously with MA (10 mg/kg × 4, n=7) or saline (0.01 ml/10 g × 4, n=7). At 30 to 40 hours after injection, animals were anesthetized with chloral hydrate (400 mg/kg, i.p.). The right MCA was ligated with a 10-O suture. After 90 minutes of ischemia, the suture on the MCA was removed to allow reperfusion. Core body temperature was monitored with a thermistor probe and maintained at 37 °C with a heating pad during anesthesia. After recovery from the anesthesia, body temperature was maintained at 37 °C using a temperature-controlled incubator. Immediately after the recovery from anesthesia, an elevated body swing test was used to evaluate the success of MCAo surgery. All animals used for this study demonstrated prominent motor bias contralateral to the lesion side.
Two days after the onset of reperfusion, animals were sacrificed. The brain tissue was then removed, immersed in cold saline for 5 minutes, and sliced into 1.0-mm sections. The brain slices were incubated in a 2% tri-phenyltetrazolium chloride (TTC) dissolved in saline for 15 minutes at room temperature, and then transferred to 4 % formaldehyde solution for fixation. The area of infarction in each slice was measured using a digital scanner. The volume of infarction in each animal was obtained from the product of average slice thickness (1 mm) and sum of infarction areas in all brain slices.
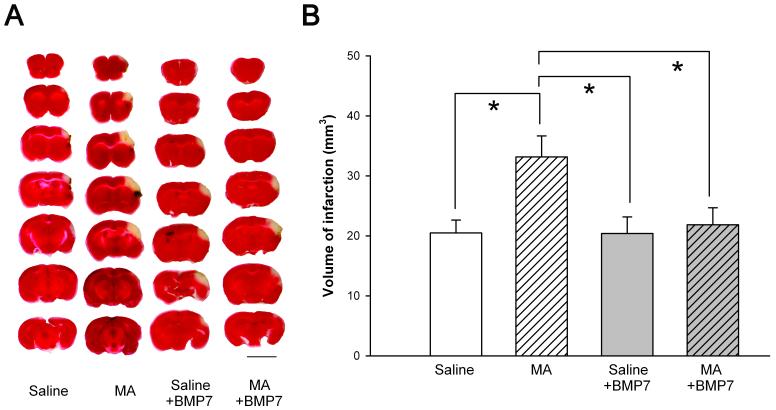
We found that the average infarction volume was 20.5 ± 2.1 mm3 in saline -treated mice. Pre-exposure to MA significantly increased the size of infarction (33.2 ± 3.5 mm3, p<0.05, t test). Typical TTC staining is illustrated in Fig 2A. We previously demonstrated that MA, given immediately before MCAo, extended the area of infarction in mice [1]. In this study, we found that this synergistic effect can occur even in animals pretreated with MA 1 to 2 days earlier. These data suggest that early exposure to MA potentiates ischemic brain injury in mice.
Fig. 2.
Pretreatment with BMP7 reduced MA- facilitated infarction in stroke mice. (A) TTC staining demonstrating that pre-exposure to high doses of MA 2 days before MCAo potentiated cortical infarction in mice. Administration of BMP7 attenuated the infarction. Calibration= 5 mm. (B) MA pretreatment significantly increased infarction induced by MCA ligation. Pretreatment with BMP7 attenuated the infarction. The volume of infarction equals 1 mm [thickness of the slice] × [sum of the infarction area in all brain slices (mm2)]. *p<0.05, 1- way ANOVA + Newman-Keuls test.
Since BMP7 is neuroprotective against MA and ischemia and, furthermore, MA suppressed BMP7 expression in the cerebral cortex, it is possible that exogenous administration of BMP7 can reduce this synergistic effect of MA and ischemia in brain. BMP7 (5 μg/ 5 μl) was injected using a Hamilton syringe into the lateral cerebroventricule in 22 mice. MA (10 mg/kg × 4) or saline was given 15 min later. All animals were subjected to a 90 min MCAo at 30 to 40 hours after injections and were sacrificed for TTC staining 2 days after reperfusion. BMP7 significantly attenuated volume of infarction in animals pre-exposed to high doses of MA (Fig 2A and 2B, p=0.021, F(3,32)= 3.739 1-way ANOVA, p<0.05, post-hoc Newman Keuls test). These data suggest that BMP7 reduced the synergistic neurodegenerative effect of cerebral ischemia and MA.
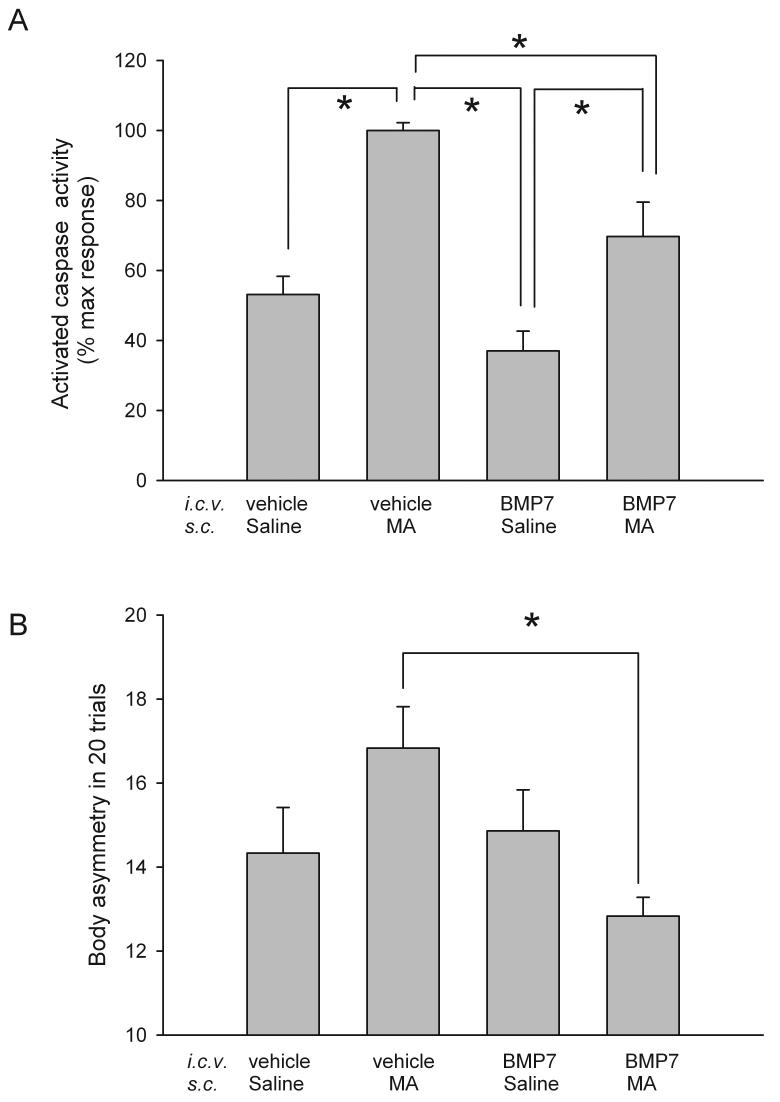
Animals develop body asymmetry after focal ischemia [17]. Another set of mice (n=30) were examined for lateral movements/turning when their bodies were suspended 10 cm above the testing table by lifting their tails. The frequency of initial turning of the head or upper body contralateral to the ischemic side was counted in 20 consecutive trials. Three measurements were conducted on days 4, 5, and 6 after MCAo; data from these three tests were averaged. BMP7 pretreatment significantly reduced body asymmetry in stroke mice pre-exposed to MA (p=0.026, F3,26=3.649; P<0.05, post-hoc Fisher-LSD test; Fig 3B), suggesting that BMP7 pretreatment reduces the behavioral deficits in these animals.
Fig 3.
BMP7 reduces caspase-3/7 activity and body asymmetry in stroke mice pretreated with MA. (A) Cortical caspase 3/7 activity was examined 1 hour afterMCAo or sham surgery. Basal activity was obtained from the sham mice. Data from all stroke mice were normalized by comparison to the mean activity in stroke animals pretreated with MA. Administration of MA significantly increased caspase 3/7 activity in stroke mice. BMP7 significantly reduced caspase-3/7 activation in stroke animals pretreated with MA. (B) BMP7 significantly reduced the body asymmetry after ischemia/MA injury. The frequency of initial turning of the head or upper body contralateral to the ischemic side was counted in 20 consecutive trials. Three measurements were conducted on days 4, 5, and 6 after MCAo; data from these three tests were averaged. In stroke mice pre-exposed to MA, BMP7 pretreatment significantly reduced body asymmetry. *p<0.05, 1- way ANOVA + Newman-Keuls test.
Since BMP7 has been known to suppress neuronal damage through antiapoptotic pathways [12;14], we next examined if MA/stroke -induced caspase activation can be suppressed by pretreatment with BMP7. Mice were treated with BMP7 or vehicle and then injected with MA or saline as previously described. A 90-min MCAo was carried out one day after injection. Previous studies have indicated that caspase enzymatic activity can be activated at one hour after focal ischemia [18]. All mice were sacrificed at 1 hour after MCAo or sham surgery. Cortical brain tissues were collected from the ischemic hemisphere (2 to 5 mm from the rostral end). Brain extracts were prepared by homogenization in extraction buffer (25 mM HEPES, pH 7.5, 0.1% Triton X-100, 5 mM MgCl2, 1 mM EGTA, 1.3 mM EDTA, 2 mM dithiothreitol (DTT) and protease inhibitors (Sigma) and then centrifuged at 15,000 × g. Caspase 3/7 activity was measured in brain tissue using the Caspase-Glo 3/7 assay kit according to the manufacturer’s instructions (Promega, Madison, WI). Caspase Glo 3/7 reagents and cytosolic protein were added to a 96-well plate and incubated at room temperature for 1 h. The luminescence of each sample was measured in a plate-reading luminometer (FLx800, BIO-TEK Instrument Inc.).
Data obtained from the sham mice (n=3) were averaged and used as basal activity. Data from all stroke mice (n=19) were normalized by comparison to the maximal response (i.e. mean activity in stroke animals pretreated with MA) using the following calculation.
Activated caspase 3/7 activity (% max response) = (caspase activity of each animal - mean activity in sham mice) / (mean activity in stroke animals pretreated with MA - mean activity in sham mice) × 100%
Similar to the data on brain infarction, caspase-3/7 activity was significantly reduced by pretreatment with BMP7 in stroke animals receiving MA (Fig 3A, p<0.001, F(3,15)=15.265, One way ANOVA; p<0.05. post-hoc Newman-Keuls test). These data suggest that BMP7 -induced protection against MA/stroke injury may be mediated through anti-apoptotic pathways.
We have previously reported that, in the absence of MA, BMP7 has no protective effect when it was given at one day after MCAo [19]. However, BMP7, given either intracerebroventricularly or systemically after MCAo, promotes behavioral recovery and proliferation of new neuronal precursors 7 days after MCAo [8;19]. It is possible that BMP7 can induce similar neuroregeneration in stroke/MA animals, which requires further investigation.
We previously demonstrated that MA potentiated the expression of p53 mRNA while it decreased GDNF levels in ischemic striatum [1]. In this study, we found that the expression of the neuroprotective ligand BMP7 mRNA is also suppressed by MA and exogenous administration of BMP7 before MA administration reduced the MA -induced increment in cerebral infarction, caspase3/7 activation, and behavioral deficits. These data suggest that MA facilitates cerebral degeneration after ischemia possibly mediated, at least in part, through the suppression of BMP7. The present data further support the clinical observations that patients with an acute or chronic MA abuse history can develop cerebral hemorrhages in striatum and infarction in middle cerebral arterial distribution [5]. In conclusion, our data suggests that the use of MA exacerbates stroke-induced damage in the brain and that BMP7 suppression maybe involved in this response.
Acknowledgment
This work was supported by the National Institute on Drug Abuse, NIH.
Reference
- [1].Wang Y, Hayashi T, Chang CF, Chiang YH, Tsao LI, Su TP, Borlongan CV, Lin SZ. Methamphetamine potentiates ischemia/reperfusion insults after transient middle cerebral artery ligation. Stroke. 2001;32:775–782. doi: 10.1161/01.str.32.3.775. [DOI] [PubMed] [Google Scholar]
- [2].Anderson CA, Sung GY. Central retinal artery occlusion associated with intranasal methamphetamine use. J Stroke Cerebrovasc. Dis. 2003;12:207–208. doi: 10.1016/S1052-3057(03)00073-9. [DOI] [PubMed] [Google Scholar]
- [3].Perez JA, Jr., Arsura EL, Strategos S. Methamphetamine-related stroke: four cases. J Emerg. Med. 1999;17:469–471. doi: 10.1016/s0736-4679(99)00009-8. [DOI] [PubMed] [Google Scholar]
- [4].Yen DJ, Wang SJ, Ju TH, Chen CC, Liao KK, Fuh JL, Hu HH. Stroke associated with methamphetamine inhalation. Eur. Neurol. 1994;34:16–22. doi: 10.1159/000117002. [DOI] [PubMed] [Google Scholar]
- [5].Rothrock JF, Rubenstein R, Lyden PD. Ischemic stroke associated with methamphetamine inhalation. Neurology. 1988;38:589–592. doi: 10.1212/wnl.38.4.589. [DOI] [PubMed] [Google Scholar]
- [6].Kokkinos J, Levine SR. Possible association of ischemic stroke with phentermine. Stroke. 1993;24:310–313. doi: 10.1161/01.str.24.2.310. [DOI] [PubMed] [Google Scholar]
- [7].Soderstrom S, Bengtsson H, Ebendal T. Expression of serine/threonine kinase receptors including the bone morphogenetic factor type II receptor in the developing and adult rat brain. Cell Tissue Res. 1996;286:269–279. doi: 10.1007/s004410050697. [DOI] [PubMed] [Google Scholar]
- [8].Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous admininstration of Bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- [9].Charytoniuk DA, Traiffort E, Pinard E, Issertial O, Seylaz J, Ruat M. Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and up-regulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience. 2000;100:33–43. doi: 10.1016/s0306-4522(00)00246-3. [DOI] [PubMed] [Google Scholar]
- [10].Perides G, Jensen FE, Edgecomb P, Rueger DC, Charness ME. Neuroprotective effect of human osteogenic protein-1 in a rat model of cerebral hypoxia/ischemia. Neurosci. Lett. 1995;187:21–24. doi: 10.1016/0304-3940(95)11327-s. [DOI] [PubMed] [Google Scholar]
- [11].Lin SZ, Hoffer BJ, Kaplan P, Wang Y. Osteogenic protein-1 protects against cerebral infarction induced by MCA-ligation in adult rats. Stroke. 1999;30:126–133. doi: 10.1161/01.str.30.1.126. [DOI] [PubMed] [Google Scholar]
- [12].Chang CF, Morales M, Chou J, Chen HL, Hoffer BJ, Wang Y. Bone morphogenetic proteins are involved in fetal kidney tissue transplantation - induced neuroprotection in stroke rats. Neuropharmacology. 2002;43:418–426. doi: 10.1016/s0028-3908(02)00092-8. [DOI] [PubMed] [Google Scholar]
- [13].Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- [14].Chou J, Lu Y, Kuo CC, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience. 2008;151:92–103. doi: 10.1016/j.neuroscience.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chou J, Harvey BK, Ebendal T, Hoffer BJ, Wang Y. Nigrostriatal alterations in bone morphogenetic protein receptor II dominant negative mice. Acta Neurochirurgica. 2008 doi: 10.1007/978-3-211-78205-7_16. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ. Glial cell line-derived neurotrophic factor protects against ischemia- induced injury in the cerebral cortex. J. Neurosci. 1997;17:4341–4348. doi: 10.1523/JNEUROSCI.17-11-04341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Borlongan CV, Hida H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- [18].Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chou J, Harvey BK, Chang CF, Shen H, Morales M, Wang Y. Neuroregenerative effects of BMP7 after stroke in rats. J. Neurol. Sci. 2006;240:21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]