Abstract
A sequence of 1,647 base pairs in length of S. mutans DNA that encodes for a 63 kDa protein with significant amino acid similarity with fibronectin-binding proteins of S. pyogenes and S. gordonii was cloned. The putative recombinant fibronectin-binding protein of S. mutans was purified using affinity chromatography and the cloned protein was used to prepare polyclonal antibodies against the recombinant protein. In immunoblot assays, antibodies against the S. pyogenes fibronectin-binding protein, FBP54, were cross-reactive with the S. mutans protein that was designated SmFnB. Additionally, antibodies to the S. mutans SmFnB protein reacted with the S. pyogenes FBP54 protein. The S. mutans SmFnB protein was found to bind to immobilized fibronectin in a concentration dependant manner. A mutant strain of S. mutans M51 that was constructed by alleleic exchange did not express the SmFnB protein. This mutant strain, S. mutans ΔSmFnB, was determined in an ELISA to bind to immobilized fibronectin 30% less when compared to the parental strain S. mutans M51. The results are consistent with the conclusion that the 63 kDa SmFnB protein of S. mutans is a fibronectin-binding protein that may contribute to the interaction of S. mutans with damaged heart tissue during pathogenesis of infective endocarditis. Also, the study suggests that multiple molecules may mediate the interaction of S. mutans with fibronectin.
Keywords: Streptococcus mutans, fibronectin, binding, endocarditis
1. Introduction
Infective endocarditis is an infection of the cardiac tissue that occurs when various species of microorganisms attach in the form of a vegetation to cardiac tissue. Viridans streptococci account for over 50% of all infective endocarditis cases. Streptococcus mutans, a member of the mutans group of streptococci and historically a member of the viridans streptococci, is the causative agent of dental caries and is responsible for about 20% of the cases of oral streptococci-associated endocarditis [1]. Oral streptococci can enter the bloodstream after trauma to the oral cavity, such as a dental procedure, and attach to platelet-fibrin matrices formed on injured endothelial tissue. Certain oral streptococci have been shown to induce platelet aggregation, a trait that is believed to be crucial in the pathogenesis of streptococcal-mediated infective endocarditis [2].
The adherence of bacteria to damaged heart tissue is a significant event in the pathogenesis of subacute (chronic) infective endocarditis caused generally by oral streptococci. Adherence is mediated by structures on the bacterial surface and specific structures associated with host cells. Since the oral bacteria that cause chronic infective endocarditis primarily adhere to damaged heart tissue, it is believed that extracellular matrix molecules act as receptors for bacterial attachment. Previous studies have shown that a number of species of bacteria, including S. mutans, adhere to certain extracellular matrix proteins such as, fibronectin, laminin, collagen, and the blood clotting component, fibrinogen [3–9]. Fibronectin is a 440 kDa dimeric glycoprotein that is a common substrate for the attachment of bacteria to the host cell surface. There are two main forms of fibronectin, the secreted insoluble matrix protein and the soluble form present in plasma and extracellular fluids. Streptococci and staphylococci express various fibronectin binding adhesins [7, 10–14]. The binding of pathogenic Streptococcus pyogenes and Staphylococcus aureus to epithelial cells via fibronectin facilitates their entry into cells [15–17]. The structural organization of the best characterized fibronectin-binding proteins of streptococci and staphylococci are similar [18]. Proteins from both bacteria are surface proteins that have a signal peptide sequence for secretion, a LPXTG motif for cell wall anchoring, and a fibronectin-binding domain composed of several amino acid repeat sequences. Also, several species of streptococci express atypical fibronectin-binding proteins that do not contain a secretion signal, anchoring motif, or repeat sequences for binding to fibronectin. These atypical fibronectin-binding proteins include FBP54 of S. pyogenes, PavA of S. pneumoniae, and FbpA of S. gordonii [7, 10, 19]. Chia et al. observed that S. mutans cells could adsorb on their surface soluble fibronectin present in plasma [14]. Furthermore, these investigators demonstrated that S. mutans can bind soluble and immobilized fibronectin and identified a cell wall-associated protein, FBP-130, as a receptor that bound fibronectin. However, the gene that encodes FBP-130 is not known. In this study, we have identified a 63 kDa protein of S. mutans that binds to fibronectin that appears to be different from the fibronectin protein identified by Chia et al. These studies report the first cloning and characterization of a fibronectin-binding protein of S. mutans and support the rationale of exploring the possibility that multiple fibronectin-binding proteins expressed by S. mutans may facilitate adherence of S. mutans cells to cardiac tissue.
2. Results
2.1. Identification of a gene for a putative fibronectin-binding protein of S. mutans
The genome sequence of S. mutans UA159 was searched for a gene that encoded for a fibronectin-binding protein. This search yielded a sequence of 1,647 base pairs that when translated generated a protein of 549 amino acids with a molecular weight of approximately 63 kDa. Sequence alignment showed that the 63 kDa protein of S. mutans had strong amino acid sequence homology with several known fibronectin-binding proteins. Comparison of the S. mutans amino acid sequence to the sequence of the fibronectin/fibrinogen binding protein of S. pyogenes FBP54 showed that 333 of 473 (70%) amino acids are identical and 61 are conserved substitutions indicating an overall similarity of 83% [7]. When the translated sequence of the S. mutans fibronectin-binding protein was compared to the fibronectin-binding protein of S. gordonii (FbpA), the alignment showed that 409 of 549 (74%) amino acids were identical and 70 were conserved substitutions indicating an overall similarity of 87% [10]. Similar levels of identity were found for the fibronectin-binding PavA protein of S. pneumoniae and the putative fibronectin binding protein of the oral streptococcus, Streptococcus sanguinis [19, 20]. Fig. 1 shows sequence comparisons using the Clustal W multiple sequence alignment tool of S. mutans (SmFnB), S. gordonii (FbpA), and S. pyogenes (FBP54) fibronectin-binding proteins.
Fig. 1.
Clustal W sequence alignment of the translated protein sequences of S. mutans (SmFnB), S. gordonii (FbpA), and S. pyogenes (FBP54) fibronectin-binding proteins.
The identified sequence that encoded for the putative fibronectin-binding protein of S. mutans was PCR amplified and cloned into an expression vector for purification of the protein product. Initially, the expressed protein was purified under denaturing conditions using nickel affinity chromatography. The purified rSmFnB protein was used to produce antibodies against the putative S. mutans fibronectin-binding protein. Western blot analysis demonstrated that the anti-SmFnB sera were able to react with purified rSmFnB protein and also with the FBP54 fibronectin-binding protein of S. pyogenes (Fig. 2B). Antibody against the FBP54 protein detected both the purified FBP54 protein and the purified rSmFnB protein (Fig. 2C). These results provide evidence that the SmFnB protein has antigenic determinants in common with a known fibronectin-binding protein.
Fig. 2.
Reactivity of antibodies against the S. mutans and S. pyogenes fibronectin-binding proteins with purified SmFnB protein of S. mutans and FBP54 protein of S. pyogenes. (A) SDS-PAGE of purified SmFnB and FBP54 proteins. (B) Immunoblot probed with anti-SmFnB sera. The reactive band of approximately 25 kDa in lane 3 is probably a truncated form of the recombinant SmFnB protein generated during expression of the protein in E. coli. (C) Immunoblot probed with anti-FBP54 sera. Lanes: (1), Molecular weight markers; (2), FBP54; (3) SmFnB.
2.2. Binding of the SmFnB protein to immobilized fibronectin
We wanted to demonstrate that SmFnB protein had the ability to bind to fibronectin. For these studies, the sequence of smFnB gene was cloned into an expression vector that allowed for the expression of the SmFnB protein fused to maltose-binding protein and purification of the protein in a native conformation using amylose affinity chromatography. The purified rSmFnB-maltose-binding protein fusion protein was used in an ELISA binding assay to determine if the protein interacts with immobilized fibronectin. The assay demonstrated that the binding of the SmFnB fusion protein to immobilized fibronectin occurs in a dose-dependant manner (Fig. 3). In contrast to the SmFnB fusion protein, the interaction of maltose-binding protein with immobilized fibronectin was significantly less. This result reinforces the conclusion that the smFnB gene of S. mutans encodes for a fibronectin-binding protein.
Fig. 3.
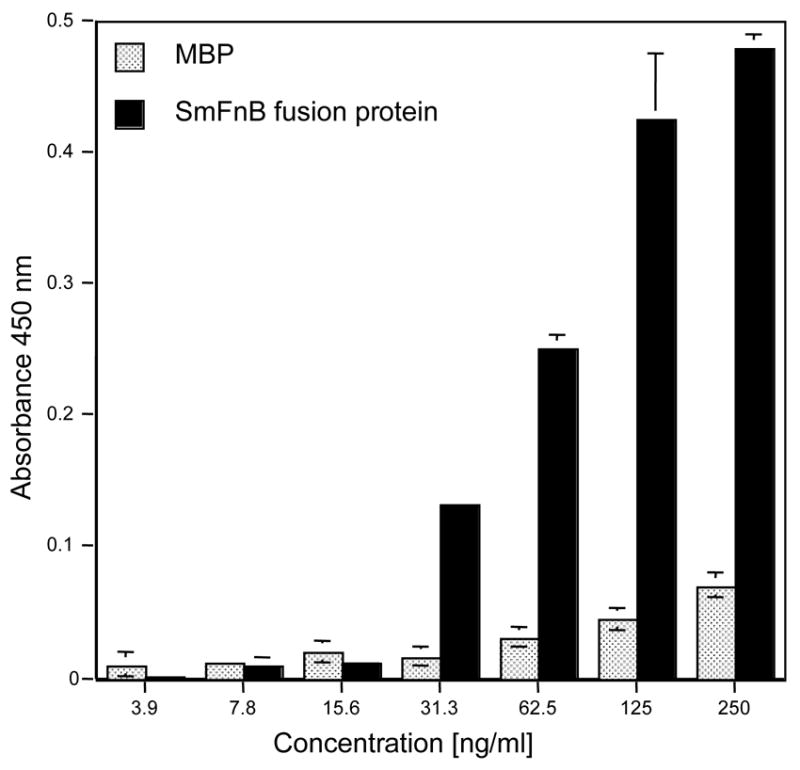
Binding of recombinant SmFnB fusion protein to immobilized fibronectin. The wells of microtiter plates were coated with fibronectin and reacted with various concentrations of SmFnB fusion protein and maltose binding protein (control). Absorbance values represent means of at least triplicate samples and are representative of three experiments.
2.3. Binding of smfnb mutant strain to immobilized fibronectin
To determine if expression of the SmFnB protein contributes to the binding of S. mutans cells to fibronectin, an isogenic mutant of S. mutans M51 that does not express the fibronectin-binding protein was constructed by allelic exchange. The mutated strain was constructed by the removal of approximately 1,100 base pairs from the central region of the gene and insertion of a DNA fragment containing a kanamycin resistance determinant within the gene. Once transformed into S. mutans cells, the mutated gene replaced the wild-type sequence on the chromosome by a double crossover recombinational event. To verify that the recombinational event had occurred in the mutated S. mutans M51 strain, chromosomal DNAs from the wild-type and mutated strains were isolated and used as a template in PCR amplification and Southern blot analysis (Fig 4A). The results demonstrated that the kanamycin resistance cassette was inserted into the fibronectin-binding gene of the mutated S. mutans strain. SDS-PAGE and Western blot analysis with the total protein lysates of the wild-type M51 and ΔSmFnB strains was used to determine if the modification in the genome interrupted the expression of the 63 kDa SmFnB protein. The results showed that in the total protein lysate of the mutated strain that there was not a protein that reacted with anti-SmFnB antibodies (Fig. 4B). The ELISA technique was used to determine if the abrogation of protein expression in the mutant strain results in a decrease in binding to fibronectin. The results from the binding assay showed that binding to immobilized fibronectin by the mutant strain was reduced by about 30 % when compared to the wild-type strain (Fig. 4C).
Fig. 4.
Properties of strain with the mutated smFnB gene. Verification of the allelic exchange event by PCR amplification and Southern blot analysis (Panel A). Procedures were performed as described in Materials and Methods. Lane 1, 10 kb DNA ladder; Lane 2, wild-type S. mutans chromosomal DNA used as template in PCR reaction; Lane 3, mutated S. mutans chromosomal DNA used as template in PCR reaction (A) Southern blot of PCR reactions probed with digoxigenin-labeled 1.65 kb fibronectin-binding gene of S. mutans. (B) Southern blot of PCR reactions probed with digoxigenin-labeled 1.4 kb fragment containing kanamycin resistance gene. SDS-polyacrylamide gel electrophoresis and western blot analysis of S. mutans wild-type and ΔSmFnB total protein lysates (Panel B). (A) Total protein lysates separated electrophorectically on an 8% SDS-polyacrylamide gel. (B) Immunoblot of gel probed with affinity purified anti-SmFnB sera. Lanes: (M) Molecular weight marker; (1) lysate from wild-type cells; (2) lysate from ΔSmFnB cells; (3) purified his-tagged SmFnB protein. Binding of S. mutans M51 wild-type and the isogenic mutant ΔSmFnB to immobilized fibronectin (Panel C). Values represent means of at least triplicate samples and are representative of three experiments.
3. Discussion
The overall goal of this work was to use a genetic approach to identify and study genes of the oral streptococcus, S. mutans, that could play a role in the pathogenesis of infective endocarditis caused by this microorganism. Previous studies have shown that various Gram-positive bacteria express multiple receptors that bind to fibronectin and have a critical role in the infectious process [21]. However, information highlighting what role fibronectin binding by S. mutans plays in the pathogenesis of infective endocarditis is sparse. Therefore, identification of genes that encode for proteins of S. mutans that bind extracellular matrix molecule and for proteins that induce platelet aggregation and binding to platelets will assist in understanding the mechanism by which this traditionally non-life threatening microorganism colonizes damaged endocardial surfaces.
Amino acid alignment comparisons demonstrated that the amino acid sequence of the putative fibronectin-binding protein of S. mutans shares significant sequence homology with the fibronectin-binding proteins of several streptococcal species. Analysis of the amino acid sequence of the S. mutans fibronectin-binding protein described in this study indicates that the S. mutans protein is a member of the atypical fibronectin-binding proteins of bacteria.
Antibodies against the FBP54 protein of S. pyogenes were able to detect the rSmFnB protein of S. mutans and SmFnB antibodies were cross-reactive with the FBP54 protein. This finding suggests that the two proteins are immunologically similar. Previous studies have shown that the FBP54 protein protects rats from developing infection in an experimental model of infective endocarditis [22]. Future studies could be directed toward identifying common epitopes of the proteins that could be used in the development of a vaccine protective against multiple infectious organisms.
The results of this study demonstrate that the 63 kDa SmFnB protein expressed by S. mutans cells binds to fibronectin and this interaction could facilitate bacterial adherence to host tissue. Comparative analysis between the wild type and the mutated SmFnB strain showed that binding of the mutant strain to fibronectin was not totally ablated. This finding is similar to that observed for a S. gordonii mutant strain that had a mutation in the fibronectin-binding protein, FbpA [10]. The most obvious explanation for this result is that S. mutans cells express multiple fibronectin-binding proteins. In support of this, Chia et al. showed that fibronectin bound to 130 and 55 kDa proteins expressed by S. mutans. The investigators observed that antibodies for the FBP-130 protein were not reactive against the 55 kDa protein demonstrating that another protein also binds to fibronectin. Our search did not identify a protein of 130 kDa with characteristics of a known fibronectin-binding protein. The 63 kDa protein fibronectin-binding protein identified in this study may be the same protein identified earlier by Chia et al. Therefore, further studies are required to verify that the fibronectin-binding protein identified in this study is or is not the 55 kDa protein previously described. A study by Sciotti et al. reported that the N-terminal region of antigen I/II bound to various extracellular matrix proteins such as, fibronectin, laminin and collagen [23]. Our laboratory has compared the ability of parental strain, S. mutans NG8, and its isogenic antigen I/II-deficient mutant strain, 834, to bind fibronectin [9]. We find that the mutant NG8 strain demonstrated a decreased adherence to immobilized fibronectin. Collectively, these studies suggest that S. mutans has multiple fibronectin-binding proteins.
In summary, S. mutans cells express a 63 kDa protein that shares amino acid sequence homology with atypical fibronectin-binding proteins of several streptococcal species. A mutant strain of S. mutans that possessed a mutation in the gene for a fibronectin-binding possessed a reduced ability to bind fibronectin that suggests that the 63 kDa fibronectin-binding protein contributes to the ability of S. mutans cells to bind fibronectin and possibly adherence to host cells. It remains to be determined whether the SmFnB protein is involved in the pathogenesis of infective endocarditis.
4. Materials and methods
4.1. Bacterial strains and media
S. mutans M51 has a spontaneously generated streptomycin-resistance phenotype and was obtained from the Centers for Disease Control and Prevention and was isolated from the peripheral blood of a patient clinically diagnosed with infective endocarditis [9]. The isogenic mutant of S. mutans M51, ΔSmFnB, was constructed by allelic exchange and has a deficiency in the expression of the SmFnB protein. S. mutans M51 and the isogenic ΔSmFnB strains were anaerobically grown at 37°C in Todd-Hewitt broth in the presence of 500 μg/ml of streptomycin or 500 μg/ml each of streptomycin and kanamycin, respectively. E. coli TG1 cells that were used to maintain the recombinant pQE31 constructs were grown in LB media containing kanamycin and ampicillin at a concentration of 25 μg/ml and 100 μg/ml, respectively. E. coli M15 (pREP4) cells that were used to express the SmFnB protein cloned into the his-tagged expression vector pQE31 and the E. coli BL21Star (DE3) strain that contains the recombinant pMAL-c2x vector with the inserted smfnb gene were grown in LB media containing 100 μg/ml ampicillin.
4.2. Isolation of plasmid DNA and S. mutans chromosomal DNA
Chromosomal DNA of S. mutans strains was isolated using the method previously described [24]. Plasmid DNA was isolated according to the Qiagen plasmid DNA protocol. The PCR products were isolated and purified according to the protocol from the Wizard PCR preps purification kit (Promega).
4.3. Cloning of the gene for a fibronectin-binding protein of S. mutans
The DNA sequence for a putative fibronectin-binding protein of S. mutans was obtained from a BLAST search of the S. mutans genome sequence ([25]; GenBank accession no. AE014133). To clone the DNA sequence that encoded the putative fibronectin-binding protein, the sequence was amplified with Taq DNA polymerase by PCR using the primer SM 10 [5′-ATGTCTTTTGATGGCTTTTTTTTACAT-35′ (forward primer; bases 1–27 of the sequence)] and SM 11 [55′-TTACATCTTCATCGCTTTTATCTTAGC-35′ (backward primer, bases 1624–1650 of the sequence and includes termination codon)] and S. mutans M51 chromosomal DNA as template.
The 1,650 base pair product was then cloned into the T/A cloning vector pCR T7/NT TopoTA and transformed into E. coli One Shot TOP10 cells and transformants were selected on LB agar plates containing 50 μg/ml ampicillin. The DNA of transformants was isolated and digested with BamHI and HindIII restriction enzymes to release inserted DNA. The insert DNA was gel purified and then cloned into BamHI and HindIII digested expression vector pQE31. For stable expression of the histidine tagged SmFnB protein, plasmid pQE31(SmFnB) was transformed into E. coli M15 (pREP4) cells. The S. mutans fibronectin-binding protein that we have designated SmFnB is assigned the name SMU.1449 in the published S. mutans genomic sequence [25].
Cloning of the smFnB gene into the maltose binding protein fusion vector, pMAL-c2x, was accomplished by amplifying the gene with Pfx DNA polymerase using the primer pair SM 10 and SM 11 and S. mutans genomic DNA as template. The amplified PCR product was purified and then ligated into XmnI linearized pMAL-c2x vector (New England Biolabs). Plasmid DNA was isolated from transformants and the presence of insert DNA was verified by restriction enzyme digestion and DNA sequence analysis.
4.4.Expression and purification of S. mutans fibronectin-binding protein
Recombinant S. mutans fibronectin-binding protein (rSmFnB) was purified from 1 liter cultures induced and processed using denaturing conditions as described previously using the Qiagen protocol [26]. To determine the purity of eluted protein, samples collected from the metal affinity column were separated on a 10% SDS-PAGE gel and transferred onto a nitrocellulose membrane and probed with mouse anti-His antibodies. Detection of reactive protein bands was achieved by incubation of membranes with alkaline phosphatase conjugated goat anti-mouse IgG followed by a substrate combination of nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate in dimethylformamide.
For purification of the maltose binding protein (MBP)-SmFnB fusion protein, one liter cultures were induced with IPTG and the cell pellet was suspended in column buffer (20 mM Tris-HCl, pH 8.0, 200 mM NaCl and 1 mM EDTA) and lysed by sonication. The cell extract containing the fusion protein was loaded onto an amylose resin column, washed with column buffer, and the fusion protein was eluted with buffer containing 10 mM maltose. The fractions containing purified fusion protein were detected by SDS-PAGE and Western blot analysis using anti-MBP serum (New England Biolabs).
Total cell lysates of S. mutans M51 wild-type and the ΔSmFnB strains were prepared from 20 ml cultures. Cells in suspension were lysed using a Mini-Beadbeater 3110BX and 0.4 mg of 0.1 mm zirconia/silica beads per ml of cell suspension. After cell lysis, the beads were allowed to settle and the supernatant fluid (total cell extract) was removed.
4.5. Preparation of antiserum to the purified SmFnB protein
Antibodies against the purified recombinant SmFnB protein were raised in New Zealand white rabbits by subcutaneous injections of 80 μg of protein emulsified in Freund’s incomplete adjuvant containing muramyl dipeptide. Rabbits were boosted 3 weeks after the initial injection with 40 μg/ml of the rSmFnB protein in Freund’s incomplete adjuvant. After an additional two weeks, the animals were bled and the immunoglobulin fraction of the serum was isolated. Antibodies used to obtain the results in Fig. 4B were further purified by affinity chromatography on a column containing purified rSmFnB coupled to Sepharose 4B beads.
4.6. Detection of rSmFnB protein with antibodies for the S. pyogenes fibronectin-binding protein, FBP54
Purified S. pyogenes FBP54 protein and anti-sera against this protein were obtained from Harry Courtney [7]. Purified rSmFnB and S. pyogenes FBP54 proteins were separated on a 10% SDS-PAGE and transferred to nitrocellulose membranes. Detection of the S. mutans and S. pyogenes fibronectin-binding proteins fixed on the nitrocellulose membrane was preformed by separately incubating each blot for one hour with rabbit anti-FBP54 and anti-SmFnB sera and processing the blots as described above using alkaline phosphatase conjugated goat anti-rabbit IgG.
4.7. Construction by allelic exchange of S. mutans ΔSmFnB strain
To construct a fibronectin-binding deficient strain of S. mutans M51, 1054 base pairs of the smFnB gene was removed by restriction enzyme digestion with HincII. A 1,394 base pair kanamycin resistance gene residing on the plasmid pVA2592 was amplified by PCR using the primer pair 55′-GATAAACCCAGCGAACCATTTG-35′ (forward primer) and 55′-GGATCCCGAGCTTTTTAGAC-35′ (backward primer) [27]. The amplified kanamycin resistance gene was inserted into the deleted sequence of the smfnb gene in the recombinant pQE31 construct. The modified sequence was then PCR amplified with the primer pair SM 10 and SM 11 and S. mutans M51 cells were transformed with the PCR product as described by Perry and Kuramitsu and kanamycin resistant transformants were selected [28]. The presence of the kanamycin resistance determinant in the fibronectin-binding gene of S. mutans M51 was verified by PCR amplification and Southern blot analysis.
4.8. PCR and Southern blot analysis verifying the insertion of the kanamycin resistance cassette into the mutated smFnB gene of S. mutans that encodes for a fibronectin-binding protein
The insertion of the kanamycin resistant cassette into the deleted sequence of S. mutans that encodes for the fibronectin-binding protein was verified by PCR using primers SM 10 and SM 11. The PCR amplified products were ran on a 0.8% agarose gel and transferred to a nylon membrane by capillary transfer. The blots were probed with a digoxigenin-labeled 1.65 kb DNA fragment containing the smFnB gene of S. mutans M51 and a 1.4 kb fragment containing the kanamycin resistance cassette according to the manufacturers protocol (Roche Molecular Biochemicals).
4.9. Fibronectin-binding assays
To evaluate the ability of the recombinant SmFnB fusion protein to bind fibronectin, an ELISA was utilized. Human plasma fibronectin was immobilized onto microtiter plate wells by adding 100 μl of the protein solution (10 μg/ml in 0.05 M carbonate buffer, pH.9.6) to each well and incubating for 16 hours at room temperature. After blocking for one hour at 25°C with 10 mM PBS containing 0.05% BSA and 0.025% Tween 20, various concentrations of the SmFnB-MBP fusion protein were added and the plates were incubated for 1 h at room temperature. Purified maltose-binding protein was used as a control and was added to the plate in a similar manner as the SmFnB fusion protein. After washing, the plates were incubated for one hour at room temperature with antibodies against maltose-binding protein. The plates were washed and 100 μl of goat anti-rabbit IgG conjugated with horseradish peroxidase was added to each well. The plates were developed with the chromogenic substrate tetramethylbenzidine and the absorbance read at 450 nm. The ELISA technique previously described was used to measure the comparative ability of wild-type S. mutans M51 and the isogenic strain with the mutated gene for the fibronectin-binding protein to bind to fibronectin [9].
Acknowledgments
This work was supported by grant GM008037 from the National Institutes of Health. T. M. T. was supported by grant T32-HL07737 from the National Institutes of Health and a GAAN Foundation Fellowship (P200A010123). We thank Latha Raju for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 2.Herzberg MC. Platelet-streptococcal interactions in endocarditis. Crit Rev Oral Biol Med. 1996;7:222–36. doi: 10.1177/10454411960070030201. [DOI] [PubMed] [Google Scholar]
- 3.Speziale P, Hook M, Wadstrom T, Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982;146:55–8. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- 4.Vercellotti GM, McCarthy JB, Lindholm P, Peterson PK, Jacob HS, Furcht LT. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985;120:13–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Kuypers JM, Proctor RA. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–12. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talay SR, Valentin-Weigand P, Jerlstrom PG, Timmis KN, Chhatwal GS. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–44. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney HS, Li Y, Dale JB, Hasty DL. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–46. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–8. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 9.Beg AM, Jones MN, Miller-Torbert T, Holt RG. Binding of Streptococcus mutans to extracellular matrix molecules and fibrinogen. Biochem Biophys Res Commun. 2002;298:75–9. doi: 10.1016/s0006-291x(02)02390-2. [DOI] [PubMed] [Google Scholar]
- 10.Christie J, McNab R, Jenkinson HF. Expression of fibronectin-binding protein FbpA modulates adhesion in Streptococcus gordonii. Microbiology. 2002;148:1615–25. doi: 10.1099/00221287-148-6-1615. [DOI] [PubMed] [Google Scholar]
- 11.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992;89:6172–6. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J Bacteriol. 2003;185:1208–17. doi: 10.1128/JB.185.4.1208-1217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Signas C, Raucci G, Jonsson K, Lindgren PE, Anantharamaiah GM, Hook M, et al. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia JS, Yeh CY, Chen JY. Identification of a fibronectin binding protein from Streptococcus mutans. Infect Immun. 2000;68:1864–70. doi: 10.1128/iai.68.4.1864-1870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler T, Wann ER, Joh D, Johansson S, Foster TJ, Hook M. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur J Cell Biol. 2000;79:672–9. doi: 10.1078/0171-9335-00104. [DOI] [PubMed] [Google Scholar]
- 16.Molinari G, Talay SR, Valentin-Weigand P, Rohde M, Chhatwal GS. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–63. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol Microbiol. 2001;42:75–86. doi: 10.1046/j.1365-2958.2001.02579.x. [DOI] [PubMed] [Google Scholar]
- 18.Joh D, Wann ER, Kreikemeyer B, Speziale P, Hook M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–23. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 19.Holmes AR, McNab R, Millsap KW, Rohde M, Hammerschmidt S, Mawdsley JL, et al. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol Microbiol. 2001;41:1395–408. doi: 10.1046/j.1365-2958.2001.02610.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu P, Alves JM, Kitten T, Brown A, Chen Z, Ozaki LS, et al. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol. 2007;189:3166–75. doi: 10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz-Linek U, Hook M, Potts JR. The molecular basis of fibronectin-mediated bacterial adherence to host cells. Mol Microbiol. 2004;52:631–41. doi: 10.1111/j.1365-2958.2004.04027.x. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata S, Kunitomo E, Terao Y, Nakagawa I, Kikuchi K, Totsuka K, et al. Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against Streptococcus pyogenes challenge in mice. Infect Immun. 2001;69:924–30. doi: 10.1128/IAI.69.2.924-930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sciotti MA, Yamodo I, Klein JP, Ogier JA. The N-terminal half part of the oral streptococcal antigen I/IIf contains two distinct binding domains. FEMS Microbiol Lett. 1997;153:439–45. doi: 10.1111/j.1574-6968.1997.tb12608.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogundipe JO, Holt RG. Molecular and immunochemical characterization of recombinant Escherichia coli containing the spaA gene region of Streptococcus sobrinus. Infect Immun. 1989;57:1906–15. doi: 10.1128/iai.57.7.1906-1915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–9. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones MN, Holt RG. Cloning and characterization of an alpha-enolase of the oral pathogen Streptococcus mutans that binds human plasminogen. Biochem Biophys Res Commun. 2007;364:924–9. doi: 10.1016/j.bbrc.2007.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol. 2003;185:5967–75. doi: 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry D, Kuramitsu HK. Genetic transformation of Streptococcus mutans. Infect Immun. 1981;32:1295–7. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]





