Abstract
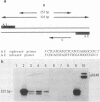
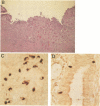
To test the hypothesis that herpes simplex virus type 1 (HSV-1) may be associated with peptic ulcer disease, we examined ulcerative lesions of the distal stomach and proximal duodenum for the presence of nucleic acids and antibodies specific for HSV-1. Utilizing in situ hybridization, immunocytochemistry, and polymerase chain reaction with sequencing, gastric or duodenal tissues from 4 of 22 patients (18%) with documented peptic ulcer disease demonstrated the presence of both specific HSV-1 nucleic acid sequences and proteins. HSV-1 was found restricted in clusters of cells near the margin of the ulcer but was absent at sites distal to the lesion. Several of such HSV-1-infected cells also contained cholecystokinin. These cholecystokinin-containing cells are of neuroendocrine origin and receive contact from the vagal nerve. Campylobacter pylori bacteria were not found in three of the four peptic ulcer tissues that harbored HSV-1. Further, none of the stomach or duodenal tissue samples from 33 patients undergoing clinical evaluation, but having no evidence of peptic ulcer disease, had HSV-1 materials. Thus, our data suggest that a subset of peptic ulcer disease may be associated with HSV-1 and raise the possibility that some peptic ulcers may be caused by this virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Crocket A., Doyle D., Joffe S. N. Electron microscopy of gastric mucosal innervation in rats. Experientia. 1981 Mar 15;37(3):270–271. doi: 10.1007/BF01991649. [DOI] [PubMed] [Google Scholar]
- Dargan D. J., Subak-Sharpe J. H. The effect of triterpenoid compounds on uninfected and herpes simplex virus-infected cells in culture. I. Effect on cell growth, virus particles and virus replication. J Gen Virol. 1985 Aug;66(Pt 8):1771–1784. doi: 10.1099/0022-1317-66-8-1771. [DOI] [PubMed] [Google Scholar]
- Dubeau L., Chandler L. A., Gralow J. R., Nichols P. W., Jones P. A. Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res. 1986 Jun;46(6):2964–2969. [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- OI M., OSHIDA K., SUGIMURA S. The location of gastric ulcer. Gastroenterology. 1959 Jan;36(1):45–56. [PubMed] [Google Scholar]
- Price R. W. Viral infections of the autonomic nervous system and its target organs: pathogenetic mechanisms. Med Hypotheses. 1977 Jan-Feb;3(1):33–36. doi: 10.1016/0306-9877(77)90049-4. [DOI] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Nesburn A. B., Cook M. L. Latent herpes simplex virus from trigeminal ganglia of rabbits with recurrent eye infection. Nat New Biol. 1972 Feb 16;235(59):216–217. doi: 10.1038/newbio235216a0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Ugolini G., Kuypers H. G., Strick P. L. Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science. 1989 Jan 6;243(4887):89–91. doi: 10.1126/science.2536188. [DOI] [PubMed] [Google Scholar]
- Waldum H. L., Bjorvatn B., Burhol P. G. Gastritis, peptic ulcer disease, inflammatory bowel disease, and stomach and colon cancers- are they all caused by viral infections? Med Hypotheses. 1981 Nov;7(11):1329–1338. doi: 10.1016/0306-9877(81)90123-7. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Denaro F. J., Nelson J. A., Lampert P. W., Oldstone M. B. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J Neuropathol Exp Neurol. 1986 Mar;45(2):127–139. doi: 10.1097/00005072-198603000-00003. [DOI] [PubMed] [Google Scholar]
- van der Spuy S., Levy D. W., Levin W. Cimetidine in the treatment of herpesvirus infections. S Afr Med J. 1980 Jul 19;58(3):112–116. [PubMed] [Google Scholar]