Abstract
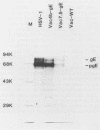
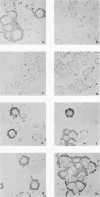
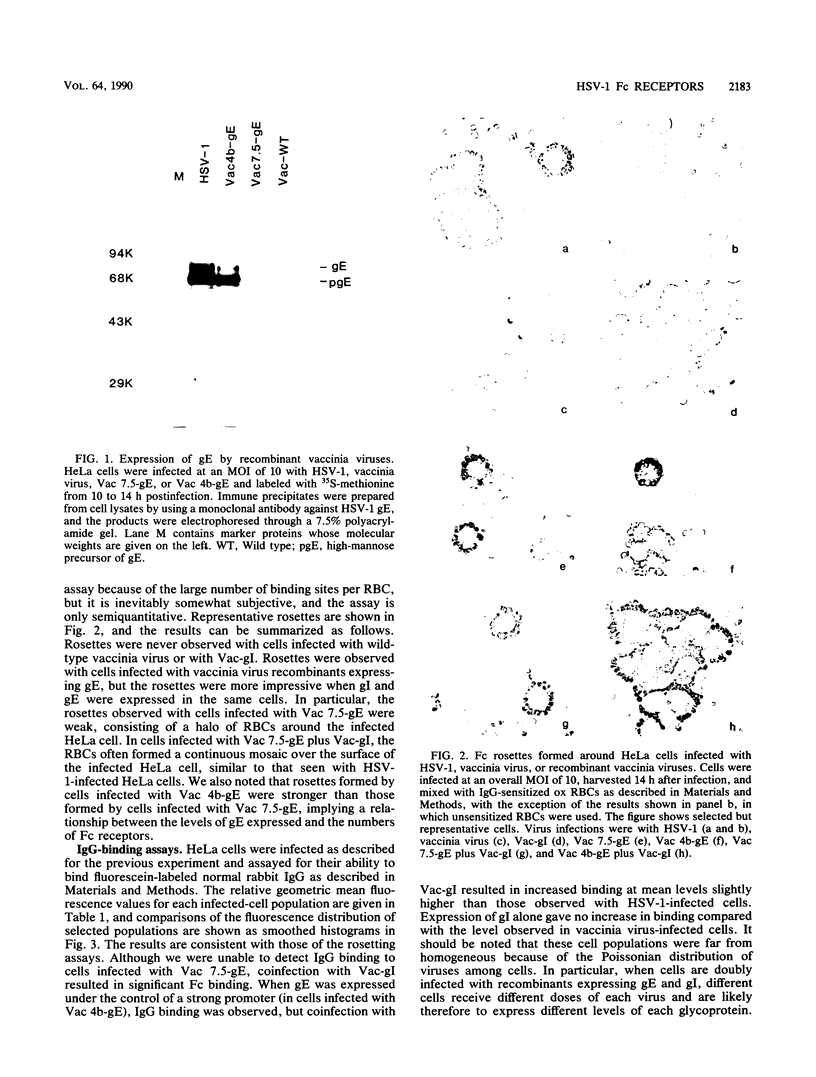
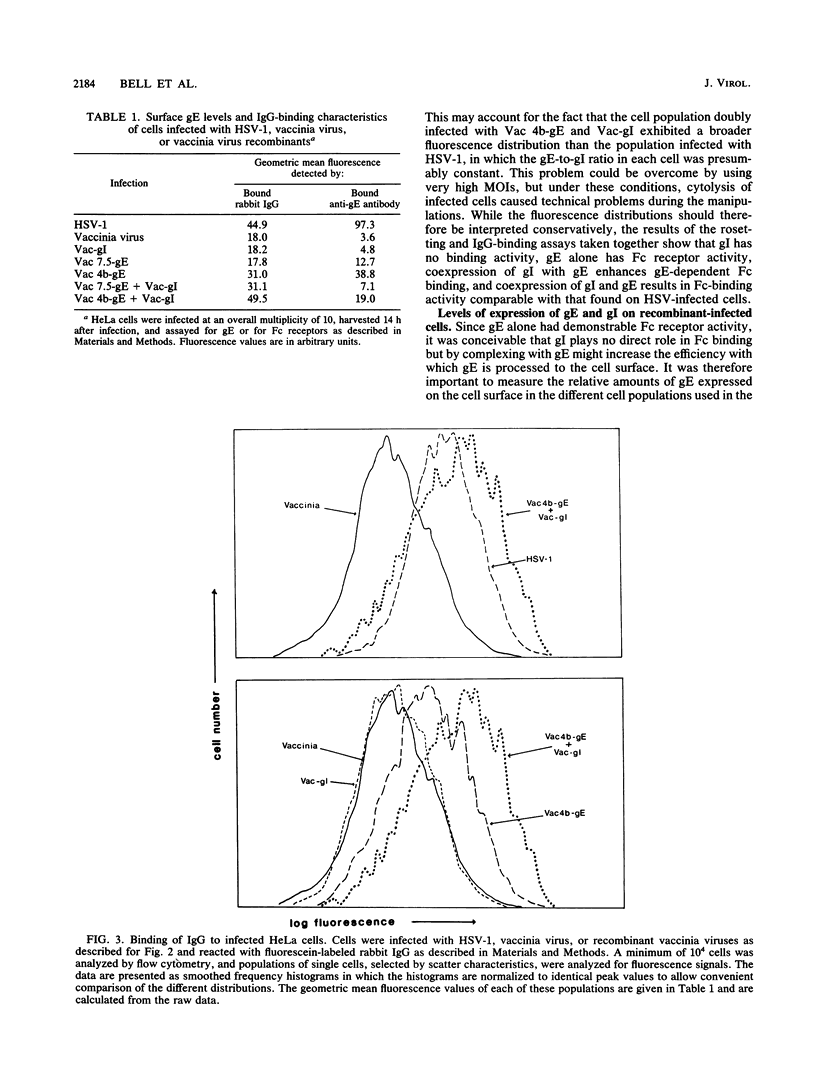
Glycoprotein E (gE) of herpes simplex virus type 1 (HSV-1) will bind immunoglobulin G (IgG) (Fc) affinity columns (R. B. Bauke and P. G. Spear, J. Virol. 32:779-789, 1979), but recent evidence suggests that the HSV-1 Fc receptor is composed of a complex of gE and glycoprotein I (gI) and that both gI and gE are required for Fc receptor activity (D. C. Johnson and V. Feenstra, J. Virol. 61:2208-2216, 1987; D. C. Johnson, M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow, J. Virol. 62:1347-1354, 1988). We have expressed gE and gI, either alone or in combination, on the surface of HeLa cells by using recombinant vaccinia viruses and have measured Fc receptor activity by Fc-rosetting or IgG-binding assays. Expression of gE alone resulted in the induction of Fc receptor activity, while expression of gI alone gave no detectable Fc binding. Coexpression of gE and gI resulted in higher levels of IgG binding than did expression of gE alone, despite the fact that under conditions of coexpression, the levels of surface gE were reduced. We propose that gE and gI together form a receptor of higher affinity than gE alone and that HSV-1 therefore has the potential to induce two Fc receptors of different affinities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Glorioso J. C., Cossman J., Levine M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978 Aug;21(2):442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. M., Hope R. G., Marsden H. S. Generation and properties of the glycoprotein E-related 32K/34K/35K and 55K/57K polypeptides encoded by herpes simplex virus type 1. J Gen Virol. 1987 Aug;68(Pt 8):2093–2104. doi: 10.1099/0022-1317-68-8-2093. [DOI] [PubMed] [Google Scholar]
- Frank I., Friedman H. M. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989 Nov;63(11):4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987 Jul;61(7):2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet J. P. Antibody-cell interactions: Fc receptors. Cell. 1989 May 5;57(3):351–354. doi: 10.1016/0092-8674(89)90910-0. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McTaggart S. P., Burns W. H., White D. O., Jackson D. C. Fc receptors induced by herpes simplex virus. I. Biologic and biochemical properties. J Immunol. 1978 Aug;121(2):726–730. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Letter: Protein cleavage and poxvirus morphogenesis: tryptic peptide analysis of core precursors accumulated by blocking assembly with rifampicin. J Mol Biol. 1973 Dec 5;81(2):267–269. doi: 10.1016/0022-2836(73)90195-2. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Goldstein L., Spear P. G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982 Jan;41(1):137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel J., Moss B. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J Virol. 1985 Dec;56(3):830–838. doi: 10.1128/jvi.56.3.830-838.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Smith G. L. The herpes simplex virus type 1 US7 gene product is a 66K glycoprotein and is a target for complement-dependent virus neutralization. J Gen Virol. 1988 Apr;69(Pt 4):859–867. doi: 10.1099/0022-1317-69-4-859. [DOI] [PubMed] [Google Scholar]
- WATKINS J. F. ADSORPTION OF SENSITIZED SHEEP ERYTHROCYTES TO HELA CELLS INFECTED WITH HERPES SIMPLEX VIRUS. Nature. 1964 Jun 27;202:1364–1365. doi: 10.1038/2021364a0. [DOI] [PubMed] [Google Scholar]
- Westmoreland D., Watkins J. F. The IgG receptor induced by herpes simplex virus: studies using radioiodinated IgG. J Gen Virol. 1974 Jul;24(1):167–178. doi: 10.1099/0022-1317-24-1-167. [DOI] [PubMed] [Google Scholar]