Abstract
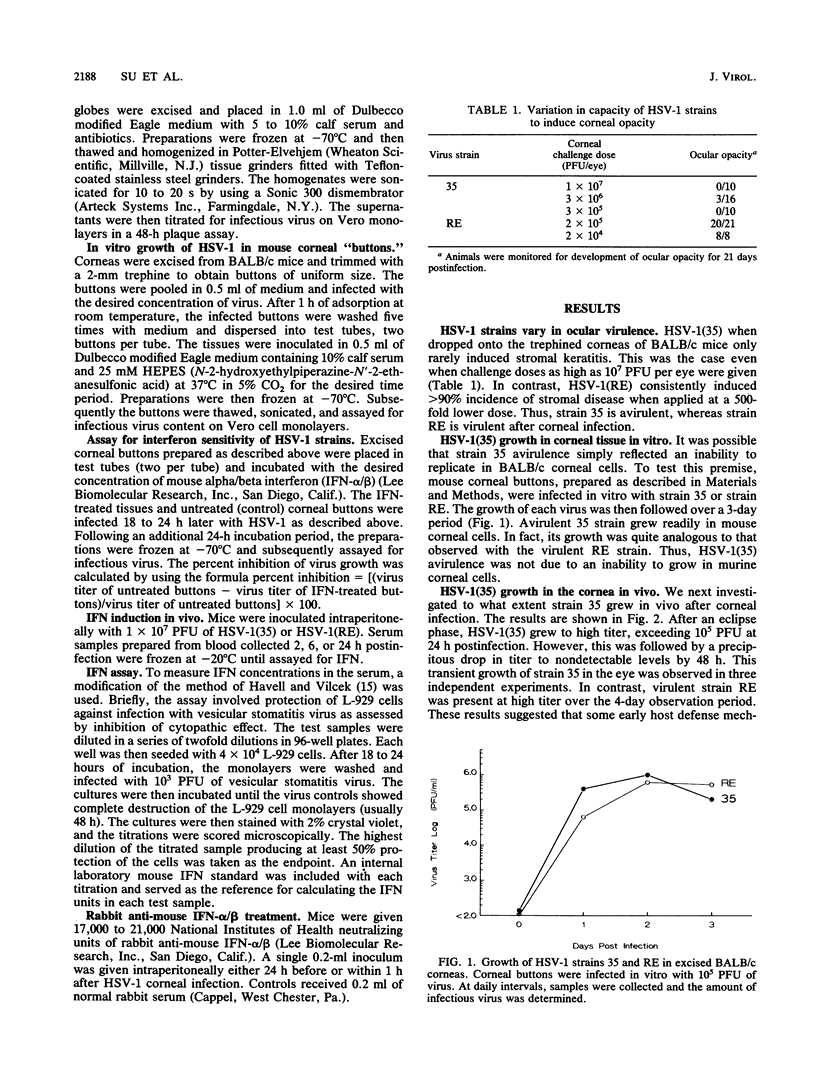
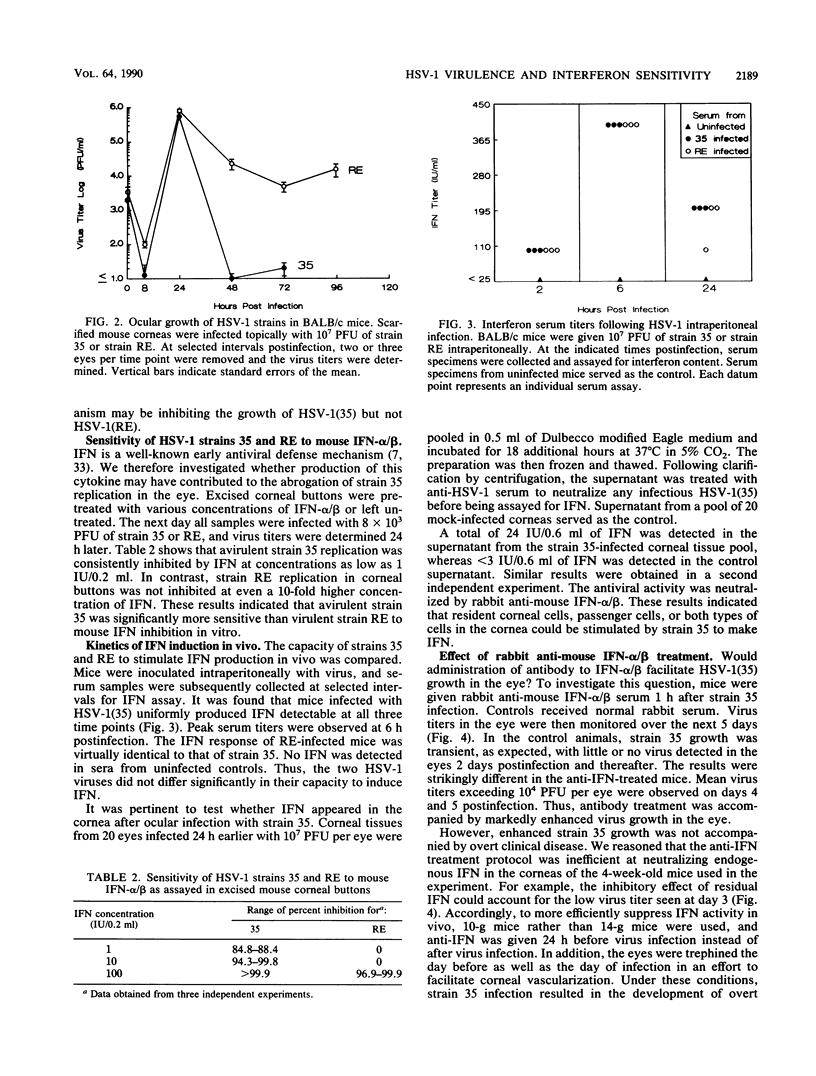
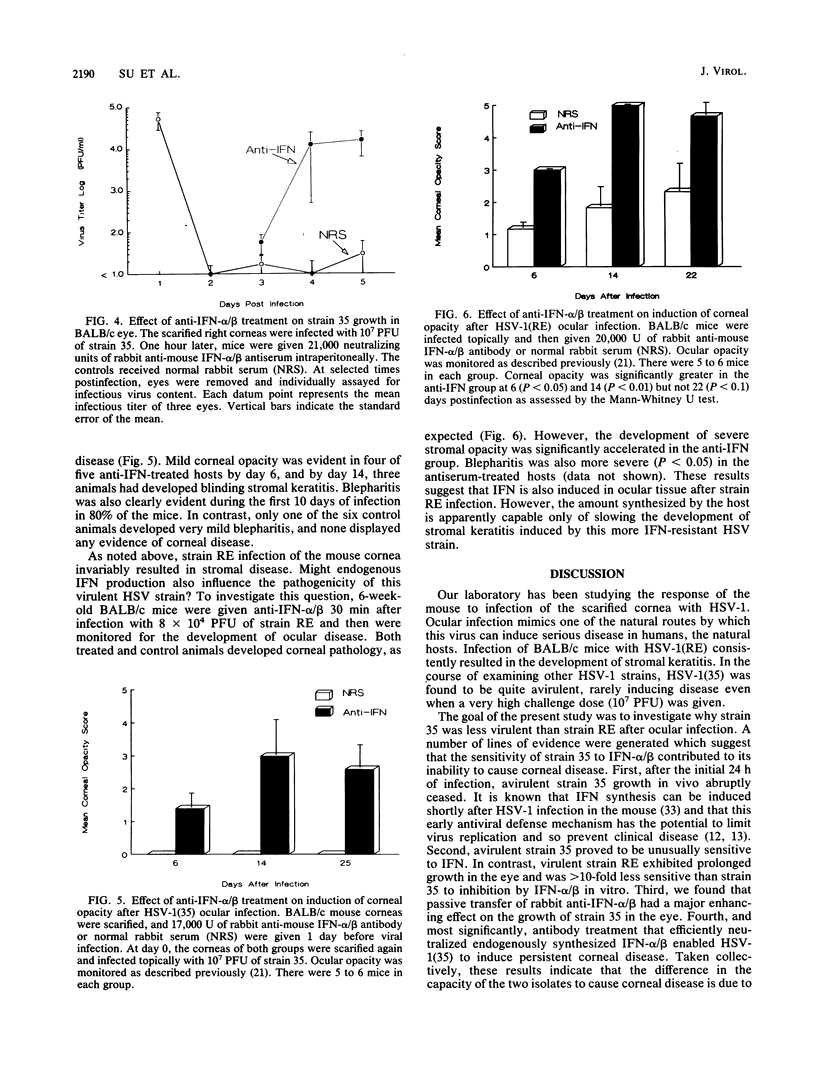
BALB/c mice infected on the scarified cornea with herpes simplex virus type 1 strain 35 [HSV-1(35)] rarely developed ocular disease even at challenge doses as high as 10(7) PFU per eye. In contrast, HSV-1(RE) consistently induced stromal keratitis at an inoculum of 2 x 10(4) PFU. The goal of this study was to determine the reason for the difference in virulence between the two HSV strains. Both HSV-1 strains replicated to similar titers in excised corneal "buttons." However, after in vivo infection of the cornea, the growth of strain 35 was evident only during the first 24 h postinfection, whereas the replication of strain RE persisted for at least 4 days. In vitro tests revealed that HSV-1(35) was greater than 10 times more sensitive to alpha/beta interferon (IFN-alpha/beta) than HSV-1(RE). Both strains induced comparable serum levels of IFN after intraperitoneal inoculation. The kinetics of HSV-1(35) clearance from the eye was markedly altered by treatment with rabbit anti-IFN-alpha/beta. Virus titers exceeding 10(4) PFU per eye could be demonstrated 4 to 5 days postinfection in mice given a single inoculation of antiserum 1 h after infection. Furthermore, anti-IFN treatment in 3-week-old mice infected with HSV-1(35) led to the development of clinically apparent corneal disease which subsequently progressed to stromal keratitis in the majority of recipients. These results indicate that the striking difference in the capacity of HSV-1(35) and HSV-1(RE) to induce corneal disease was related to the inherently greater sensitivity of strain 35 to IFN-alpha/beta produced by the host in response to infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukowski J. F., Welsh R. M. The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J Immunol. 1986 May 1;136(9):3481–3485. [PubMed] [Google Scholar]
- Cameron J. M., McDougall I., Marsden H. S., Preston V. G., Ryan D. M., Subak-Sharpe J. H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988 Oct;69(Pt 10):2607–2612. doi: 10.1099/0022-1317-69-10-2607. [DOI] [PubMed] [Google Scholar]
- Cayley P. J., Davies J. A., McCullagh K. G., Kerr I. M. Activation of the ppp(A2'p)nA system in interferon-treated, herpes simplex virus-infected cells and evidence for novel inhibitors of the ppp(A2'p)nA-dependent RNase. Eur J Biochem. 1984 Aug 15;143(1):165–174. doi: 10.1111/j.1432-1033.1984.tb08355.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E., Whitley R. Effect of cloned human interferons on protein synthesis and morphogenesis of herpes simplex virus. J Virol. 1985 Nov;56(2):419–425. doi: 10.1128/jvi.56.2.419-425.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Lakeman A. D., Whitley R. J., Hunter E. Effect of cloned human interferons on the replication of and cell fusion induced by herpes simplex virus. Virus Res. 1984 Jan;1(1):81–87. doi: 10.1016/0168-1702(84)90037-6. [DOI] [PubMed] [Google Scholar]
- Day S. P., Lausch R. N., Oakes J. E. Evidence that the gene for herpes simplex virus type 1 DNA polymerase accounts for the capacity of an intertypic recombinant to spread from eye to central nervous system. Virology. 1988 Mar;163(1):166–173. doi: 10.1016/0042-6822(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domke-Opitz I., Straub P., Kirchner H. Effect of interferon on replication of herpes simplex virus types 1 and 2 in human macrophages. J Virol. 1986 Oct;60(1):37–42. doi: 10.1128/jvi.60.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Coen D. M. Pathogenicity of herpes simplex virus mutants containing drug resistance mutations in the viral DNA polymerase gene. J Virol. 1986 Oct;60(1):286–289. doi: 10.1128/jvi.60.1.286-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines K. L., Kayes S. G., Wilson G. L. Factors affecting the infection of the D variant of encephalomyocarditis virus in the B cells of C57BL/6J mice. Diabetologia. 1987 Jun;30(6):419–425. doi: 10.1007/BF00292545. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausch R. N., Kleinschradt W. R., Monteiro C., Kayes S. G., Oakes J. E. Resolution of HSV corneal infection in the absence of delayed-type hypersensitivity. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1509–1515. [PubMed] [Google Scholar]
- Lopez C., Ryshke R., Bennett M. Marrow-dependent cells depleted by 89Sr mediate genetic resistance to herpes simplex virus type 1 infection in mice. Infect Immun. 1980 Jun;28(3):1028–1032. doi: 10.1128/iai.28.3.1028-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight J. L., Kristie T. M., Roizman B. Binding of the virion protein mediating alpha gene induction in herpes simplex virus 1-infected cells to its cis site requires cellular proteins. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7061–7065. doi: 10.1073/pnas.84.20.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. F., Koga J., Chatterjee S., Whitley R. J. Passive immunization with monoclonal antibodies against herpes simplex virus glycoproteins protects mice against herpetic ocular disease. Curr Eye Res. 1987 Jan;6(1):173–177. doi: 10.3109/02713688709020086. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Straub P., Kirchner H., Jacobsen H. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology. 1988 May;164(1):201–210. doi: 10.1016/0042-6822(88)90637-x. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Carrasco L. Formation of non-infective herpesvirus particles in cultured cells treated with human interferon. J Gen Virol. 1984 Jun;65(Pt 6):1069–1078. doi: 10.1099/0022-1317-65-6-1069. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin D., Lenz T., Sundmacher R. Strain characteristics and features of ocular infection of herpes simplex virus type 1 isolates. Med Microbiol Immunol. 1979;167(4):239–250. doi: 10.1007/BF02120809. [DOI] [PubMed] [Google Scholar]
- Orchansky P., Rubinstein M., Fischer D. G. The interferon-gamma receptor in human monocytes is different from the one in nonhematopoietic cells. J Immunol. 1986 Jan;136(1):169–173. [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Richards J. T., Kern E. R., Overall J. C., Jr, Glasgow L. A. Differences in neurovirulence among isolates of Herpes simplex virus types 1 and 2 in mice using four routes of infection. J Infect Dis. 1981 Nov;144(5):464–471. doi: 10.1093/infdis/144.5.464. [DOI] [PubMed] [Google Scholar]
- Stanton G. J., Jordan C., Hart A., Heard H., Langford M. P., Baron S. Nondetectable levels of interferon gamma is a critical host defense during the first day of herpes simplex virus infection. Microb Pathog. 1987 Sep;3(3):179–183. doi: 10.1016/0882-4010(87)90094-5. [DOI] [PubMed] [Google Scholar]
- Taylor J. L., O'Brien W. J. Interferon production in inbred mice during herpetic eye disease. Curr Eye Res. 1987 Jan;6(1):259–264. doi: 10.3109/02713688709020101. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Engler H., Kirchner H. Experimental infection of inbred mice with herpes simplex virus. III. Comparison between newborn and adult C57BL/6 mice. J Gen Virol. 1982 May;60(Pt 1):25–29. doi: 10.1099/0022-1317-60-1-25. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Gresser I., DeMaeyer E., Kirchner H. The role of interferon in the resistance of C57BL/6 mice to various doses of herpes simplex virus type 1. J Infect Dis. 1982 Sep;146(3):405–410. doi: 10.1093/infdis/146.3.405. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Marcucci F., Kirchner H. Experimental infection of inbred mice with herpes simplex virus type 1. I. Investigation of humoral and cellular immunity and of interferon induction. J Gen Virol. 1981 Mar;53(Pt 1):31–38. doi: 10.1099/0022-1317-53-1-31. [DOI] [PubMed] [Google Scholar]



