Abstract
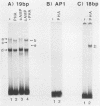
The 19-base-pair enhancer repeat of the human cytomegalovirus immediate-early 1 gene mediates cyclic AMP- and phytohemagglutinin-induced expression in Jurkat T cells. Synergistic activity was observed in the presence of both drugs, suggesting a convergence of the protein kinase A and C pathways on this transcription element. In addition, the immunosuppressive drug cyclosporine strongly reduced the ability of the 19-base-pair repeat to activate gene expression in phytohemagglutinin-stimulated T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Braun R. W., Reiser H. C. Replication of human cytomegalovirus in human peripheral blood T cells. J Virol. 1986 Oct;60(1):29–36. doi: 10.1128/jvi.60.1.29-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning J. H., Bruggeman C. A., van Boven C. P., van Breda Vriesman P. J. Reactivation of latent rat cytomegalovirus by a combination of immunosuppression and administration of allogeneic immunocompetent cells. Transplantation. 1989 May;47(5):917–918. doi: 10.1097/00007890-198905000-00040. [DOI] [PubMed] [Google Scholar]
- Bruning J. H., Bruggeman C. A., van Breda Vriesman P. J. The transfer of cytomegalovirus infection in rats by latently infected renal allografts, and the role of various immunosuppressive regimens in virus reactivation. Transplantation. 1988 Oct;46(4):623–624. doi: 10.1097/00007890-198810000-00041. [DOI] [PubMed] [Google Scholar]
- Bunce C. M., Thick J. A., Lord J. M., Mills D., Brown G. A rapid procedure for isolating hemopoietic cell nuclei. Anal Biochem. 1988 Nov 15;175(1):67–73. doi: 10.1016/0003-2697(88)90362-4. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Crawford S., Stall J., Rawlins D. R., Jeang K. T., Hayward G. S. The palindromic series I repeats in the simian cytomegalovirus major immediate-early promoter behave as both strong basal enhancers and cyclic AMP response elements. J Virol. 1990 Jan;64(1):264–277. doi: 10.1128/jvi.64.1.264-277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Keil G. M., Weber F., Jasin M., Schaffner W., Koszinowski U. H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Pohajdak B., Talbot D. J., Shaw J., Paetkau V. Phorbol diester-inducible, cyclosporine-suppressible transcription from a novel promoter within the mouse mammary tumor virus env gene. J Virol. 1988 Apr;62(4):1373–1380. doi: 10.1128/jvi.62.4.1373-1380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Fickenscher H., Stamminger T., Rüger R., Fleckenstein B. The role of a repetitive palindromic sequence element in the human cytomegalovirus major immediate early enhancer. J Gen Virol. 1989 Jan;70(Pt 1):107–123. doi: 10.1099/0022-1317-70-1-107. [DOI] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Fleckenstein B., Hennighausen L. Binding of transcription factors and creation of a large nucleoprotein complex on the human cytomegalovirus enhancer. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3658–3662. doi: 10.1073/pnas.84.11.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Multiple sequence-specific transcription factors modulate cytomegalovirus enhancer activity in vitro. Mol Cell Biol. 1988 Apr;8(4):1809–1811. doi: 10.1128/mcb.8.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Specific interactions between transcription factors and the promoter-regulatory region of the human cytomegalovirus major immediate-early gene. J Virol. 1988 Mar;62(3):1076–1079. doi: 10.1128/jvi.62.3.1076-1079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Lubon H. Interaction of protein with DNA in vitro. Methods Enzymol. 1987;152:721–735. doi: 10.1016/0076-6879(87)52076-6. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Monick M. M., Liu B., Stinski M. F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989 Jul;63(7):3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Irving S. G., June C. H., Zipfel P. F., Siebenlist U., Kelly K. Mitogen-induced genes are subject to multiple pathways of regulation in the initial stages of T-cell activation. Mol Cell Biol. 1989 Mar;9(3):1034–1040. doi: 10.1128/mcb.9.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Rawlins D. R., Rosenfeld P. J., Shero J. H., Kelly T. J., Hayward G. S. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J Virol. 1987 May;61(5):1559–1570. doi: 10.1128/jvi.61.5.1559-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Poggi A., Olive D., Bottino C., Fortis C., Pantaleo G., Moretta L. Selection and characterization of T-cell variants lacking molecules involved in T-cell activation (T3 T-cell receptor, T44, and T11): analysis of the functional relationship among different pathways of activation. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1654–1658. doi: 10.1073/pnas.84.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Roehr T. J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985 Aug;55(2):431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starkesen S. E., Luciw P. A., Peterlin B. M. Signaling through T lymphocyte surface proteins, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J Immunol. 1989 Jan 15;142(2):702–707. [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]