Abstract
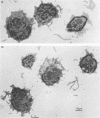
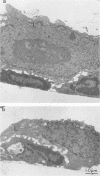
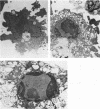
Lysis of virus-infected L929 target cells transfected with the H-2 class II IAk gene by class II-restricted influenza virus-specific murine cytotoxic T lymphocyte (CTL) clones was studied by electron microscopy and compared with lysis of L929 cells by class I-restricted CTL clones. T lymphocytes predominantly approached the basal surface of target cells grown on a plastic dish and also approached uninfected L929 target cells, although virus maturation exhibited no polarity with respect to the cell surface site. After incubation for 30 min, the target cell nuclei began to change: chromatin became irregularly redistributed and aggregated, and the nuclei appeared swollen. Later, electron-dense and -light areas of nuclei became segregated, and the cytoplasm became disorganized with many vacuoles. The ultrastructural changes of target cells during lysis by class I- and class II-restricted CTL clones appeared to be similar. These findings and other cytotoxicity data of class I and class II CTLs are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Taylor P. M., Esquivel F. Cytotoxic T cells in influenza infection. Ann N Y Acad Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- Berke G., Rosen D. Highly lytic in vivo primed cytolytic T lymphocytes devoid of lytic granules and BLT-esterase activity acquire these constituents in the presence of T cell growth factors upon blast transformation in vitro. J Immunol. 1988 Sep 1;141(5):1429–1436. [PubMed] [Google Scholar]
- Bourgault I., Gomez A., Gomard E., Picard F., Levy J. P., Gomrad E. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. J Immunol. 1989 Jan 1;142(1):252–256. [PubMed] [Google Scholar]
- Braciale T. J., Andrew M. E., Braciale V. L. Heterogeneity and specificity of cloned lines of influenza-virus specific cytotoxic T lymphocytes. J Exp Med. 1981 Apr 1;153(4):910–923. doi: 10.1084/jem.153.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykovskaja S. N., Rytenko A. N., Rauschenbach M. O., Bykovsky A. F. Ultrastructural alteration of cytolytic T lymphocytes following their interaction with target cells. I. Hypertrophy and change of orientation of the Golgi apparatus. Cell Immunol. 1978 Sep 15;40(1):164–174. doi: 10.1016/0008-8749(78)90324-6. [DOI] [PubMed] [Google Scholar]
- Bykovskaja S. N., Rytenko A. N., Rauschenbach M. O., Bykovsky A. F. Ultrastructural alteration of cytolytic T lymphocytes following their interaction with target cells. II. Morphogenesis of secretory granules and intracellular vacuoles. Cell Immunol. 1978 Sep 15;40(1):175–185. doi: 10.1016/0008-8749(78)90325-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sanz J. A., Plaetinck G., Velotti F., Masson D., Tschopp J., MacDonald H. R., Nabholz M. Perforin is present only in normal activated Lyt2+ T lymphocytes and not in L3T4+ cells, but the serine protease granzyme A is made by both subsets. EMBO J. 1987 Apr;6(4):933–938. doi: 10.1002/j.1460-2075.1987.tb04841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkowski S. H., Brown T. C., Cerutti P. A., Cerottini J. C. DNA of human Raji target cells is damaged upon lymphocyte-mediated lysis. J Immunol. 1986 Feb 1;136(3):752–756. [PubMed] [Google Scholar]
- Groscurth P., Qiao B. Y., Podack E. R., Hengartner H. Cellular localization of perforin 1 in murine cloned cytotoxic T lymphocytes. J Immunol. 1987 May 1;138(9):2749–2752. [PubMed] [Google Scholar]
- Hackett C. J., Sullivan K., Lin Y. L. Ultrastructure of an influenza virus-specific cytotoxic T-cell clone and its interaction with P815 and macrophage targets. Cell Immunol. 1982 Apr;68(2):276–286. doi: 10.1016/0008-8749(82)90112-5. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Hioe C. E., Hinshaw V. S. Induction and activity of class II-restricted, Lyt-2+ cytolytic T lymphocytes specific for the influenza H5 hemagglutinin. J Immunol. 1989 Apr 1;142(7):2482–2488. [PubMed] [Google Scholar]
- Howell D. M., Martz E. Nuclear disintegration induced by cytotoxic T lymphocytes. Evidence against damage to the nuclear envelope of the target cell. J Immunol. 1988 Feb 1;140(3):689–692. [PubMed] [Google Scholar]
- Kalina M., Berke G. Contact regions of cytotoxic T lymphocyte-target cell conjugates. Cell Immunol. 1976 Jul;25(1):41–51. doi: 10.1016/0008-8749(76)90095-2. [DOI] [PubMed] [Google Scholar]
- Maimone M. M., Morrison L. A., Braciale V. L., Braciale T. J. Features of target cell lysis by class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1986 Dec 1;137(11):3639–3643. [PubMed] [Google Scholar]
- Malissen B., Price M. P., Goverman J. M., McMillan M., White J., Kappler J., Marrack P., Pierres A., Pierres M., Hood L. Gene transfer of H-2 class II genes: antigen presentation by mouse fibroblast and hamster B-cell lines. Cell. 1984 Feb;36(2):319–327. doi: 10.1016/0092-8674(84)90225-3. [DOI] [PubMed] [Google Scholar]
- Matter A. Microcinematographic and electron microscopic analysis of target cell lysis induced by cytotoxic T lymphocytes. Immunology. 1979 Feb;36(2):179–190. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Fitzgerald K., Snow P., Terhorst C., Schlossman S. F. Antibody directed at a surface structure inhibits cytolytic but not suppressor function of human T lymphocytes. Nature. 1981 Nov 12;294(5837):168–170. doi: 10.1038/294168a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., Dobos C. B. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980 Sep;125(3):1256–1261. [PubMed] [Google Scholar]
- Russell J. H., Masakowski V. R., Dobos C. B. Mechanisms of immune lysis. I. Physiological distinction between target cell death mediated by cytotoxic T lymphocytes and antibody plus complement. J Immunol. 1980 Mar;124(3):1100–1105. [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Sanderson C. J., Glauert A. M. The mechanism of T-cell mediated cytotoxicity. VI. T-cell projections and their role in target cell killing. Immunology. 1979 Jan;36(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Tite J. P., Ruddle N. H. DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1881–1885. doi: 10.1073/pnas.83.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y., Hosaka Y., Fukami Y., Fukai K. Immunoelectron microscopic study on interactions of noninfectious sendai virus and murine cells. J Virol. 1981 Jul;39(1):273–281. doi: 10.1128/jvi.39.1.273-281.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Cell-mediated killing: a common mechanism? Cell. 1986 Aug 29;46(5):641–642. doi: 10.1016/0092-8674(86)90336-3. [DOI] [PubMed] [Google Scholar]
- Young J. D., Liu C. C. Multiple mechanisms of lymphocyte-mediated killing. Immunol Today. 1988 May;9(5):140–144. doi: 10.1016/0167-5699(88)91201-7. [DOI] [PubMed] [Google Scholar]
- Young J. D., Podack E. R., Cohn Z. A. Properties of a purified pore-forming protein (perforin 1) isolated from H-2-restricted cytotoxic T cell granules. J Exp Med. 1986 Jul 1;164(1):144–155. doi: 10.1084/jem.164.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]