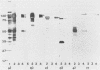
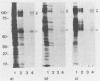
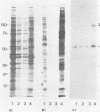
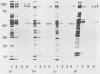
Abstract
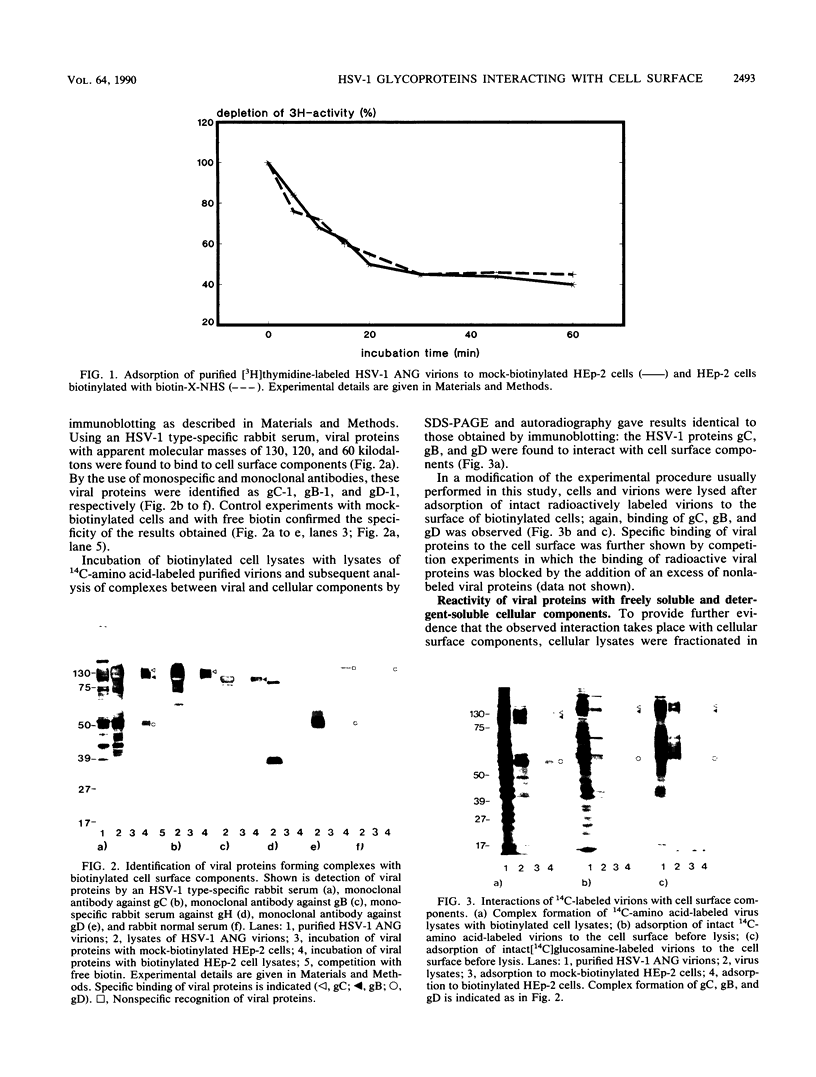
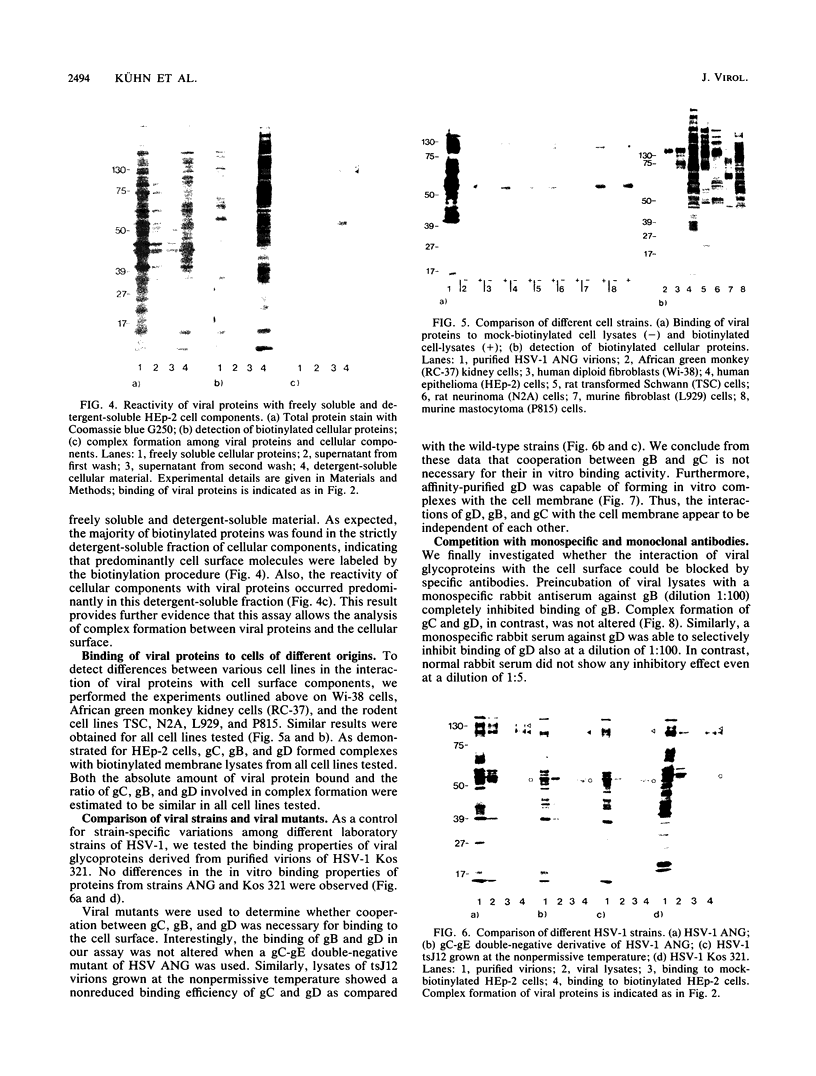
To investigate the interaction of herpes simplex virus type 1 (HSV-1) with the cell surface, we studied the formation of complexes by HSV-1 virion proteins with biotinylated cell membrane components. HSV-1 virion proteins reactive with surface components of HEp-2 and other cells were identified as gC, gB, and gD. Results from competition experiments suggested that binding of gC, gB, and gD occurred in a noncooperative way. The observed complex formation could be specifically blocked by monospecific rabbit antisera against gB and gD. The interaction of gD with the cell surface was also inhibited by monoclonal antibody IV3.4., whereas other gD-specific monoclonal antibodies, despite their high neutralizing activity, were not able to inhibit this interaction. Taken together, these data provide direct evidence that at least three of the seven known HSV-1 glycoproteins are able to form complexes with cellular surface structures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Skutelsky E., Wilchek M. The ultrastructural visualization of cell surface glycoconjugates. Methods Enzymol. 1982;83:195–215. doi: 10.1016/0076-6879(82)83014-0. [DOI] [PubMed] [Google Scholar]
- Buckie J. W., Cook G. M. Specific isolation of surface glycoproteins from intact cells by biotinylated concanavalin A and immobilized streptavidin. Anal Biochem. 1986 Aug 1;156(2):463–472. doi: 10.1016/0003-2697(86)90280-0. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988 Jan;62(1):159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Eing B. R., Kühn J. E., Braun R. W. Neutralizing activity of antibodies against the major herpes simplex virus type 1 glycoproteins. J Med Virol. 1989 Jan;27(1):59–65. doi: 10.1002/jmv.1890270113. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Ligas M. W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988 Dec;62(12):4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Wittels M., Spear P. G. Binding to cells of virosomes containing herpes simplex virus type 1 glycoproteins and evidence for fusion. J Virol. 1984 Oct;52(1):238–247. doi: 10.1128/jvi.52.1.238-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Spear P. G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989 Feb;63(2):819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn J. E., Dunkler G., Munk K., Braun R. W. Analysis of the IgM and IgG antibody response against herpes simplex virus type 1 (HSV-1) structural and nonstructural proteins. J Med Virol. 1987 Oct;23(2):135–150. doi: 10.1002/jmv.1890230206. [DOI] [PubMed] [Google Scholar]
- Kühn J. E., Eing B. R., Brossmer R., Munk K., Braun R. W. Removal of N-linked carbohydrates decreases the infectivity of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2847–2858. doi: 10.1099/0022-1317-69-11-2847. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk K., Ludwig G. Properties of plaque variants of herpes virus hominis strains of genital origin. Arch Gesamte Virusforsch. 1972;37(4):308–315. doi: 10.1007/BF01241453. [DOI] [PubMed] [Google Scholar]
- Neidhardt H., Schröder C. H., Kaerner H. C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J Virol. 1987 Feb;61(2):600–603. doi: 10.1128/jvi.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Meyer A. L., Post L. E. Reduced yield of infectious pseudorabies virus and herpes simplex virus from cell lines producing viral glycoprotein gp50. J Virol. 1988 Jun;62(6):2196–2199. doi: 10.1128/jvi.62.6.2196-2199.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. S., Killius J., Hodnichak C. M., Venetta T. M., Gyurgyik L., Janiga K. Mild acidic pH inhibition of the major pathway of herpes simplex virus entry into HEp-2 cells. J Gen Virol. 1989 Apr;70(Pt 4):857–867. doi: 10.1099/0022-1317-70-4-857. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus glycoprotein gIII is required for efficient virus growth in tissue culture. J Virol. 1988 Jul;62(7):2512–2515. doi: 10.1128/jvi.62.7.2512-2515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Labeling glycoconjugates with hydrazide reagents. Methods Enzymol. 1987;138:429–442. doi: 10.1016/0076-6879(87)38037-1. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F., Zsak L., Reilly L., Sugg N., Ben-Porat T. Early interactions of pseudorabies virus with host cells: functions of glycoprotein gIII. J Virol. 1989 Aug;63(8):3323–3329. doi: 10.1128/jvi.63.8.3323-3329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]