Abstract
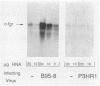
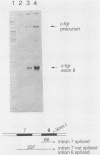
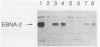
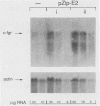
The efficient immortalization of primary resting human B lymphocytes by Epstein-Barr virus (EBV) requires several viral genes and presumably the altered expression of an unknown number of cellular genes as well. In this paper, I show that infection of primary human B cells with EBV increased the transcript level of the proto-oncogene, c-fgr, 10-fold. This effect on the level of c-fgr transcripts in B cells was not secondary to blast formation, because levels of c-fgr RNA were also increased 10-fold in two proliferating EBV-negative Burkitt's lymphoma-derived cell lines, Ramos and BJAB, 2 days after infection with EBV. Two lines of evidence indicated that EBV nuclear antigen 2 (EBNA-2) mediates this increase in c-fgr RNA levels: acute infection of BJAB and Ramos cells by a mutant strain of EBV that lacked the EBNA-2 open reading frame, P3HR1, did not affect c-fgr RNA levels; and cell lines constitutively expressing only the EBNA-2 gene of EBV had increased levels of c-fgr RNA relative to those in the parental cell lines. Since P3HR1, a nonimmortalizing strain of EBV, failed to affect c-fgr RNA levels and since a viral gene required for B-cell immortalization was responsible for the induction of c-fgr, the data indicate a possible role of c-fgr expression in B-lymphocyte immortalization by EBV and a mechanism by which EBNA-2 contributes to the immortalizing activity of EBV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Posttranslational processing of an Epstein-Barr virus-encoded membrane protein expressed in cells transformed by Epstein-Barr virus. J Virol. 1987 Mar;61(3):866–875. doi: 10.1128/jvi.61.3.866-875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Wang F., Hennessy K., Woodland E., Rickinson A., Kieff E. Expression of the Epstein-Barr virus nuclear protein 2 in rodent cells. J Virol. 1986 Aug;59(2):453–462. doi: 10.1128/jvi.59.2.453-462.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kappes B., Ziemiecki A., Müller R. G., Theilen G. H., Bauer H., Barnekow A. The TP1 isolate of feline sarcoma virus encodes a fgr-related oncogene lacking gamma actin sequences. Oncogene. 1989 Mar;4(3):363–372. [PubMed] [Google Scholar]
- Kawakami T., Pennington C. Y., Robbins K. C. Isolation and oncogenic potential of a novel human src-like gene. Mol Cell Biol. 1986 Dec;6(12):4195–4201. doi: 10.1128/mcb.6.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Klein C., Busson P., Tursz T., Young L. S., Raab-Traub N. Expression of the c-fgr related transcripts in Epstein-Barr virus-associated malignancies. Int J Cancer. 1988 Jul 15;42(1):29–35. doi: 10.1002/ijc.2910420107. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson J. C., Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987 Jul;164(1):44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Connolly N. L., Katamine S., Cheah M. S., Senior R. M., Robbins K. C. Tissue-specific expression and developmental regulation of the human fgr proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):92–99. doi: 10.1128/mcb.9.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Lewis D. B., Wilson C. B., Gearn M. E., Krebs E. G., Perlmutter R. M. Regulation of pp56lck during T-cell activation: functional implications for the src-like protein tyrosine kinases. EMBO J. 1987 Sep;6(9):2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Overell R. W., Meier K. E., Krebs E. G., Perlmutter R. M. Translational activation of the lck proto-oncogene. Nature. 1988 Mar 10;332(6160):171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yoshida M. C., Yamamoto T., Sasaki M., Toyoshima K. Structure, expression, and chromosomal location of the human c-fgr gene. Mol Cell Biol. 1986 Feb;6(2):511–517. doi: 10.1128/mcb.6.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Lebo R., Varmus H., Bishop J. M., Pettenati M. J., Le Beau M. M., Diaz M. O., Rowley J. D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987 Jun;7(6):2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Heston L., Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Barbacid M., Aaronson S., Gardner M. B. Origin and biological properties of a new feline sarcoma virus. Virology. 1982 Feb;117(1):238–244. doi: 10.1016/0042-6822(82)90522-0. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Reisman D., Sugden B. An EBNA-negative, EBV-genome-positive human lymphoblast cell line in which superinfecting EBV DNA is not maintained. Virology. 1984 Aug;137(1):113–126. doi: 10.1016/0042-6822(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Reisman D., Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986 Nov;6(11):3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Howe J. G., Speck S. H., Miller G. Influence of Burkitt's lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989 Apr;63(4):1531–1539. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K., Nishizawa M., Miyajima N., Yoshida M. C., Sukegawa J., Yamanashi Y., Sasaki M., Yamamoto T., Toyoshima K. yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5459–5463. doi: 10.1073/pnas.83.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Pfitzner A., Strominger J. L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Metzenberg S. Characterization of an antigen whose cell surface expression is induced by infection with Epstein-Barr virus. J Virol. 1983 Jun;46(3):800–807. doi: 10.1128/jvi.46.3.800-807.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Phelps M., Domoradzki J. Epstein-Barr virus DNA is amplified in transformed lymphocytes. J Virol. 1979 Sep;31(3):590–595. doi: 10.1128/jvi.31.3.590-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J Virol. 1989 Jun;63(6):2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Popescu N. C., Cheah M. S., Swan D. C., Amsbaugh S. C., Lengel C. R., DiPaolo J. A., Robbins K. C. Isolation and chromosomal localization of the human fgr protooncogene, a distinct member of the tyrosine kinase gene family. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6595–6599. doi: 10.1073/pnas.82.19.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Horak E. M., Bookman M. A., Bolen J. B. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988 Oct;8(10):4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willman C. L., Stewart C. C., Griffith J. K., Stewart S. J., Tomasi T. B. Differential expression and regulation of the c-src and c-fgr protooncogenes in myelomonocytic cells. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4480–4484. doi: 10.1073/pnas.84.13.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Marth J. D., Lewis D. B., Perlmutter R. M. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol Cell Biol. 1987 Jun;7(6):2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]