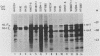
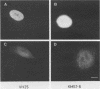
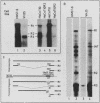
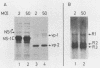
Abstract
Human fibroblasts and epithelial cells differing in their susceptibility to killing by the autonomous parvoviruses H-1 and minute virus of mice were compared for their capacity to express viral mRNAs and proteins. The transition from a parvovirus-resistant to a parvovirus-sensitive phenotype correlated with a proportional increase in the production of the three major viral transcripts and of structural and nonstructural proteins. In contrast, cell sensitization to parvovirus could not be correlated with detectable changes in virus uptake, intracellular localization of gene products, stability of viral mRNAs, or phosphorylation of viral nonstructural polypeptides. Moreover, the H-1 virus-sensitive keratinocyte line studied did not sustain a greater level of viral DNA amplification than its resistant derivative. Therefore, the differential susceptibility of the human cells tested to parvovirus infection appears to be mainly controlled at the level of transcription of the viral genome. Parvoviral gene expression could not be elevated by increasing the input multiplicity of infection in either of the cell systems analyzed. Together, these data suggest that a cellular factor(s) regulating parvoviral transcription may be modulated by oncogenic transformation or by differentiation, as both features have been shown to affect cell susceptibility to parvoviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D. Oncogenes in development. Development. 1987 Apr;99(4):449–471. doi: 10.1242/dev.99.4.449. [DOI] [PubMed] [Google Scholar]
- Antonietti J. P., Sahli R., Beard P., Hirt B. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J Virol. 1988 Feb;62(2):552–557. doi: 10.1128/jvi.62.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburger A., Legendre D., Avalosse B., Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990 Feb;174(2):576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Chen Y. Q., Tuynder M. C., Cornelis J. J., Boukamp P., Fusenig N. E., Rommelaere J. Sensitization of human keratinocytes to killing by parvovirus H-1 takes place during their malignant transformation but does not require them to be tumorigenic. Carcinogenesis. 1989 Jan;10(1):163–167. doi: 10.1093/carcin/10.1.163. [DOI] [PubMed] [Google Scholar]
- Chen Y. Q., de Foresta F., Hertoghs J., Avalosse B. L., Cornelis J. J., Rommelaere J. Selective killing of simian virus 40-transformed human fibroblasts by parvovirus H-1. Cancer Res. 1986 Jul;46(7):3574–3579. [PubMed] [Google Scholar]
- Cornelis J. J., Becquart P., Duponchel N., Salomé N., Avalosse B. L., Namba M., Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988 May;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Spruyt N., Spegelaere P., Guetta E., Darawshi T., Cotmore S. F., Tal J., Rommelaere J. Sensitization of transformed rat fibroblasts to killing by parvovirus minute virus of mice correlates with an increase in viral gene expression. J Virol. 1988 Sep;62(9):3438–3444. doi: 10.1128/jvi.62.9.3438-3444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of the autonomous parvovirus MVM is a nuclear phosphoprotein. Virus Res. 1986 May;4(3):243–250. doi: 10.1016/0168-1702(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Antonietti J. P., Beard P. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J Virol. 1990 Jan;64(1):387–396. doi: 10.1128/jvi.64.1.387-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Beard P., Antonietti J. P. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J Gen Virol. 1988 Oct;69(Pt 10):2563–2573. doi: 10.1099/0022-1317-69-10-2563. [DOI] [PubMed] [Google Scholar]
- Dupressoir T., Vanacker J. M., Cornelis J. J., Duponchel N., Rommelaere J. Inhibition by parvovirus H-1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res. 1989 Jun 15;49(12):3203–3208. [PubMed] [Google Scholar]
- Faisst S., Schlehofer J. R., zur Hausen H. Transformation of human cells by oncogenic viruses supports permissiveness for parvovirus H-1 propagation. J Virol. 1989 May;63(5):2152–2158. doi: 10.1128/jvi.63.5.2152-2158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner E. M., Tattersall P. Evidence that developmentally regulated control of gene expression by a parvoviral allotropic determinant is particle mediated. J Virol. 1988 May;62(5):1713–1722. doi: 10.1128/jvi.62.5.1713-1722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labieniec-Pintel L., Pintel D. The minute virus of mice P39 transcription unit can encode both capsid proteins. J Virol. 1986 Mar;57(3):1163–1167. doi: 10.1128/jvi.57.3.1163-1167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R. M., Roeder R. G. Parvovirus H-1 expression: mapping of the abundant cytoplasmic transcripts and identification of promoter sites and overlapping transcription units. J Virol. 1986 May;58(2):271–280. doi: 10.1128/jvi.58.2.271-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Cotmore S. F., Stout E. R., Bates R. C. Detection of bovine parvovirus proteins homologous to the nonstructural NS-1 proteins of other autonomous parvoviruses. J Virol. 1987 Nov;61(11):3612–3616. doi: 10.1128/jvi.61.11.3612-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Patton J. T., Stout E. R., Bates R. C. Virally coded noncapsid protein associated with bovine parvovirus infection. J Virol. 1984 Feb;49(2):315–318. doi: 10.1128/jvi.49.2.315-318.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Ward D. C., Ruddle F. H. Embryonal carcinoma cells (and their somatic cell hybrids) are resistant to infection by the murine parvovirus MVM, which does infect other teratocarcinoma-derived cell lines. J Cell Physiol. 1977 Jun;91(3):393–401. doi: 10.1002/jcp.1040910309. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Identification and characterization of a porcine parvovirus nonstructural polypeptide. J Virol. 1985 Sep;55(3):554–559. doi: 10.1128/jvi.55.3.554-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982 Dec 9;300(5892):537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- Ohlsson R. I., Pfeifer-Ohlsson S. B. Cancer genes, proto-oncogenes, and development. Exp Cell Res. 1987 Nov;173(1):1–16. doi: 10.1016/0014-4827(87)90327-2. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Identification of multiple forms of the noncapsid parvovirus protein NCVP1 in H-1 parvovirus-infected cells. J Virol. 1984 Oct;52(1):82–87. doi: 10.1128/jvi.52.1.82-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P. S., Mengeling W. L., Brown T. T., Jr Replication of porcine parvovirus in peripheral blood lymphocytes, monocytes, and peritoneal macrophages. Infect Immun. 1979 Sep;25(3):1003–1007. doi: 10.1128/iai.25.3.1003-1007.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Hirai S., Yaniv M. Constitutive synthesis of activator protein 1 transcription factor after viral transformation of mouse fibroblasts. Proc Natl Acad Sci U S A. 1988 May;85(10):3401–3405. doi: 10.1073/pnas.85.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Gesquière J. C., Stéhelin D., Ghysdael J. Mitogenic stimulation of thymocytes results in the calcium-dependent phosphorylation of c-ets-1 proteins. EMBO J. 1988 Apr;7(4):977–983. doi: 10.1002/j.1460-2075.1988.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Construction of a genetic switch for inducible trans-activation of gene expression in eucaryotic cells. J Virol. 1987 May;61(5):1448–1456. doi: 10.1128/jvi.61.5.1448-1456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus replication in normal and transformed human cells correlates with the nuclear translocation of the early protein NS1. J Virol. 1989 Jan;63(1):349–355. doi: 10.1128/jvi.63.1.349-355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Richard S. M. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J Virol. 1987 Sep;61(9):2807–2815. doi: 10.1128/jvi.61.9.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N., van Hille B., Geuskens M., Rommelaere J. Partial reversion of conditional transformation correlates with a decrease in the sensitivity of rat cells to killing by the parvovirus minute virus of mice but not in their capacity for virus production: effect of a temperature-sensitive v-src oncogene. J Virol. 1989 Nov;63(11):4797–4807. doi: 10.1128/jvi.63.11.4797-4807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H., Ledinko N. Growth and cytopathogenicity of H-viruses in human and simian cell cultures. Nature. 1965 Nov 20;208(5012):812–813. doi: 10.1038/208812a0. [DOI] [PubMed] [Google Scholar]
- Tullis G. E., Labieniec-Pintel L., Clemens K. E., Pintel D. Generation and characterization of a temperature-sensitive mutation in the NS-1 gene of the autonomous parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2736–2744. doi: 10.1128/jvi.62.8.2736-2744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hille B., Duponchel N., Salomé N., Spruyt N., Cotmore S. F., Tattersall P., Cornelis J. J., Rommelaere J. Limitations to the expression of parvoviral nonstructural proteins may determine the extent of sensitization of EJ-ras-transformed rat cells to minute virus of mice. Virology. 1989 Jul;171(1):89–97. doi: 10.1016/0042-6822(89)90514-x. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988 Aug;7(8):2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Callaham M. F., Huberman E. Perturbation of the cell cycle by adeno-associated virus. Virology. 1988 Dec;167(2):393–399. [PubMed] [Google Scholar]
- Wolter S., Richards R., Armentrout R. W. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim Biophys Acta. 1980 May 30;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]
- Yalkinoglu A. O., Heilbronn R., Bürkle A., Schlehofer J. R., zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988 Jun 1;48(11):3123–3129. [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]