Abstract
We carried out a yeast two-hybrid screen to identify proteins that interact with large-conductance Ca2+-activated K+ (BKCa) channels encoded by the Slo1 gene. Nephrin, an essential adhesion and scaffolding molecule expressed in podocytes, emerged in this screen. The Slo1-nephrin interaction was confirmed by coimmunoprecipitation from the brain and kidney, from HEK-293T cells expressing both proteins, and by glutathione S-transferase pull-down assays. We detected nephrin binding to the Slo1VEDEC splice variant, which is typically retained in intracellular stores, and to the β4-subunit. However, we did not detect significant binding of nephrin to the Slo1QEERL or Slo1EMVYR splice variants. Coexpression of nephrin with Slo1VEDEC increased expression of functional BKCa channels on the surface of HEK-293T cells but did not affect steady-state surface expression of the other COOH-terminal Slo1 variants. Nephrin did not affect the kinetics or voltage dependence of channel activation in HEK-293T cells expressing Slo1. Stimulation of Slo1VEDEC surface expression in HEK-293T cells was also observed by coexpressing a small construct encoding only the distal COOH-terminal domains of nephrin that interact with Slo1. Reduction of endogenous nephrin expression by application of small interfering RNA to differentiated cells of an immortalized podocyte cell line markedly reduced the steady-state surface expression of Slo1 as assessed by electrophysiology and cell-surface biotinylation assays. Nephrin therefore plays a role in organizing the surface expression of ion channel proteins in podocytes and may play a role in outside-in signaling to allow podocytes to adapt to mechanical or neurohumoral stimuli originating in neighboring cells.
Keywords: podocyte, Slo1, calcium, trafficking
the initial steps in renal function entail filtration of blood in the glomerulus, which acts as a sieve to exclude macromolecules from the urinary space in the lumen of Bowman's capsule. Normal glomerular filtration requires a population of specialized cells called podocytes, whose interdigitating foot processes attach to the glomerular basement membrane and form rectangular zipper-shaped structures known as slit diaphragms (38). Glomerular slit diaphragms are permeable to small inorganic and organic solutes but normally inhibit passage of macromolecules such as albumin and other plasma proteins into the filtrate. Mutations in several genes encoding podocyte proteins lead to congenital nephrotic syndromes. One form, originally described in Finnish patients, is associated with mutations in the gene encoding nephrin, a key structural and signaling protein expressed in podocyte foot processes (12, 21, 33). Nephrin interacts with a number of proteins in podocytes, and deletion of nephrin in mice results in a massive and lethal neonatal proteinuria (36).
Surprisingly little is known about the cellular physiology of podocytes, but there is evidence that these cells play a dynamic role in the regulation of glomerular filtration. The arrangement of F-actin, myosin, and α-actinin within foot processes allows podocytes to generate a contractile force that is postulated to facilitate adaptation of the glomerulus to changes in intraluminal hydrostatic pressure or to modify the surface area for filtration (5, 18, 31). Proteins of the slit diaphragm complex, including nephrin, are coupled to underlying contractile elements by a variety of adaptor proteins (7, 13, 35) that are postulated to allow for outside-in signaling from the nephrin ectodomains. Moreover, the size-selective barrier properties of podocyte monolayers are regulated by Ca2+-dependent changes in proteins that regulate the cytoskeleton and the slit diaphragm (11). Notably, congenital proteinurias have been associated with at least three different gain-of-function mutations in the gene encoding the Ca2+-permeable cation channel TRPC6, which is heavily expressed in podocytes (27, 37, 50), and overexpression of wild-type TRPC6 channels causes marked changes in podocyte actin dynamics and function (27).
TRPC6 channels have complex permeability properties. At more negative membrane potentials, they are permeable to Ca2+, but moderate depolarization causes them to function essentially as monovalent cation channels (6). For this reason, TRPC6 cannot be an efficient pathway for Ca2+ influx in most nonexcitable cells unless there is a mechanism in place to limit the amount of depolarization that occurs as a result of their own activation. Large-conductance Ca2+-activated K+ (BKCa) channels are ideally situated to play that role. BKCa channels are widely expressed in excitable and nonexcitable cells, including podocytes (29). Their gating is markedly voltage dependent, and binding of Ca2+ to sites on the large cytosolic COOH-terminal portion of the molecule shifts the activation voltage dependence into the physiological range. BKCa channels in podocytes (29) and in other cell types (8, 45) also can be activated by membrane stretch. The pore-forming subunits of BKCa channels are encoded by the Slo1 gene (also known as KCNMA1), which is expressed in a large number (≥20) of splice variants. The large COOH-terminal domains of Slo1 channels contain multiple high- and low-affinity sites for Ca2+ binding, as well as many phosphorylation and protein-protein interaction sites (24). In addition, at least four genes encode β-subunits that modulate BKCa gating and trafficking but that are not essential to make functional channels (24). Among these, the β3- and β4-subunits are expressed in podocytes (29).
Alternative splicing in the extreme COOH terminus of Slo1 gives rise to channels with markedly different patterns of trafficking to the plasma membrane (15, 16, 26). For example, one variant, known as VEDEC (Slo1VEDEC), after the last five residues at the COOH terminus, tends to be retained in intracellular compartments but can be translocated to the plasma membrane by treating cells with appropriate growth factors (15, 16). Two other COOH-terminal splice variants, referred to as Slo1QEERL and Slo1EMVYR, show much higher levels of steady-state expression in the plasma membrane (15, 16, 26). Coexpression of at least some BKCa β-subunits also can stimulate trafficking of Slo1 to the plasma membrane (15). For this reason, we have carried out a series of yeast two-hybrid screens in search of proteins that exhibit isoform-specific binding to Slo1 subunits. Nephrin emerged in this screen.
In the present study we have confirmed using several methods that nephrin binds to the Slo1VEDEC isoform but not to Slo1QEERL or Slo1EMVYR, and we also have shown that it colocalizes with endogenous Slo1 channels in differentiated cells of an immortalized podocyte cell line. Moreover, we have shown that coexpression of nephrin stimulates steady-state surface expression of Slo1VEDEC channels in a heterologous expression system, whereas small interfering (si)RNA-mediated nephrin knockdown reduces surface expression of functional Slo1 channels in cultured podocytes.
MATERIALS AND METHODS
Yeast two-hybrid screen.
Yeast two-hybrid analysis was carried out using the Matchmaker system (BD Biosciences, San Jose, CA) according to the manufacturer's instructions, as described in detail previously (17). We screened a cDNA library of the embryonic day 9 (E9) chick ciliary ganglion transcriptome homologously recombined into the pGADT7-rec plasmid. The resulting library was transformed into the AH109 yeast strain and selected on a SD/Leu single dropout medium. We obtained nephrin using a bait construct encoding amino acids 175-200 of the chick β1-subunit of BKCa channels (15), cloned in-frame into the pGBKT7 bait vector in the Matchmaker system. The bait vectors were transformed into the Y187 strain, which were selected on SD/Trp- single dropout medium. The transformed AH109 cDNA library cells were then mated with the transformed Y187 bait cells. Positive colonies expressing putative interacting proteins were selected by blue-white selection carried out on a quadruple dropout medium (SD/Ade-/His-/Trp-/Leu-) supplemented with a chromogenic substrate. After selection, the pGADT7 plasmids encoding fragments of putative interacting proteins were isolated using standard methods, transformed into Escherichia coli Top Ten cells (Invitrogen, Carlsbad, CA), sequenced, and subjected to BLAST search analysis.
Plasmid constructs and siRNA.
Expression plasmids encoding NH2-terminal Myc-tagged Slo1VEDEC, Slo1EMVYR, and Slo1QEERL isoforms of Slo1 were kindly provided by Dr. Min Li (Department of Neuroscience, Johns Hopkins University, Baltimore, MD) and encode proteins identical except at the extreme COOH termini (16, 26). Constructs encoding green fluorescent protein (GFP), β1-GFP fusion proteins, and a glutathione S-transferase (GST)-β1 fusion protein (comprising residues 175-200 of chicken β1) were described previously (15), as were plasmids encoding GST-Slo1VEDEC, GST-Slo1EMVYR, and GST-Slo1QEERL (17). Plasmids encoding full-length nephrin were obtained from Dr. Gerd Walz (Universitätsklinikum, Freiburg, Germany). Plasmids encoding GST-nephrin Ig-like domains 1-2 (P27–V234), 3-4 (P242–I434), 5-6 (P440–S635), and 7-8 (P740–S939), as well as GST-nephrin fibronectin type III domain (P941–T1035), GST-nephrin-CT1 (N1077–S1159), and GST-nephrin-CT2 (R1160–V1241) were generated by polymerase chain reaction from full-length nephrin and cloned into pGEX-6P-1 expression vector (GE Healthcare, Slough, UK). A construct encoding NH2-terminal GFP-tagged truncated nephrin protein (GFP-nephrin-CT2) was generated by subcloning the COOH-terminal fragment-encoding residues (R1160–V1241) into NT-GFP-TOPO vector (Invitrogen). The fidelity of all constructs was confirmed by sequencing. We obtained siRNAs directed against mouse nephrin from Santa Cruz Biotechnology (Santa Cruz, CA). We obtained a negative control siRNA composed of a scrambled sequence from the same vendor.
Cell culture and transfection.
HEK-293T (human embryonic kidney) cells were grown in Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO) containing 10% heat-inactivated fetal bovine serum at 37°C in a 5% CO2 incubator. These cells were transiently transfected using Lipofectamine-2000 (Invitrogen) in serum-reduced medium (Opti-MEM; Invitrogen) following the manufacturer's instructions. The DNA concentration in the transfection medium was 1 μg/ml of each plasmid. Cells were used for physiology or biochemistry 24–48 h after transfection. A podocyte cell line was obtained from Dr. Peter Mundel (Mount Sinai School of Medicine, New York, NY). It was maintained in RPMI 1640 medium (GIBCO) supplemented with 10% fetal bovine serum and 100 U/ml penicillin-streptomycin, with or without recombinant mouse γ-interferon (Sigma), in humidified 5% CO2 incubators. Podocytes were propagated on collagen I-coated plates at 33°C in the presence of recombinant mouse γ-interferon (10 U/ml). Removal of γ-interferon and a temperature switch to 37°C inactivated the SV40T antigen and induced podocytes to differentiate, a process that was complete in 2 wk. In experiments using siRNA, differentiated podocytes were transiently transfected in 6- (for biochemistry) or 24-well plates (for electrophysiology) with siRNA targeting nephrin, or control siRNA, using Oligofectamine (Invitrogen) in Opti-MEM medium following the manufacturer's instructions. To do this, Oligofectamine was combined with Opti-MEM for 5 min before being combined with a solution containing 20 μM siRNA in Opti-MEM. This was left to form complexes for 20 min, and a portion of this complex was then added to the wells in a final concentration of 1:5 relative to the amount of medium in the wells. After 4 h, a saturating volume of growth medium containing 30% fetal bovine serum concentration was added to the transfection mixture to stop the reaction. Cells were maintained in this medium and used 48–72 h after transfection.
Electrophysiology.
Whole cell recordings were made from HEK-293T cells as described previously (15). Plasmids encoding GFP were cotransfected with other constructs to allow identification of transfected cells in the recording chamber. The bathing solution contained (in mM) 150 NaCl, 0.08 KCl, 0.8 MgCl2, 5.4 CaCl2, 10 glucose, and 10 HEPES, and the pH was adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 145 NaCl, 2 KCl, 6.2 MgCl2, 10 HEPES, and 5.0 H-EDTA, pH 7.2. The free Ca2+ concentration in this solution was titrated to a concentration of 5 μM by addition of CaCl2 using an Orion 97-20 calcium electrode (Thermo Fisher Scientific, Waltham, MA) calibrated using solution standards obtained from World Precision Instruments (Sarasota, FL). HEK-293T cells do not express endogenous voltage-activated Ca2+ currents, and these ionic conditions were chosen to provide sufficient intracellular Ca2+ for activation of BKCa channels by depolarizing step pulses while at the same time keeping the resulting macroscopic currents sufficiently small to avoid saturation of the patch-clamp amplifier or significant series resistance errors (15). In the absence of Ca2+ in the pipette, no whole cell currents were seen. For podocytes, the bath solution contained 150 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 5.4 mM CaCl2, and 10 mM HEPES, pH 7.4. Pipette solutions contained 10 mM NaCl, 125 mM KCl, 6.2 mM MgCl2, 10 mM HEPES, pH 7.2, and 5 μM free Ca2+ buffered with 10 mM H-EDTA, as determined with the calcium electrode. Voltage-activated whole cell currents in podocytes were not detectable with those ionic gradients when recording pipettes contained no added CaCl2 and 10 mM EGTA (data not shown). The recording electrodes had resistances of 3–4 MΩ, and it was possible to compensate up to 85% of this without introducing oscillations into the current output of the patch-clamp amplifier (Axopatch 1D; Axon Instruments). In podocytes and HEK-293T cells, whole cell currents were evoked by a series of eight 450-ms depolarizing steps (from −25 to +80 mV in 15-mV increments) from a holding potential of −60 mV.
Cell-surface biotinylation assays.
Cell-surface biotinylation assays were carried out as described in detail previously (15, 16, 17). Briefly, intact cells were treated with a membrane-impermeable biotinylation reagent, sulfo-N-hydroxy-succinimidobiotin (1 mg/ml in PBS buffer; Pierce Biotechnology, Rockford, IL) for 1 h on ice with gentle shaking. The reaction was stopped, cells were lysed, and biotinylated proteins from the cell surface were recovered from lysates by incubation with immobilized streptavidin-agarose beads (Pierce Biotechnology). A sample of the initial cell lysate also was retained for analysis of total proteins. These samples were separated on SDS-PAGE, and proteins were quantified by immunoblot analysis. Protein bands in immunoblots were quantified by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD). For Slo1, we analyzed the most intense band associated with Slo1 monomers (∼135 kDa). Higher molecular mass bands represent multimers of Slo1 (48). These and all other biochemical experiments were repeated at least three times.
Coimmunoprecipitation and immunoblot analysis.
For coimmunoprecipitation, NH2-terminal Myc-tagged Slo1VEDEC, Slo1QEERL, and Slo1EMVYR were expressed in HEK-293T cells. Cells were lysed in 50 mM Tris-Cl, pH 7.6, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 2 mM EDTA, 1 mM PMSF, and protease inhibitor mixture (Sigma). Cell extracts (500–700 μg of protein) were incubated in the presence of primary antibodies anti-Myc (Cell Signaling) or IgG (1–2 μg) for 4 h at 4°C, followed by the addition of 20 μl of protein A/G-agarose (Santa Cruz Biotechnology) for 12 h. Pellets were washed four times, boiled for 5 min in SDS sample buffer, and subjected to SDS-PAGE on 10% gels. Cell-extracted protein (50–100 μg) was used as control in each experiment. Blots were blocked with 5% nonfat dry milk dissolved in TBST buffer (10 mM Tris, 150 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature, washed three times with TBST, incubated with the primary antibody overnight at 4°C, and washed again with TBST, and the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The proteins were visualized using a chemiluminescent substrate (SuperSignal West Pico; Pierce Biotechnology). Mouse kidney and brain were lysed, and the soluble cell extracts (300 μg of protein) were incubated in the presence of primary antibodies anti-Slo1VEDEC (16), anti-β4 (Alomone, Jerusalem, Israel), or IgG (1–2 μg) for 4 h at 4°C, followed by the addition of 20 μl of protein A/G-agarose for 12 h at 4°C with gentle rotation. Pellets were washed and boiled in sample buffer, separated on 10% SDS-PAGE, and analyzed by immunoblot as described above. Anti-nephrin and anti-NEPH1 used in immunoblot assays were obtained from Santa Cruz Biotechnology.
GST pull-down assay.
GST and GST-nephrin fusion proteins composed of nephrin residues P27–V234, P242–I434, P440–S635, P740–S939, P941–T1035, N1077–S1159, and R1160–V1241, as well as GST-Slo1VEDEC, GST-Slo1QEERL, and GST-Slo1EMVYR fusion proteins (17), were expressed and extracted from E. coli strain BL21, and 100–200 μg of each were bound to glutathione-Sepharose 4B beads according to the manufacturer's instructions (Amersham Biosciences). Differentiated podocytes were lysed, and the soluble cell extracts (300–500 μg of protein) were added to the beads and incubated overnight at 4°C with gentle rotation. Beads were washed three times with PBS containing 0.1–0.5% Triton X-100 (PBST) before the bound proteins were eluted with glutathione elution buffer. Eluates were incubated in sample buffer and separated on 10% SDS-PAGE gels, which is optimal for analysis of Slo1 monomers under reducing conditions. A sample of lysate was used to visualize electrophoretic mobility of the interacting proteins and was labeled as “input” in Figs. 1 and 7. It is not intended for quantification of the amounts of protein present.
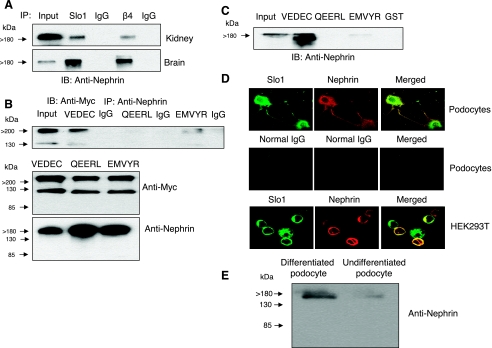
Fig. 1.
Nephrin binds to large-conductance Ca2+-activated K+ (BKCa) channel subunits. A: coimmunoprecipitation of nephrin observed by immunoblot analysis after immunoprecipitation using antibodies against Slo1 or the β4-subunit of BKCa channels from mouse kidney or mouse brain, as indicated. The “input” lane contained a diluted sample of cell extract used to illustrate electrophoretic mobility; it was not designed for quantification of signal intensity. B: coimmunoprecipitation of Slo1 observed by immunoblot analysis after immunoprecipitation with an antibody against nephrin (top). This procedure was carried out on HEK-293T cells transiently expressing Myc-tagged isoforms Slo1VEDEC, Slo1QEERL, or Slo1EMVYR, as indicated, and channels were detected using anti-Myc. Note that a strong signal was only detected from cells expressing the Slo1VEDEC isoform. Interaction with Slo1EMVYR was detectable but much weaker. IB analysis shows that the various Slo1 variants (middle) and nephrin (bottom) were expressed at comparable levels throughout the assay. C: glutathione S-transferase (GST) pull-down assay showing that a GST fusion protein containing the COOH-terminal sequences of Slo1VEDEC could bind to nephrin, whereas GST and a GST fusion protein containing the COOH-terminal sequences of Slo1QEERL did not. The interaction with Slo1EMVYR with nephrin was very weak. D: confocal microscopy showing colocalization of endogenous Slo1 and nephrin in differentiated cells of a podocyte cell line (top) and in HEK-293T cells transiently expressing nephrin and Slo1VEDEC (bottom). Middle, control images from podocytes in which the primary antibody was a species-appropriate IgG. E: immunoblot using the same commercial antibody against nephrin on extracts of differentiated and undifferentiated cells from a podocyte cell line. IB, immunoblot; IP, immunoprecipitation.
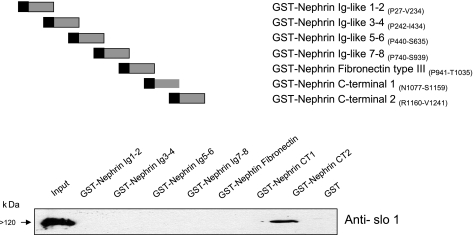
Fig. 7.
Evidence that the distal COOH-terminal portion of nephrin binds to Slo1VEDEC. Composition of a series GST-fusion proteins is indicated above a representative GST pull-down assay showing binding of the nephrin-CT2 construct (composed of residues R1160–V1241) to Slo1VEDEC. The assay was carried out on lysates of HEK-293T cells expressing this Slo1 splice variant.
Confocal microscopy.
For immunofluorescent labeling, HEK-293T cells were transfected with Myc-tagged Slo1VEDEC, GFP-nephrin-CT2, or full-length nephrin. These cells, or differentiated podocytes, were fixed in 4% paraformaldehyde, blocked, permeabilized in PBST, and exposed to the anti-mSlo1 (NeuroMAB, Davis, CA) and anti-nephrin (Santa Cruz Biotechnology). To examine the role of nephrin in surface expression of BKCa channels, HEK-293T cells were transiently transfected with NH2-terminal Myc-tagged Slo1VEDEC or Slo1QEERL constructs, either alone or together with constructs encoding full-length nephrin as described above. Cells were subsequently exposed to fluorescein-conjugated goat anti-Myc (1:500; Abcam, Cambridge, MA) in Opti-MEM medium for 1 h at 37° to label surface Slo1 channels. Cells were then washed in PBS, fixed by 30 min of exposure to 4% paraformaldehyde in PBS, rinsed in PBS, blocked with 10% normal goat serum, and then permeabilized in PBS containing 0.5% Triton X-100. They were then incubated with mouse anti-Myc antibody (1:1,000; antibody 9B11) for 1 h and exposed to Alexa568-conjugated anti-mouse IgG (1:1,000; Molecular Probes) for 1 h to label intracellular Slo1 channels. The cells were then rinsed in PBS and mounted using Vectashield (Vector Laboratories, Burlingame, CA). Control samples were treated with species-appropriate IgG instead of primary antibody. All images were collected on an Olympus FV-1,000 inverted stage confocal microscope using a Plan Apo N ×60 1.42-NA oil-immersion objective. Green fluorescence (from FITC) was evoked using an excitation wavelength of 495 nm while monitoring emission at 519 nm. Red fluorescence (from Alexa568) was evoked by excitation at 580 nm, and emission was monitored at 620 nm.
Statistics.
All quantitative data are means ± SE. Electrophysiological data were compiled from 9–25 cells in each group. Data were analyzed using Student's unpaired t-test when each group had its own control or one-way ANOVA, followed by post hoc analysis using Tukey's honest significant difference test for unequal sample size when multiple comparisons were made. Analyses were carried out using Statistica software (Statsoft, Tulsa, OK). Throughout, P < 0.05 was regarded as significant. Fitting of conductance-voltage curves and the rising phase of macroscopic currents was done by nonlinear least squares using Origin version 7.0 software (Northhampton, MA) as described in detail previously (15).
RESULTS
Nephrin emerged in a yeast two-hybrid screen of a neuronal (chick ciliary ganglion) transcriptome that was probed with a bait derived from the COOH-terminal domain (amino acids 175–200) of the chicken BKCa channel β1-subunit (15). Because yeast two-hybrid screens can yield false positives, we confirmed that nephrin binds to Slo1 channel subunits by several independent experimental approaches. Initially, we observed that nephrin could be detected in immunoprecipitates of mouse kidney and mouse brain that were prepared using commercially available antibodies against either Slo1 or the β4-subunits of BKCa channels (Fig. 1A). We chose to use anti-β4 for this experiment because nephrin is a podocyte protein, and β4-subunits are expressed in both podocytes (29) and brain (1). This result suggests that nephrin can interact with endogenously expressed BKCa complexes in multiple tissues. However, the Slo1 antibody used in those experiments does not discriminate between different Slo1 COOH-terminal splice variants. Therefore, we carried out coimmunoprecipitation experiments in HEK-293T cells transiently overexpressing nephrin together with one of three different Slo1 splice variants: Slo1VEDEC, Slo1QEERL, or Slo1EMVYR. It was previously reported that these Slo1 isoforms diverge at the extreme COOH terminus and exhibit markedly different patterns of intracellular trafficking (15, 16, 26). These expression constructs encode full-length Slo1 channels with an NH2-terminal (ectofacial) Myc tag, which allows us to use the same antibody to detect the different splice variants in a variety of experimental conditions. In these experiments, immunoprecipitation was carried out with anti-nephrin, and Slo1 interactions were detected by probing the resulting samples with an antibody against the Myc tags. We obtained a much more intense signal from HEK-293T cells expressing Slo1VEDEC channels than from cells expressing either Slo1QEERL (which could not be detected in immunoprecipitates) or Slo1EMVYR (which was only present at trace levels) (Fig. 1B, top). Together with the data on native tissues, this result shows that Slo1-nephrin coimmunoprecipitation can be detected regardless of which protein is used for the initial precipitation, and it can be seen with multiple antibodies. In control experiments we observed that the Myc-tagged Slo1 channels and nephrin were expressed at comparable levels in the HEK-293T cells (Fig. 1B, bottom), and therefore, the differences in the amount of channel protein that coimmunoprecipitated with anti-nephrin probably reflect differential affinity of various Slo1 isoforms for nephrin. This was addressed more directly by means of GST pull-down assays. To perform these assays, we prepared a series of GST-Slo1 fusion proteins that contained the unique COOH-terminal portions of Slo1VEDEC, Slo1QEERL, and Slo1EMVYR, as described elsewhere (17), and used them to probe lysates of HEK-293T cells overexpressing nephrin. We observed that the GST-Slo1VEDEC fusion protein was able to pull nephrin out of the lysates, whereas GST, GST-Slo1QEERL, and GST-Slo1EMVYR were ineffective (Fig. 1C). Thus the Slo1-nephrin interaction appears to be isoform specific. In addition, we observed using confocal microscopy that endogenous Slo1 channels and nephrin were extensively colocalized in differentiated cells of an immortalized podocyte cell line (Fig. 1D, top), as well as in HEK-293T cells coexpressing nephrin and Slo1VEDEC (Fig. 1D, bottom). The characteristics of the nephrin antibody are shown by immunoblot analysis of differentiated and undifferentiated cells of the podocyte cell line (Fig. 1E).
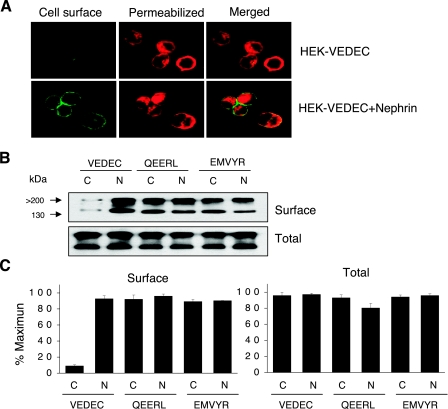
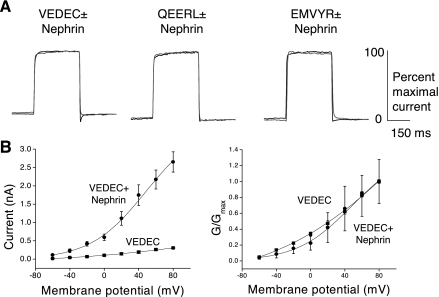
Is the nephrin-Slo1 interaction functionally significant? To address this question, we transiently expressed Slo1 channels in HEK-293T cells in the presence or absence of nephrin and examined the resulting distribution of Slo1 channels using confocal microscopy (Fig. 2A) and cell surface biotinylation assays (Fig. 2, B and C). We previously established that nontransfected HEK-293T cells do not express detectable levels of endogenous nephrin (data not shown). Before the use of confocal microscopy, Slo1 channels were labeled with a fluorescein-conjugated anti-Myc antibody. The cells were then fixed, permeabilized, and labeled with a nonconjugated anti-Myc raised in a different species, which allowed us to obtain a distinct fluorescence signal from tagged Slo1 channels in intracellular pools. Note that we used identical processing procedures, Myc antibodies, and laser excitation intensities to determine the distribution of the Slo1 isoforms. As with our previous studies (15, 16, 17), we observed that Slo1VEDEC channels expressed by themselves in HEK-293T cells were almost exclusively in intracellular locations (red fluorescence) and exhibited comparatively low constitutive expression on the plasma membrane (green fluorescence). However, enhanced surface expression was observed in HEK-293T cells transiently coexpressing nephrin with Slo1VEDEC (Fig. 2A). Nephrin coexpression had no obvious effect on the distribution of fluorescence in HEK-293T cells expressing either the Slo1QEERL or Slo1EMVYR isoforms (data not shown).
Fig. 2.
Coexpression of nephrin with Slo1VEDEC increases steady-state expression on the surface of HEK-293T cells. A: confocal immunofluorescence using antibodies against the Myc tags of transiently expressed Slo1VEDEC channels. Cell surface channels in cells expressing Myc-tagged Slo1VEDEC were labeled with an FITC-conjugated goat anti-Myc applied to intact cells (green). After fixation and permeabilization, intracellular channels were stained using a mouse anti-Myc revealed using Alexa568-conjugated anti-mouse IgG (red). Identical laser excitation intensities and detection sensitivities were used for image collection from all of these samples. B: representative cell surface biotinylation assay shows that nephrin coexpression caused marked increase in surface expression of Slo1VEDEC but had no effect on Slo1QEERL or Slo1EMVYR, which had high levels of constitutive surface expression even in the absence of nephrin. C: densitometric quantification of 3 repetitions of the experiment shown in B. Data are means ± SE of relative Slo1 expression in the absence (C) or presence (N) of nephrin.
A similar pattern was observed using cell surface biotinylation assays (Fig. 2B). We observed that biotinylated Slo1VEDEC channels on the cell surface represent a small fraction of the total signal, especially compared with what is observed in HEK-293T cells expressing either Slo1QEERL or Slo1EMVYR. However, coexpression of nephrin caused a large increase in the relative surface expression of Slo1VEDEC such that its steady-state expression on the surface was indistinguishable from that of the other isoforms. Coexpression of nephrin had no discernible effect on the surface expression of Slo1QEERL or Slo1EMVYR. These were consistent observations, as can be seen by the results of densitometric quantification of three repetitions of this experiment (Fig. 2C).
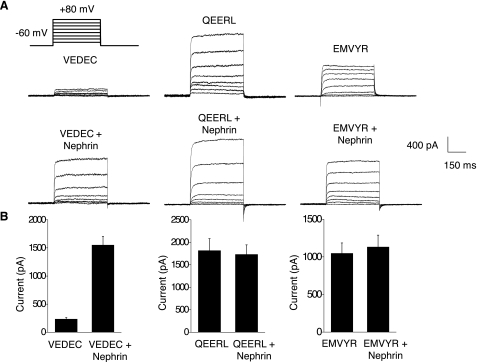
Nephrin-induced stimulation of Slo1VEDEC surface expression could also be observed using whole cell recordings from HEK-293T cells. In these experiments, we examined whole cell currents using methods described previously (15, 16). The recording pipettes contained 5 μM free Ca2+ in a solution buffered with H-EDTA to allow activation of BKCa channels by step pulses from a holding potential of −60 mV, and the concentrations of permeant ions were reduced to keep current amplitudes in a range that did not saturate our recording amplifier. Currents evoked by voltage steps to +80 mV were used for quantification of surface expression. We have previously shown that these currents are not detectable in nontransfected HEK 293T cells or when cells overexpressing Slo1 are examined using recording electrodes filled with Ca2+-free solutions and EGTA buffer. We observed that HEK-293T cells expressing Slo1VEDEC had much smaller macroscopic outward currents than cells expressing Slo1QEERL or Slo1EMVYR (Fig. 3A, top), consistent with previous reports (16, 26). However, these differences were not seen in cells coexpressing nephrin with the channel constructs, which can be attributed to a fivefold increase in mean currents observed in cells expressing Slo1VEDEC. Coexpression of nephrin had no effect on the mean current amplitudes in cells expressing Slo1QEERL or Slo1EMVYR (Fig. 3B). Moreover, nephrin coexpression had no discernible effect on the activation kinetics or voltage dependence measured in HEK-293T cells expressing any of the Slo1 splice variants (Fig. 4). This can be seen when amplitude-normalized traces are superimposed (Fig. 4A), and data from groups of cells are summarized in Table 1. These were obtained by fitting the rising phase of macroscopic currents with a single exponential, as described previously (15), and comparing the resulting time constants. Coexpression of nephrin had no significant effect on the mean time constant for any of the three Slo1 splice variants. Conductance-voltage curves show that nephrin had no effect on the voltage-dependence of Slo1VEDEC activation (Fig. 4B). Nephrin coexpression caused an increase in mean current at all membrane potentials in cells expressing Slo1VEDEC. Collectively, these data suggest that nephrin can stimulate the steady-state surface expression of Slo1VEDEC channels on the cell surface.
Fig. 3.
Effects of nephrin coexpression on whole cell currents recorded from HEK-293T cells expressing various Slo1 splice variants. Recording electrodes contained 5 μM free Ca2+ to allow for activation of BKCa channels by families of depolarizing voltage steps (shown in A) from a holding potential of −60 mV. A: representative traces illustrating a marked increase of currents in cells coexpressing nephrin with Slo1VEDEC. Nephrin had no effect in cells expressing the other isoforms, which exhibited large currents even in the absence nephrin. B: quantification of results from many cells. Data are means ± SE (n > 15 cells in each group). The only significant effect of nephrin was in cells expressing Slo1VEDEC (P < 0.05).
Fig. 4.
Nephrin does not substantially alter gating properties of BKCa channels. A: representative traces of whole cell currents evoked by depolarizing steps to +80 mV from a holding potential of −60 mV in HEK-293T cells expressing different Slo1 isoforms, as indicated, in the absence (solid traces) or presence (shaded traces) of nephrin. Amplitudes of traces are scaled to allow comparison of their time courses. Recording electrodes contained 5 μM Ca2+. B: activation curves constructed from HEK-293T cells expressing Slo1VEDEC in the presence and absence of nephrin. Nephrin markedly increased mean current at all membrane potentials but did not produce any substantial effects on the voltage dependence of activation.
Table 1.
Nephrin coexpression and voltage dependence in HEK-293T cells expressing Slo1 splice variants
| Splice Variant |
Activation Time Constant, ms |
|
|---|---|---|
| Control | Nephrin | |
| Slo1VEDEC | 6.41±0.31 | 8.60±0.84 |
| Slo1QEERL | 7.63±0.46 | 7.62±0.56 |
| Slo1EMVYR | 6.25±0.54 | 6.75±0.51 |
Coexpression of nephrin did not affect the activation kinetics of currents evoked by a depolarizing step pulse in HEK-293T cells expressing various Slo1 splice variants. Time constants were calculated from single-exponential fits to the rising phase of currents evoked by a depolarizing step to +80 mV from a holding potential of −60 mV. Examples of the types of current traces used for these analyses are shown in Fig. 4A. Data are means ± SE of the time constant derived from 10 cells in each group. None of these data are statistically significant at the 0.05 level as determined using Student's unpaired t-test.
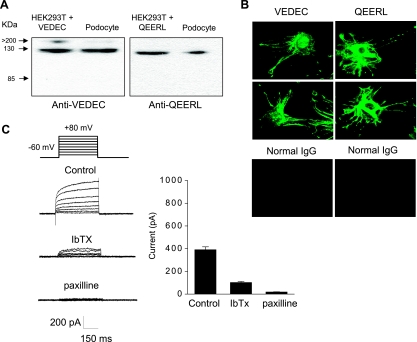
That hypothesis predicts that knocking down endogenous nephrin expression should reduce surface expression of Slo1VEDEC channels in cells that endogenously express that isoform. Differentiated cells derived from a conditionally immortalized podocyte cell line (32) are a useful model to test that prediction, since we observed that the Slo1VEDEC and Slo1QEERL isoforms were expressed in a differentiated podocyte cell line by using immunoblot analysis (Fig. 5A) and confocal microscopy (Fig. 5B). The isoform-specific antibodies used for this analysis were described previously (16). Antibodies selective for Slo1EMVYR are not available. In addition, we observed that voltage-evoked outward currents with the characteristics of Slo1 channels could be quantified in whole cell recordings from differentiated podocytes. In these experiments, the recording pipettes contained 5 μM free Ca2+ in a solution buffered with H-EDTA, K+ gradients were physiological, and the resulting voltage-evoked outward currents were partially inhibited by iberiotoxin and completely blocked by paxilline (Fig. 5C). These outward currents could not be detected when the recordings were made with electrodes containing Ca2+-free solutions (data not shown). These data indicate that all of the voltage-evoked outward current under our experimental conditions is carried by Slo1 channels. The activation kinetics of these macroscopic currents are quite slow, at least compared with what is typically seen in neuronal preparations (2) or in HEK-293T cells expressing Slo1 variants recorded under similar conditions (Fig. 4), and this observation is consistent with earlier observations made in inside-out patches (29). The slow activation kinetics and the incomplete blockade by iberiotoxin may reflect the presence of the β4-subunit in podocytes (1, 29, 49). In any case, it is clear that Slo1VEDEC and nephrin are both endogenously expressed in podocytes, which are therefore a useful cell line for testing the functional significance of the nephrin interaction.
Fig. 5.
Differentiated cells from a podocyte cell line express functional BKCa channels. A: immunoblot analysis using isoform-specific antibodies showing expression of Slo1VEDEC and Slo1QEERL in differentiated cells of a podocyte cell line. Adjacent lines are extracts of HEK-293T cells expressing the corresponding Slo1 isoform. The antibodies used in this analysis were described previously and do not cross-react (16). B: confocal analysis using the same antibodies also shows that both isoforms were expressed in differentiated podocytes. Note signal in paranuclear intracellular compartments and in foot processes. Control images are from cells in which rabbit IgG was used as primary antibody. C: endogenous BKCa currents in podocytes revealed by whole cell recordings using microelectrodes containing 5 μM free Ca2+. Note very slow activation kinetics and partial blockade after treatment with 500 nM iberiotoxin (IbTX) and complete blockade after 1 μM paxilline. Bar graph at right shows means ± SE for repetitions of this experiment (n > 10 cells per group). Mean currents in all 3 groups are significantly (P < 0.05) different from each other, as determined using one-way ANOVA and Tukey's honest significant difference test for unequal sample size.
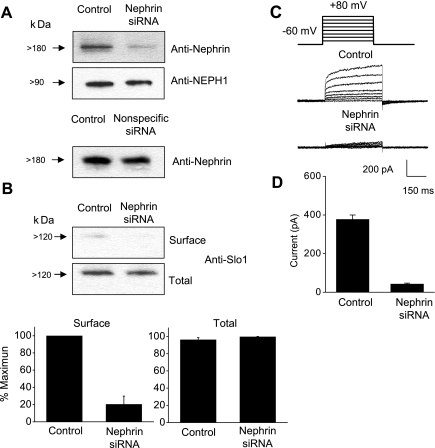
To test the hypothesis more directly, we used siRNA to knock down nephrin expression in differentiated podocytes. The effectiveness of the knockdown strategy was ascertained using immunoblot analysis, which showed that nephrin expression was substantially reduced in podocytes treated with nephrin siRNA but not in cells treated with control siRNA (Fig. 6A). Total expression of a closely related protein, NEPH1, was not altered by these procedures. We observed using cell surface biotinylation assays that surface expression of Slo1 was markedly attenuated in podocytes with reduced nephrin expression (Fig. 6B), and macroscopic outward currents were reduced by an order of magnitude (Fig. 6, C and D). These data therefore demonstrate that nephrin is required for the normal steady-state expression of functional BKCa channels and Slo1 subunits on the surface of differentiated cells in a conditionally immortalized cell line derived from podocytes.
Fig. 6.
Nephrin is required for normal surface expression of BKCa channels in differentiated cells of a podocyte cell line. A: immunoblot analysis showing that treatment with a small interfering (si)RNA directed against nephrin markedly reduced expression of nephrin but had no effect on total expression of NEPH1 in podocytes. A control siRNA had no effect. B: application of nephrin siRNA reduced surface expression of Slo1 channels as determined by cell surface biotinylation assay. Representative blots are shown at top, and densitometric analyses of 3 repetitions of this experiment are shown at bottom. C: representative traces illustrating that nephrin siRNA caused marked reduction in BKCa currents measured using whole cell recordings as Fig. 5. D: means ± SE of BKCa currents (n > 15 cells per group). The means are significantly different (P < 0.05), as determined using Student's unpaired t-test.
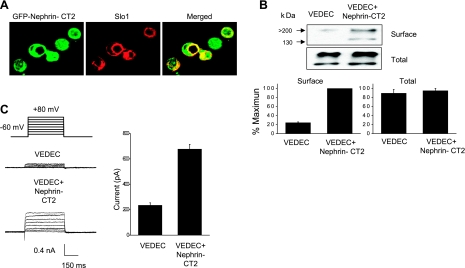
One question that emerged from these results pertains to the portions of nephrin that bind to the COOH-terminal domains of Slo1VEDEC and that stimulate its expression on the cell surface. Nephrin is a 180-kDa transmembrane protein that has structural features in common with many other adhesion molecules (14). The extracellular portion contains eight Ig-like motifs and one type III-fibronectin domain that are involved in cell adhesion and formation of the glomerular slit diaphragm (39), whereas the cytoplasmic COOH terminus appears to play a role in cell signaling (10). One could be concerned that the stickiness of the extracellular domains in some way caused an artifactual alteration in Slo1VEDEC trafficking. To address this, we prepared a series of GST fusion-proteins that comprise portions of nephrin, including each of the extracellular Ig-like and fibronectin domains, as well as two portions of the COOH terminus, and we used these in pull-down assays to determine and identify regions that bind to Slo1 (Fig. 7). We observed that only the fusion protein containing the most distal COOH-terminal portions of nephrin (nephrin-CT2, composed of R1160–V1241) was able to bind to Slo1 in this assay. Interestingly, this relatively small portion of nephrin was biologically active by itself, and its coexpression was sufficient to cause a significant increase in Slo1 on the cell surface (Fig. 8), although it was not as effective as full-length nephrin (data not shown). Specifically, we observed that a GFP-nephrin-CT2 fusion protein colocalized with Slo1VEDEC when they were coexpressed in HEK-293T cells (Fig. 8A). The signal appeared especially concentrated in paranuclear regions. Nephrin-CT2 coexpression in HEK-293T cells caused an increase in cell surface expression of Slo1VEDEC as measured using cell surface biotinylation assays (Fig. 8B) and whole cell recordings (Fig. 8C).
Fig. 8.
Coexpression of a green fluorescent protein (GFP) fusion protein containing only the distal COOH-terminal residues of nephrin (GFP-nephrin-CT2) causes an increase in surface expression of Slo1VEDEC in HEK-293T cells but is not as effective as full-length nephrin. A: colocalization of the GFP-nephrin-CT2 with Myc-tagged Slo1VEDEC (red) as revealed using confocal microscopy. Note expression of both proteins on the cell surface and accumulation in perinuclear regions. B: coexpression of GFP-nephrin-CT2 caused a marked increase in steady-state surface expression of Slo1VEDEC as determined using cell surface biotinylation assays. Representative blots (top) and quantitative densitometric analysis of 3 repetitions of this experiment (bottom) are shown. Control cells expressed GFP. C: coexpression of GFP-nephrin-CT2 caused a statistically significant (P < 0.05) increase in mean whole cell currents in cells expressing Slo1VEDEC. Control cells coexpressed GFP.
DISCUSSION
In the present study we have shown that one of the COOH-terminal variants of BKCa channels encoded by the Slo1 gene binds to nephrin, a scaffolding and adhesion molecule expressed in podocytes and the brain. This interaction, which occurs at the COOH termini of both proteins, promotes the steady-state surface expression the Slo1VEDEC isoform in a podocyte cell line and in a heterologous expression system. However, coexpression of nephrin does not appear to have significant effects on the gating properties of BKCa channels, and nephrin does not appear to bind to or alter the functional properties of the Slo1QEERL variant. However, nephrin interacts with the β4-subunit of BKCa channels, which is expressed in podocytes as well as in the nervous system (29, 49).
Nephrin appears to have multiple functions in podocytes. The extracellular portions are composed of immunoglobulin- and fibronectin-like domains that contribute to the slit diaphragm and that promote adhesive interactions between the podocyte foot processes and the underlying capillary basement membrane (34, 51). The intracellular domains are much smaller but provide a rich substrate for interactions with many other proteins, including podocin (43), ZO-1 (20), P-cadherin (40), Neph1 (23), and synaptopodin (32), as well as adaptor proteins such as CD2-associated protein and Nck that tie the nephrin complex to the underlying cytoskeleton (13). Importantly, nephrin interactions with proteins such as podocin and CD2AP (9) cause recruitment of signaling proteins such as phosphoinositide 3-OH kinase (PI3K) to the plasma membrane, resulting in stimulation of PI3K-dependent Akt signaling in podocytes (10). Nephrin also has been reported to interact with TRPC6, a Ca2+-permeable stretch-sensitive cation channel known to play a role in regulation of glomerular filtration (37).
Because of these interactions, and based on precedents derived from other adhesion molecules (25), nephrin has been suggested to play a role in outside-in signaling, a process by which mechanical stimuli transduced through the nephrin ectodomains lead to changes in the status of cytosolic and plasma membrane proteins (46). Nephrin interacts with subjacent actin filaments of podocyte foot processes via adapter proteins such as CD2AP (44) and Nck (13). In this way, mechanical stimuli delivered to nephrin ectodomains could be transduced to underlying actin filaments. The gating of BKCa channels in podocytes and several other cell types is stretch sensitive (29), a process that is mediated by interactions of the channels with actin-binding proteins (45) and possibly by direct interactions between Slo1 subunits and actin microfilaments (52). In addition, the ability of nephrin to organize a variety of signaling molecules could provide a mechanism to organize changes in BKCa gating in response hormones, cytokines, or second messengers. In this regard, we have previously demonstrated that PI3K and Akt, which can be recruited to nephrin cytosolic domains (10), are essential for regulated trafficking of BKCa to the plasma membrane in neurons (3, 22).
The physiological impact of BKCa activation is paradoxically likely to be an increase in net Ca2+ influx, at least in podocytes. These cells are electrically compact, so activation of a relatively small number of BKCa channels would cause marked cell hyperpolarization. One of the key sources of Ca2+ influx in podocytes is through activated TRPC6 channels (37). TRPC6 channels have an unusual and physiologically important biophysical property: their permeability to Ca2+ relative to Na+ (PNa/PCa) is highly voltage dependent. Thus, at more negative membrane potentials, they are permeable to Ca2+, but moderate depolarization causes them to exclude divalent cations due to a combination of pore blockade and a reduction in driving force (6). Because of this, active TRPC6 channels can be a direct source of steady Ca2+ influx, but only if there is a mechanism in place to limit the amount of depolarization that occurs as a result of their own activation. BKCa channels are ideally suited to counteract TRPC6-mediated depolarization because their gating is Ca2+ sensitive and because the presence of β4-subunits will confer a steeper voltage dependence of activation (47). Moreover, TRPC6 channels also bind to nephrin (37), suggesting the existence of a multichannel complex built around nephrin scaffolds in which a subset of BKCa channels provides positive feedback to Ca2+ influx through TRPC6. This arrangement could also provide a mechanism to couple the mechanosensitivity and/or chemosensitivity of BKCa channels to local changes in Ca2+ dynamics. This in turn could trigger changes in the structure of podocyte foot processes and their cytoskeletal elements (11) to allow filtration pathways to adapt to changes in hydrostatic pressure. Note that podocytes also express a splice variant of Slo1 that does not interact with nephrin, and these channels could be targeted to other portions of the plasma membrane in such a way as to allow them to be regulated independently of the ones that bind to nephrin.
The mechanism whereby nephrin controls BKCa trafficking in podocytes or other cell types is not known. However, nephrin was recently shown to be necessary for the translocation of an insulin-sensitive GLUT4 glucose transporter to the plasma membrane of podocytes (4). Those workers also noted that nephrin can bind to a vesicle protein synapotobrevin (also known as VAMP2), a so-called vesicle-associated soluble N-ethylmaleimide-sensitive factor attachment protein receptor (V-SNARE) that is essential for docking and fusion of exocytotic vesicles in a host of cell types. Therefore, it is possible that nephrin plays a more general role in regulation of vesicle trafficking in podocytes. One possibility is that interactions between synaptobrevin and nephrin allow the vesicle to dock close to the plasma membrane; in other words, nephrin could function analogously to a plasma membrane SNARE protein to regulate vesicle trafficking in cells where it is expressed. Although we cannot exclude this hypothesis, it is worth noting that a construct containing only the distal COOH-terminal residues of nephrin is able to cause a statistically significant increase in the surface expression of BKCa channels in HEK-293T cells. That construct does not contain transmembrane domains, suggesting that nephrin does not need to bind to the plasma membrane to regulate vesicle trafficking. This suggests nephrin is not simply functioning as a membrane docking protein.
What is the role of nephrin in neuronal function? Our initial yeast two-hybrid screen was carried out on a library representing a neuronal transcriptome, and we have confirmed earlier reports that nephrin is expressed in neurons (36), as are Slo1VEDEC channels (16). Therefore, it is reasonable to propose that nephrin plays a role in trafficking of neuronal Slo1 channels, and possibly other membrane proteins, similar to the one it plays in podocytes. In this regard, nephrin can stimulate steady-state expression of Slo1VEDEC on the surface of HEK-293T cells, which do not endogenously express other podocyte proteins, suggesting that nephrin can promote trafficking to the plasma membrane in a variety of cellular contexts. Moreover, it is notable that ∼10% of Finnish patients bearing nephrin mutations also exhibit congenital neurological symptoms, especially ataxia, athetosis, and hearing defects (19). In many cases, these problems did not resolve after kidney transplantation and did not appear related to the previous nephrotic symptoms. Although these neurological problems could reflect a role for nephrin in regulation of axon guidance or fasciculation (28), it is striking that mice lacking Slo1 proteins or functional BKCa channels also show marked ataxia and alterations in Purkinje neuron firing (42), as well as a progressive loss of hearing (41). This raises the possibility that some of the extrarenal effects of nephrin deficiencies could be related to abnormalities in the function of a subset of BKCa channels.
In summary, we have shown that one of the COOH-terminal splice variants of Slo1 expressed in podocytes binds to cytosolic domains of nephrin. These interactions regulate the steady-state expression of Slo1 proteins on the cell surface of podocytes and possibly in other cell types where nephrin or related molecules are expressed.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke Grant 1-R01-NS-32748.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β-subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JS, Dryer SE. BK-type KCa channels in two parasympathetic cell types: differences in kinetic properties and developmental expression. J Neurophysiol 84: 2767–2776, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Chae KS, Martin-Caraballo M, Anderson M, Dryer SE. Akt activation is necessary for growth factor-induced trafficking of functional KCa channels in developing parasympathetic neurons. J Neurophysiol 93: 1174–1182, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59: 673–682, 1988. [PubMed] [Google Scholar]
- 6.Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP. TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol 572: 359–377, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem 276: 41543–41546, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt JL, Pollak MR, Denker BM. Cultured podocytes establish a size-selective barrier regulated by specific signaling pathways and demonstrate synchronized barrier assembly in a calcium switch model of junction formation. J Am Soc Nephrol 16: 1593–1602, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Jalanko H, Patrakka J, Tryggvason K, Holmberg C. Genetic kidney diseases disclose the pathogenesis of proteinuria. Ann Med 33: 526–533, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol 97: 3508–3516, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience 146: 1652–1661, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EY, Ridgway LD, Dryer SE. Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding. Mol Pharmacol 72: 622–630, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kriz W, Kretzler M, Nagata M, Provoost AP, Shirato I, Uiker S, Sakai T, Lemley KV. A frequent pathway to glomerulosclerosis: deterioration of tuft architecture-podocyte damage-segmental sclerosis. Kidney Blood Press Res 19: 245–253, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Laakkonen H, Lönnqvist T, Uusimaa J, Qvist E, Valanne L, Nuutinen M, Ala-Houhala M, Majamaa K, Jalanko H, Holmberg C. Muscular dystonia and athetosis in six patients with congenital nephrotic syndrome of the Finnish type (NPHS1). Pediatr Nephrol 21: 182–189, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Lehtonen S, Lehtonen E, Kudlicka K, Holthöfer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol 165: 923–936, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenkkeri U, Männikkö M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestilä M, Tryggvason K. Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 64: 51–61, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lhuillier L, Dryer SE. Developmental regulation of neuronal KCa channels by TGFβ1: an essential role for PI3 kinase signaling and membrane insertion. J Neurophysiol 88: 954–964, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 112: 209–221, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res 100: 112–120, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619–647, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett 581: 1000–1008, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa Y, Komori T, Hisaoka T, Ueno H, Kitamura T, Senba E. Expression of mKirre in the developing sensory pathways: its close apposition to nephrin-expressing cells. Neuroscience 150: 880–886, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Morton MJ, Hutchinson K, Mathieson PW, Witherden IR, Saleem MA, Hunter M. Human podocytes possess a stretch-sensitive, Ca2+-activated K+ channel: potential implications for the control of glomerular filtration. J Am Soc Nephrol 15: 2981–2987, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci USA 102: 16102–16106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 192: 385–397, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Pätäri-Sampo A, Ihalmo P, Holthöfer H. Molecular basis of the glomerular filtration: nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med 38: 483–492, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Patrakka J, Tryggvason K. Nephrin—a unique structural and signaling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60: 423–433, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 96: 7962–7967, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestilä M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H. Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 157: 1905–1916, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rüttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Müller M, Köpschall I, Pfister M, Münkner S, Rohbock K, Pfaff I, Rüsch A, Ruth P, Knipper M. Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BK β1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA 101: 12922–12927, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA 101: 9474–9478, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol 159: 2303–2308, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J 20: 2588–2590, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B, Rothberg BS, Brenner R. Mechanism of β4 subunit modulation of BK channels. J Gen Physiol 127: 449–465, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang SX, Ikeda M, Guggino WB. The cytoplasmic tail of large conductance, voltage- and Ca2+-activated K+ (MaxiK) channel is necessary for its cell surface expression. J Biol Chem 278: 2713–2722, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, Ge P, Wang C, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, DiStefano PS, Curtis R. A novel nervous system beta subunit that down regulates human large conductance calcium-dependent potassium channels. J Neurosci 20: 3563–3570, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol 106: 26–31, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol Pharmacol 73: 359–368, 2008. [DOI] [PubMed] [Google Scholar]