Abstract
We previously demonstrated that several clinically utilized volatile anesthetics including sevoflurane protected against renal ischemia-reperfusion (IR) injury by reducing necrosis and inflammation in vivo. We also demonstrated that volatile anesthetics produced direct anti-necrotic and anti-inflammatory effects in cultured renal tubules via mechanisms involving the externalization of phosphatidylserine and subsequent release of transforming growth factor (TGF)-β1. In this study, we tested the hypothesis that volatile anesthetic-mediated renal protection requires TGF-β1 and SMAD3 signaling in vivo. We subjected TGF-β1+/+, TGF-β1+/−, SMAD3+/+, or SMAD3−/− mice to renal IR under anesthesia with pentobarbital sodium or with sevoflurane. Although TGF-β1+/+ and SMAD3+/+ mice were significantly protected against renal IR injury under sevoflurane anesthesia with reduced necrosis and inflammation, TGF-β1+/− mice and SMAD3−/− mice were not protected against renal IR with sevoflurane. Furthermore, a neutralizing TGF-β1 antibody blocked renal protection with sevoflurane in TGF-β1+/+ mice. Sevoflurane caused nuclear translocation of SMAD3 and reduced the TNF-α-induced nuclear translocation of NF-κB in primary cultures of proximal tubules from TGF-β1+/+ but not in TGF-β1+/− mice. Finally, sevoflurane protected against necrosis induced with hydrogen peroxide in primary cultures of proximal tubules from TGF-β1+/+ mice or SMAD3+/+ mice but not in proximal tubules from TGF-β1+/− or SMAD3−/− mice. Therefore, we demonstrate in this study that sevoflurane-mediated renal protection in vivo requires the TGF-β1→SMAD3 signaling pathway.
Keywords: acute renal failure, inflammation, necrosis, SMAD3
perioperative acute renal failure (ARF) is a frequent clinical complication after major surgical procedures (1, 10, 26). Development of perioperative ARF greatly increases mortality and is frequently complicated by other organ failures or sepsis (3, 25, 26). The mortality from ARF and subsequent multiorgan dysfunction is extremely high ranging from 50 to 90% according to some studies (2, 3, 25). Unfortunately, the mortality and morbidity rate from perioperative ARF has changed little over the past 50 years (10).
Volatile anesthetics are an essential part of perioperative medicine as virtually all patients subjected to general anesthesia will be anesthetized with these drugs. We demonstrated previously that volatile anesthetics provide renal protection against in vivo renal ischemia-reperfusion (IR) injury by reducing necrosis and inflammation after IR in rats as well as in mice (14, 16). We also demonstrated that the anti-necrotic and anti-inflammatory effects of volatile anesthetics can be demonstrated in cultures of proximal tubules in vitro and the mechanisms involved in protection include activation of the cytoprotective kinases ERK and Akt and the induction of HSP70 (12). We subsequently demonstrated that volatile anesthetics produce anti-necrotic and anti-inflammatory effects in renal tubules in vitro via externalization of plasma membrane phosphatidylserine and by releasing transforming growth factor (TGF)-β1, a cytokine with anti-necrotic and anti-inflammatory properties, in cultured renal proximal tubule cells (13).
In this study, we wanted to determine whether the sevoflurane-mediated renal protection via TGF-β1 signaling occurred in vivo by utilizing mice lacking the key signaling components of TGF-β1. We used mice significantly (less than 50%) deficient in TGF-β1 molecules (TGF-β1+/− mice). Mice completely lacking TGF-β1 (TGF-β1−/−) are not viable to adulthood. We also utilized mice lacking the SMAD3 transcription factor (SMAD3−/−), a key distal signaling component of TGF-β1 receptor ligation. We tested the hypothesis that a volatile anesthetic, sevoflurane, would not protect these mice deficient in TGF-β1 signaling against renal IR injury in vivo. Furthermore, we tested the hypothesis that primary cultures of proximal tubules from these TGF-β1 signaling-deficient mice (TGF-β1−/−, SMAD3−/−) would not be protected with sevoflurane against oxidative necrotic injury induced with hydrogen peroxide (H2O2) in vitro.
MATERIALS AND METHODS
Mice.
All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University (New York, NY). Breeder pairs of TGF-β1 heterozygous mice were obtained from Dr. T. Doetschman (University of Cincinnati, OH) to produce wild-type (+/+) and heterozygous (+/−) mice (24). These mice are mixed in genetic background (50% 129SV and 50% CF-1) and TGF-β1+/− mice synthesize less than 50% TGF-β1 compared with the TGF-β1+/+ mice. TGF-β1 knockout mice are not viable to adulthood. Exon 8 of the SMAD3 gene was disrupted in mice of background 129SV/EV × C57BL/6 by Yang et al. (30) and heterozygous breeding pairs were provided by Dr. C. Deng (Genetics of Development and Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD) to produce SMAD3+/+ and SMAD3−/− mice. The genotypes of mice were determined by PCR analysis on tail DNA obtained from 4-wk-old animals and all experiments were performed with littermates to ensure identical genetic backgrounds.
In vivo renal IR injury in mice.
We subjected mice to renal IR injury with techniques previously described (11, 16, 18). In brief, male mice (25–30 g) were anesthetized with intraperitoneal pentobarbital sodium (50 mg/kg body wt, or to effect) or with inhaled sevoflurane [∼1 minimum alveolar concentration (MAC): defined as the concentration of the anesthetic at the alveolus that is needed to prevent movement in 50% of subjects in response to a painful stimulus; 2.2%]. All experiments were performed on an electric heating pad under a warming light. Pentobarbital sodium-anesthetized mice were allowed to breathe room air spontaneously, whereas sevoflurane-anesthetized mice breathed spontaneously while receiving ∼1 MAC sevoflurane in room air. Body temperature was monitored with a rectal probe and maintained at 37°C. To anesthetize the mice with sevoflurane, they were placed in an airtight 2-liter chamber with inflow and outflow outlets (Braintree Scientific, Braintree, MA). The chamber temperature was maintained between 36 and 38°C. They were anesthetized initially to achieve immobility. One MAC sevoflurane was delivered in room air at 5 l/min using agent-specific Datex-Ohmeda vaporizer. The sevoflurane concentration was monitored by infrared analyzer sampling gas at the outflow hose. After complete general anesthesia was achieved, the animals were removed from the chamber during anesthesia and allowed to breathe identical anesthetic concentrations through a nose cone connected in parallel to the gas chamber. Each animal was subjected to midline laparotomy, right nephrectomy, and sham operation, or 30 min left renal ischemia during anesthesia. After 30 min of left renal ischemia, occlusion clips were removed, and the abdomen was closed in two layers. Each animal was returned to the chamber and allowed to breathe the identical anesthetic concentration spontaneously for an additional 3 h. Pentobarbital sodium-treated animals were returned to their cages to recover from anesthesia.
Neutralization of TGF-β1 in vivo.
TGF-β1+/+ mice were injected with 250 μg of monoclonal anti-TGF-β1 intravenously (MAB240, R&D Systems) 15 min before anesthesia with either sevoflurane or with pentobarbital sodium. To determine the effectiveness of TGF-β1 neutralizing antibody, plasma from TGF-β1 antibody-treated mice was assayed for total TGF-β1 with ELISA 24 h after anti-TGF-β1 antibody injection.
Assessment of renal function after IR injury.
Renal function was assessed by measurement of plasma creatinine 24 h after renal ischemia using the colorimetric method based on the Jaffe reaction (11, 16, 17).
Histologic examination to detect necrosis.
Morphologic assessment was performed by an experienced renal pathologist who was unaware of the treatment that each animal had received. A renal injury score grading scale of 0 to 4 was used to assess the degree of renal tubular necrosis in the outer medullary area after renal IR injury as described previously (27, 28).
TGF-β1 ELISA.
TGF-β1 levels from TGF-β1+/+ and +/− mouse plasma were detected using ELISA kits from Promega (Madison, WI) with appropriate acidification of samples for activation of latent TGF-β1.
Semiquantitative reverse transcriptase polymerase chain reaction for TGF-β1 and GAPDH.
Total RNA from mouse kidneys was extracted using TRIzol (Invitrogen, Carlsbad, CA) reagent and RNA concentrations were determined with spectrophotometric readings at 260 nm. Primers for mouse TGF-β1 were designed based on published GenBank sequences (Table 1) and to amplify a genomic region that spans one or two introns to eliminate the confounding effect of amplifying contaminating genomic DNA. RT-PCR was performed using the Access RT-PCR System (Promega) using AMV reverse transcriptase and subsequent PCR using Tfl DNA polymerase (12, 14). The PCR cycle number was optimized to yield linear increases in the densitometric measurements of resulting bands with increasing cycles of PCR. The starting amount of RNA was also optimized to yield linear increases in the densitometric measurements of resulting bands at an established number of PCR cycles. For each experiment, we also performed semiquantitative RT-PCR under conditions yielding linear results for GAPDH (Table 1) to confirm equal RNA input. Five microliters of the RT-PCR product were analyzed on a 6% acrylamide gel stained with SYBR green (Invitrogen) for analysis with a UVP Bio-imaging System (Upland, CA). Semiquantitative analysis of mRNA expression gene was accomplished by obtaining the ratio of the band density of the TGF-β1 mRNA to that of GAPDH (a housekeeping gene) from the same sample.
Table 1.
Primer sequences
| Primers | Species | Amplicon Size, bp | Sequence (Sense/Antisense) | Annealing °C/Cycle No. |
|---|---|---|---|---|
| TGF-β1 | Mouse | 741 | 5′-AGTGGATCCACGAGCCCAAG-3′ | 58/21 |
| 5′-GCCTTTCTCACCCAATCTGTTTC-3′ | ||||
| GAPDH | Mouse | 450 | 5′-ACCACAGTCCATGCCATCAC-3′ | 65/15 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ |
TGF, transforming growth factor.
Primary cultures of mouse proximal tubules.
TGF-β1+/+ and +/− or SMAD3+/+ and −/− mice were killed with an overdose of pentobarbital sodium. Kidneys were rapidly removed sterilely and the proximal tubules were enriched using the methods of Vinay et al. (27). In brief, renal cortices were minced and suspended in sterile buffer A containing (in mM) 105 NaCl, 24 NaHCO3, 5 KCl, 1.5 CaCl2, 1 MgSO4, 2.0 NaH2PO4, 10 HEPES, and 0.2% BSA, pH 7.4, with the following additions: 8.3 glucose, 1 alanine, 0.03% collagenase (Sigma, type I), and 0.01% soybean trypsin inhibitor (Sigma), gassed with 95% O2-5% CO2 at 37°C. The cortical suspension was incubated for 45 min with gentle agitation. The suspension was strained through a large sieve, centrifuged at 50 g for 10 min, resuspended in oxygenated buffer A, and washed three times. The resulting pellet was mixed with oxygenated and chilled 40% Percoll solution with the identical ionic composition as buffer A. The Percoll solution was centrifuged at 12,200 g for 30 min at 4°C. The lowermost band of 4, enriched in proximal tubules, was washed three times in buffer A and cultured in 50:50 mix of DMEM high glucose and F12 plus 5% fetal bovine serum.
Exposure of proximal tubule cells to sevoflurane.
Confluent primary cultures of mouse proximal tubule cells were placed in an air tight, 37°C, humidified modular incubator chamber (Billups-Rothenberg, Del Mar, CA) with inflow and outflow connectors. The inlet port was connected to the in-line agent-specific calibrated agent-specific vaporizer (Datex-Ohmeda) to deliver sevoflurane mixed with 95% air-5% CO2 (carrier gas) at 10 l/min. The outlet port was connected to a Datex-Ohmeda 5250 RGM gas analyzer that measured sevoflurane concentrations. Exposure to 2.2% sevoflurane lasted 16 h. Control cells were exposed to carrier gas in a modular incubator chamber.
Induction of necrosis in primary culture of renal proximal tubules.
All of the following treatments were performed in primary cultures of renal proximal tubules isolated from TGF-β1 (+/+, +/−) and SMAD3 (+/+, −/−) mice after 24-h deprivation of fetal bovine serum. Primary murine renal proximal tubule cell necrosis was induced with 1–5 mM H2O2 for 4 h. We determined in preliminary studies that the ability of H2O2 to kill renal tubule cells is dose and time related. Our preliminary studies also showed that high-dose (1–5 mM) treatment with H2O2 (for 1–4 h) causes negligible apoptosis. Cells were treated with H2O2 after 16-h treatment with 2.2% (or 1 MAC) sevoflurane or with carrier gas (95% room air-5% CO2). We showed in previous studies that this pretreatment regimen protected HK-2 cells as well as LLC-PK1 cells against H2O2-induced necrosis (12).
Induction of proximal tubule inflammation.
Tumor necrosis factor-α (TNF-α) has been implicated in initiating an inflammatory process after ARF (21, 22). We therefore used TNF-α to mimic an inflammatory process occurring in vivo after renal IR injury (12). To determine the role of TGF-β1 signaling in mediating the anti-inflammatory effects of sevoflurane, murine proximal tubule cells isolated from TGF-β1 (+/+, +/−) and SMAD3 (+/+, −/−) mice were treated with vehicle (saline), 10 ng/ml TNF-α, or TNF-α plus 2.2% sevoflurane in carrier gas (95% room air-5% CO2) for 16 h.
Measurement of cell viability with LDH.
LDH released into cell culture media as indexes of cell death was measured using the LDH assay kit from Promega. Released LDH from cells was expressed as a percentage of total cellular LDH (LDH released into the media plus intracellular LDH released by solubilization of cells with 1% Triton X-100).
Nuclear protein extraction.
Confluent primary cultures of murine proximal tubule cells were scraped in 500 μl of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 20% glycerol, 0.2 mM PMSF, 0.5 mM DTT; Protease Inhibitor Cocktail, Roche, Indianapolis, IN) for 10 min at 4°C. The cells were homogenized using a polytron homogenizer for 5 s to release the nuclei into solution and centrifuged at 18,000 g for 5 min at 4°C. The supernatant was discarded and the pellet was resuspended in 50 μl of buffer B (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.5 mM EDTA, 25% glycerol, 0.1% Triton X-100, 0.2 mM PMSF, 0.5 mM DTT, Protease Inhibitor Cocktail) and incubated for 1 h at 4°C with occasional swirling to extract nuclear protein. The solubilized pellet was centrifuged at 16,000 g for 15 min and the supernatant-containing nuclear proteins were used for EMSAs.
EMSA.
EMSA was performed using the Gel Shift Assay Systems (Promega). The oligonucleotides for NF-κB and SMAD3 (Promega) consensus sequences were end-labeled with 10 μCi of γ32P ATP (Perkin Elmer Life Technology) and purified using a G-25 Spin Column (Amersham Biosciences). Ten micrograms of the nuclear extract were incubated with 1 μl of the labeled probe for 20 min at room temperature and electrophoresed on a 4% polyacrylamide gel (200 V at 4°C). Two micrograms of Hela Nuclear extract (Promega) were used for positive control and 1 μl of a TransCruz polyclonal antibody (Santa Cruz Biotechnology) was coincubated with the nuclear protein and probe for a super-shift reaction. One hundred-fold concentration of cold probe was coincubated as a competitor for a negative control reaction. The gel was then transferred to a blotting paper and exposed to X-ray or scanned with a Phospho Imager (Molecular Dynamics).
Protein determination.
Protein content was determined with the Pierce Chemical (Rockford, IL) bicinchoninic acid protein assay reagent with BSA as a standard.
Statistical analysis.
The data were analyzed with Student's t-test when comparing means between two groups. One-way ANOVA plus Dunnett's post hoc multiple comparison test was used when comparing multiple groups. The ordinal values of the Jablonski scale were analyzed by the Mann-Whitney nonparametric test. In all cases, a probability statistic <0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SE.
Materials.
Otherwise specified, all chemicals were obtained from Sigma (St. Louis, MO).
RESULTS
TGF-β1 mRNA expression in TGF-β1+/+ and TGF-β1+/− mice.
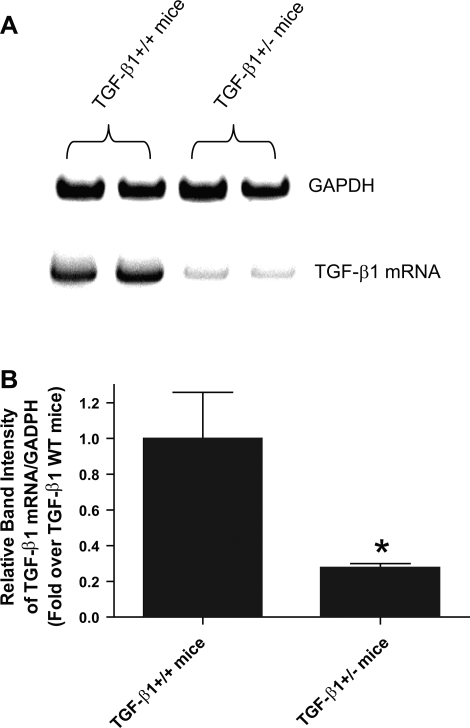
We show in Fig. 1 that TGF-β1 mRNA expression in TGF-β1+/− mice was ∼27% of TGF-β1+/+ mice.
Fig. 1.
A: representative gel images of semiquantitative RT-PCR results of transforming growth factor (TGF)-β1 and GAPDH mRNAs from TGF-β1+/+ and TGF-β1+/− mouse renal cortices. Representative images from 3 experiments are shown. B: densitometric quantifications of relative band intensities from RT-PCR reactions (n = 3). TGF-β1 mRNA band intensity was first normalized against GAPDH band intensity. *P < 0.001 vs. TGF-β1+/+ mice. Data presented as means ± SE.
TGF-β1 levels in TGF-β1 wild-type (+/+) and TGF-β1 heterozygous (+/−) mice.
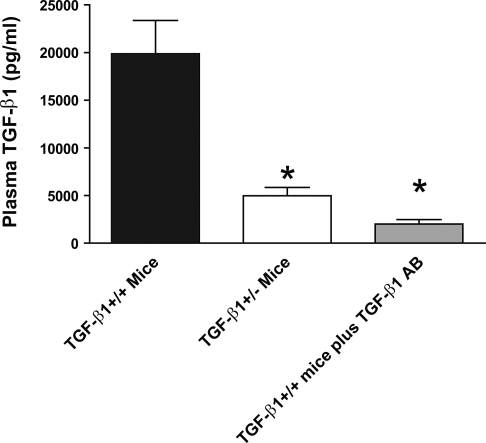
As expected, TGF-β1+/+ mice (19,883 ± 3,483 pg/ml, n = 10) had significantly higher plasma levels of TGF-β1 compared with TGF-β1+/− mice (4,968 ± 880 pg/ml, n = 10, P < 0.01 vs. TGF-β1+/+ mice; Fig. 2). TGF-β1+/+ mice injected with 250 μg anti-TGF-β1 neutralizing antibody iv showed a dramatic reduction in active plasma TGF-β1 levels in 24 h (2,004 ± 463, n = 4, P < 0.01 vs. untreated TGF-β1+/+ mice).
Fig. 2.
Mouse plasma TGF-β1 concentration (in pg/ml) in TGF-β1+/+ mice (n = 10), TGF-β1+/− mice (n = 10), and TGF-β1+/+ mice injected with TGF-β1 neutralizing antibody (n = 4). Total plasma TGF-β1 concentrations were determined with ELISA. *P < 0.01 vs. TGF-β1+/+ mice. Data presented as means ± SE.
Sevoflurane does not protect TGF-β1+/− mice and SMAD3−/− mice from renal IR injury.
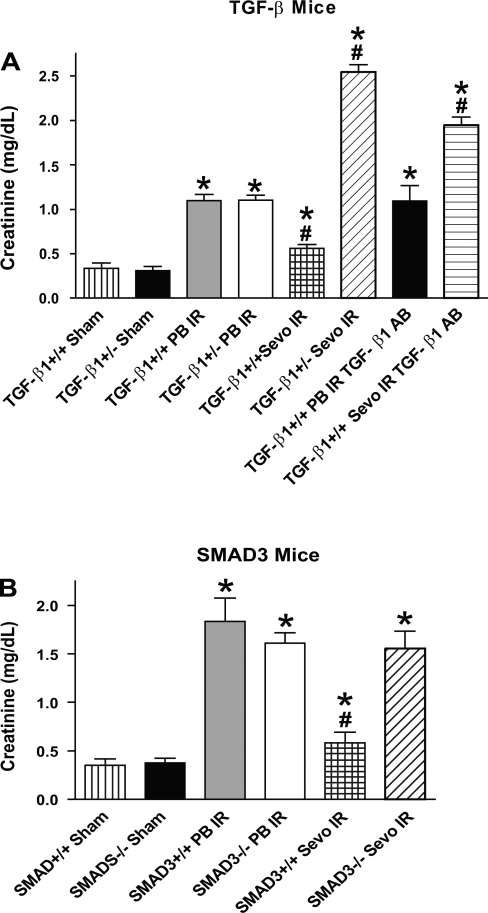
In preliminary studies, we measured systolic blood pressure (via carotid artery catheter) in mice anesthetized with sevoflurane or pentobarbital sodium (n = 2 each). There were no differences between the mice anesthetized with either anesthetic at equipotent anesthetic doses (∼1 MAC of sevoflurane or 2.2%). When the animals were anesthetized with pentobarbital sodium, renal dysfunction occurred 24 h after IR injury in both TGF-β1+/+ mice (Cr = 1.1 ± 0.1 mg/dl, n = 8 vs. sham Cr = 0.3 ± 0.1 mg/dl, n = 4, P < 0.05; Fig. 1) and TGF-β1+/− mice (Cr = 1.1 ± 0.1 mg/dl, n = 9 vs. sham Cr = 0.3 ± 0.1 mg/dl, n = 4, P < 0.05). In contrast, TGF-β1+/+ mice anesthetized with sevoflurane were protected against IR injury (Cr = 0.6 ± 0.1 mg/dl, n = 6). However, volatile anesthetics failed to protect TGF-β1+/− mice against renal IR injury (Cr = 2.5 ± 0.1 mg/dl, n = 6; Fig. 3A). In fact, plasma Cr values for TGF-β1+/− mice subjected to renal IR under sevoflurane anesthesia were significantly higher than the plasma Cr from TGF-β1+/+ mice subjected to renal IR under pentobarbital sodium anesthesia (P < 0.01).
Fig. 3.
A: plasma creatinine (Cr in mg/dl) from studies with TGF-β1+/+ and +/− mice. Twenty-four hours after renal ischemia-reperfusion (IR), plasma Cr were measured in sham-operated mice (Sham; n = 4) or mice subjected to IR under pentobarbital sodium anesthesia (PB IR; n = 8 for TGF-β1+/+ mice, n = 9 for TGF-β1+/− mice) or sevoflurane anesthesia (Sevo IR; n = 6 for TGF-β1+/+ mice, n = 6 for TGF-β1+/− mice). Some TGF-β1+/+ mice were injected with TGF-β1 neutralizing antibody and subjected to renal IR under either pentobarbital sodium or sevoflurane anesthesia (n = 4). B: plasma Cr from studies with SMAD3+/+ and −/− mice. Twenty-four hours after renal IR, plasma Cr were measured in Sham (n = 4) or mice subjected to IR under PB IR (n = 5 for SMAD3+/+ and n = 6 for SMAD3−/− mice) or Sevo IR (n = 6 for SMAD3+/+ and n = 4 for SMAD3−/− mice). *P < 0.05 vs. sham-operated +/+ mice. #P < 0.05 vs. +/+ mice subjected to IR under pentobarbital sodium anesthesia. Data presented as means ± SE.
The renal protective effects of sevoflurane were lost (Cr = 1.9 ± 0.1 mg/dl, n = 4) in TGF-β1+/+ mice treated with a neutralizing TGF-β1 antibody. The neutralizing antibody had no effect on TGF-β1+/+ mice subjected to renal IR under pentobarbital sodium anesthesia (Cr = 1.1 ± 0.2 mg/dl, n = 4).
SMAD3+/+ (Cr = 1.8 ± 0.2 mg/dl, n = 5 vs. sham Cr = 0.4 ± 0.1 mg/dl, n = 4, P < 0.05) and SMAD3−/− (Cr = 1.6 ± 0.1 mg/dl, n = 6 vs. sham Cr = 0.4 ± 0.1 mg/dl, P < 0.05, n = 4) mice anesthetized with pentobarbital sodium developed ARF 24 h after IR injury (Fig. 3B). SMAD3+/+ mice anesthetized with sevoflurane were protected against IR injury (Cr = 0.6 ± 0.1 mg/dl, n = 6, P < 0.05). However, sevoflurane failed to protect the SMAD3−/− mice against renal IR injury (Cr = 1.6 ± 0.2 mg/dl, n = 4; Fig. 3B).
Sevoflurane anesthesia reduces necrosis in TGF-β1+/+ and SMAD3+/+ mice but not in TGF-β1+/− and SMAD3−/− mice.
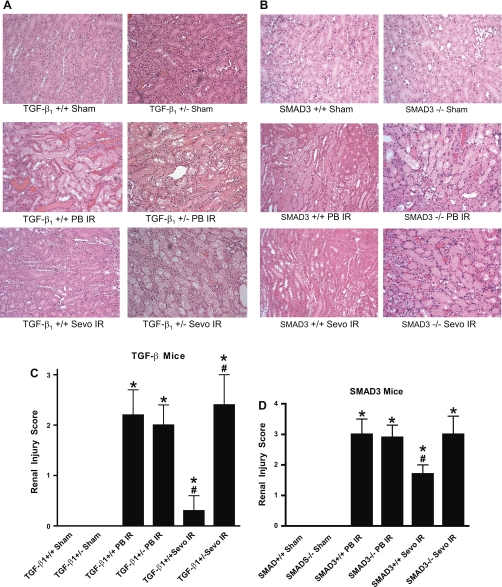
In Fig. 4, the renal protective effects of sevoflurane against renal IR are further supported by the representative histological slides (of n = 6 slide preparations). Thirty minutes of renal ischemia followed by 24-h reperfusion with pentobarbital sodium anesthesia resulted in significant renal injury in both TGF-β1+/+ mice (Fig. 4A) and SMAD3+/+ mice (Fig. 4B) as evidenced by severe tubular necrosis, medullary congestion and hemorrhage, and development of proteinaceous casts in all mouse kidney sections. Sevoflurane anesthesia reduced the degree of necrosis in TGF-β1+/+ mice and SMAD3+/+ mice but not in TGF-β1+/− and SMAD3−/− mice.
Fig. 4.
Representative of 6 photomicrographs (hematoxylin and eosin staining, magnification ×200) from studies with TGF-β1+/+ and +/− mice (A) or SMAD3+/+ and −/− mice (B). Pictures of outer medulla of the kidneys of sham-operated mice and mice subjected to renal IR injury under PB IR or Sevo IR anesthesia 24 h before. C: renal injury scores for the histologic appearance of acute tubular necrosis from sham-operated TGF-β1 mice (Sham) and mice subjected to renal IR during PB IR (n = 6 for TGF-β1+/+ mice, n = 6 for TGF-β1+/− mice) or during Sevo IR (n = 5 for TGF-β1+/+ mice, n = 6 for TGF-β1+/− mice). D: renal injury scores for the histologic appearance of acute tubular necrosis from sham-operated SMAD3 mice (Sham) and mice subjected to renal IR during PB IR (n = 4 for SMAD3+/+ and n = 4 for SMAD3−/− mice) or during Sevo IR (n = 6 for SMAD3+/+ and n = 4 for SMAD3−/− mice). *P < 0.05 vs. sham group. #P < 0.05 vs. +/+ PB IR group. Data are presented as means ± SE. Statistical analysis performed using the Kruskal-Wallis nonparametric test with Dunn posttest comparison.
The Jablonski scale histology grading scores are used to grade renal tubular necrosis after murine renal IR injury (Fig. 4, C and D). Thirty minutes of renal ischemia and 24-h reperfusion resulted in severe acute tubular necrosis in TGF-β1+/+ mice (grade = 2.2 ± 0.5, n = 6), TGF-β1+/− mice (grade = 2.0 ± 0.4, n = 6), SMAD3+/+ mice (grade = 3.0 ± 0.5, n = 4), and SMAD3−/− mice (grade = 2.9 ± 0.4, n = 4). Sevoflurane anesthesia reduced necrosis in TGF-β1+/+ mice (grade = 0.3 ± 0.3, n = 5, P < 0.01 vs. TGF-β1+/+ anesthetized with pentobarbital sodium) but not in TGF-β1+/− mice (grade = 2.4 ± 0.6, n = 6). Sevoflurane anesthesia also reduced necrosis in SMAD3+/+ mice (grade = 1.7 ± 0.3, n = 6, P < 0.05 vs. SMAD3+/+ anesthetized with pentobarbital sodium) but not in SMAD3−/− mice (grade = 3.0 ± 0.6, n = 4).
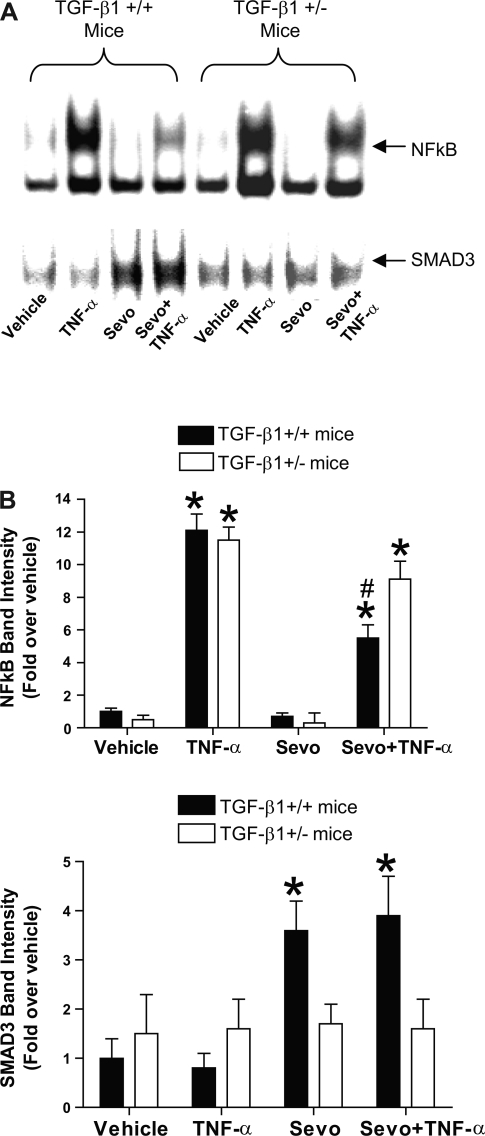
Differential regulation of NF-κB and SMAD3 in primary cultures of renal proximal tubules from TGF-β1 wild-type (+/+) and heterozygous (+/−) mice.
We demonstrated previously that a proinflammatory cytokine TNF-α causes robust nuclear translocation of a proinflammatory transcription factor NF-κB which was significantly attenuated by sevoflurane (12). To determine whether sevoflurane-mediated attenuation of nuclear translocation of NF-κB is dependent on TGF-β1 signaling, primary cultures of proximal tubules from TGF-β1+/+ and +/− mice were treated with vehicle (saline), 10 ng/ml TNF-α, 2.2% sevoflurane or TNF-α plus sevoflurane 16 h in carrier gas (95% room air-5% CO2). EMSA was performed for NF-κB (n = 4) which demonstrated the expected increase in NF-κB translocation with TNF-α in tubules from both TGF-β1+/+ and +/− mice. Sevoflurane treatment significantly reduced the translocation of NF-κB only in tubules from TGF-β1+/+ mice but not in TGF-β1+/− mice (Fig. 5).
Fig. 5.
A: representative image of EMSA studies for transcription factor NF-κB (top) and SMAD3 (bottom) binding activity in renal proximal tubules from TGF-β1+/+ and +/− mice. Percoll gradient-separated proximal tubules were maintained for 24 h and treated with vehicle (Veh), 10 ng/ml tumor necrosis factor-α (TNF-α), 2.2% sevoflurane (Sevo), or TNF-α plus 2.2% sevoflurane for 16 h. B: densitometric quantifications of relative band intensities from NF-κB (top) and SMAD3 (bottom) EMSA (n = 4). *P < 0.05 vs. vehicle-treated cells from TGF-β1+/+ mice. #P < 0.05 vs. TNF-α-treated cells from TGF-β1+/+ mice. Means ± SE.
SMAD3 is a key nuclear transcription factor for TGF-β1 signaling. Figure 5 also shows that sevoflurane increased SMAD3 binding/nuclear translocation (n = 4) only in TGF-β1+/+ mice but not in TGF-β1+/− mice supporting the hypothesis that sevoflurane causes release of TGF-β1 and activates SMAD3 translocation.
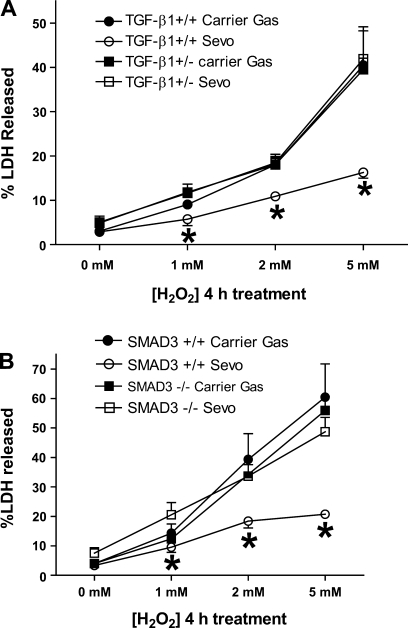
Sevoflurane failed to protect against necrosis in primary cultures of proximal tubules from mice lacking TGF-β1 signaling.
Four-hour treatments with 1–5 mM H2O2 caused rapid necrosis of proximal tubule cells isolated and cultured from mice (Fig. 6; n = 6 for each group). Sevoflurane caused significant protection against necrosis in proximal tubule cells from TGF-β1+/+ mice and SMAD3+/+ mice (Fig. 6). However, sevoflurane failed to protect against necrosis in proximal tubule cells cultured from mice deficient in TGF-β1 signaling (TGF-β1+/− mice and SMAD3−/− mice).
Fig. 6.
LDH release after vehicle or H2O2 treatment (0–5 mM) for 4 h in proximal tubule cells cultured from TGF-β1+/+ and +/− mice (A) or SMAD3+/+ and −/− mice (B). Cells were treated with H2O2 16 h after treatment with carrier gas (room air plus 5% CO2) or with 2.2% sevoflurane in carrier gas (n = 6). *P < 0.05 vs. LDH released from +/+ mice proximal tubules with H2O2 injury after carrier gas pretreatment.
DISCUSSION
The major findings of this study are that an inhalational anesthetic sevoflurane 1) protected TGF-β1+/+ and SMAD3+/+ against renal IR injury compared with mice anesthetized with pentobarbital sodium, 2) failed to protect TGF-β1+/− and SMAD3−/− mice against renal IR injury, 3) attenuated the nuclear translocation of NF-κB only in TGF-β1+/+ mice but not in TGF-β1+/− mice, 4) increased the nuclear translocation of SMAD3 in TGF-β1+/+ mice but not in TGF-β1+/− mice, and 5) protected against necrotic cell death in proximal tubules isolated from TGF-β1+/+ and SMAD3+/+ mice but not from TGF-β1+/− and SMAD3−/− mice.
Sevoflurane {2,2,2-trifluoro-1-[trifluoromethyl]ethyl fluoromethyl ether} is used frequently in the operating room for induction and maintenance of general anesthesia. In our previous studies, we showed that clinically relevant concentrations of sevoflurane protected against H2O2-mediated necrosis in cultured porcine (LLC-PK1) and human (HK-2) proximal tubule cells (12). Mechanistically, we demonstrated that sevoflurane caused the externalization of phosphatidylserine (PS) and released TGF-β1 in cultured human kidney cells in vitro (13). TGF-β1 release by sevoflurane resulted in activation of the cytoprotective kinases ERK and Akt, induction of HSP70, and nuclear translocation of SMAD3, a key transcription factor in TGF-β1 signaling. Furthermore, sevoflurane-mediated protection against H2O2-induced necrosis in HK-2 cells was dependent on TGF-β1 release as the neutralizing TGF-β1 antibody abrogated sevoflurane's protective effects in HK-2 cells (15).
We also showed previously that sevoflurane caused significant protection against renal IR injury in rats and mice in vivo (14, 16). Since in vitro studies demonstrated that TGF-β1 release and nuclear translocation of SMAD3 are signaling intermediates in sevoflurane-mediated renal tubular protection in vitro, we utilized mice deficient in TGF-β1→SMAD3 signaling to test/confirm our in vitro findings in vivo. The fact that sevoflurane failed to protect TGF-β1+/− mice and SMAD3−/− mice supports the role of TGF-β→SMAD3 signaling in sevoflurane-mediated renal protection against IR injury in vivo. Due to inherent concerns regarding compensatory changes that may occur in a mouse genetic knockout model, complimentary experiments were performed with a TGF-β1 neutralizing antibody. Our studies showed that neutralization of TGF-β1 prevented the protection with sevoflurane in vivo complementing our studies with the TGF-β1+/− mice.
In this study, we further demonstrate that sevoflurane provided protection in cultures of proximal tubules isolated from TGF-β1+/+ mice and SMAD3+/+ mice. However, TGF-β1+/− and SMAD3−/− mice proximal tubules were not protected with sevoflurane against H2O2-induced necrosis. These findings support our mechanistic studies in HK-2 cells in that sevoflurane-mediated protection against necrosis in renal tubules in culture is dependent on the release of TGF-β1 and subsequent signaling. We also conclude that even partial deficiency of TGF-β1 in renal proximal tubules prevents the renal protective signaling cascade induced with sevoflurane (Fig. 1). Moreover, we now provide evidence that sevoflurane causes direct renal tubular protection in three different species (human, pig, and mouse) (12).
Our previous studies implicated the externalization of PS and the release of TGF-β1 to mediate sevoflurane's ability to reduce necrosis and inflammation. TGF-β1 is a well-known anti-inflammatory cytokine and has been shown to produce both anti-inflammatory and anti-necrotic effects in vivo as well as in vitro (9, 28). Neutralization of TGF-β1 signaling increases the inflammatory response in many models of immune diseases in vivo (4, 20, 30). In fact, TGF-β1−/− mice do not survive to adulthood due to lack of TGF-β1. Moreover, previous studies by Fadok's group (5–7, 9, 29) consistently demonstrated that PS exposure and subsequent release of TGF-β1 provide powerful resolution of inflammation in macrophages in vitro. They also demonstrated that in mice, modulation of PS exposure and TGF-β1 levels affected the inflammatory response to antigen challenges in mice in vivo (8). These studies together with the data obtained in this study support that externalization of PS and subsequent release of TGF-β1 provide powerful anti-inflammatory signal in vitro and in vitro.
We previously demonstrated that volatile anesthetics including sevoflurane reduced markers of inflammation including transcription of proinflammatory mRNAs (TNF-α, ICAM-1, MCP-1, and MIP2) and nuclear translocation of proinflammatory transcription factors (e.g., NF-κB) in vitro (induced with TNF-α) as well as in vivo (renal IR injury) models of inflammation (12, 16). Recently, we showed that the reduction in NF-κB activation with sevoflurane is dependent on the release of TGF-β1 as a neutralizing antibody for TGF-β1 attenuated the reduction in NF-κB translocation (15). In this study, we provide evidence from freshly cultured murine proximal tubules that sevoflurane-mediated attenuation of NF-κB nuclear translocation was dependent on TGF-β1 release as TGF-β1+/− proximal tubules had significantly less attenuation of NF-κB translocation with sevoflurane treatment when compared with TGF-β1+/+ tubules.
Previously, we showed that SMAD3 (Mothers against decapentaplegic homolog 3, a key transcription factor involved in TGF-β1 signaling) nuclear translocation occurs with sevoflurane in HK-2 cells (15). SMAD proteins carry the TGF-β1 signals from the cell surface to the nucleus (19, 23). The activated heteromeric complex of TGF-β type I and type II transmembrane serine/threonine kinase receptors induces phosphorylation of SMAD3. The complexes then translocate to the nucleus and regulate transcriptional responses together with DNA binding cofactors. In this study, we demonstrate that sevoflurane treatment led to nuclear translocation of SMAD3 in primary cultures from TGF-β1+/+ mice proximal tubules (Fig. 5). Therefore, we now have evidence that sevoflurane causes nuclear translocation of SMAD3 in renal proximal tubules in vitro and in vivo. Furthermore, we demonstrated that the activation of SMAD3 occurred robustly in TGF-β1+/+ proximal tubule cells but not in TGF-β1+/− tubule cells further providing evidence that sevoflurane-mediated SMAD3 nuclear translocation requires TGF-β1.
One of the limitations of this study is that the TGF-β1+/− anesthetized with sevoflurane and subjected to renal IR had significantly higher plasma creatinine compared with TGF-β1+/− anesthetized with pentobarbital sodium and subjected to renal IR. Moreover, TGF-β1+/+ mice given neutralizing TGF-β1 antibody and subjected to renal IR under sevoflurane anesthesia also had increased plasma creatinine. It is indeed interesting that in both the creatinine measurements and the necrosis measurements, the effect of a partial loss of TGF-β1 had more striking effects than the complete loss of SMAD3. The reason for this exacerbated renal dysfunction in TGF-β1-depleted mice anesthetized with sevoflurane is not clear. We hypothesize that the TGF-β1 is affecting the sevoflurane protection through more pathways than just a SMAD3 pathway. It is also possible that neutralization or depletion of plasma TGF-β1 unmasks some detrimental effects of renal injury with sevoflurane. Further work is required to elucidate the mechanisms of these effects.
Another limitation of this study is that the exposure of primary cultures of murine proximal tubules to sevoflurane occurred for 16 h. We previously showed that in vitro, sevoflurane's protection against H2O2-mediated necrosis required at least 4 h of pretreatment. This relatively prolonged time course of sevoflurane administration required to achieve cytoprotection (4–16 h) could be a concern in utilizing sevoflurane to provide immediate clinical applicability. Identification of the specific distal effectors may provide more practical therapeutic targets requiring less pretreatment time. Moroever, identification of distal signaling pathways of sevoflurane-mediated renal tubular effects could lead to the development of drugs that could mimic sevoflurane's renal protective effects without having to deal with the systemic hemodynamic effects of inhalational anesthetics (e.g., hypotension, negative inotropic effects).
In this study, we utilized both in vivo (renal IR) as well as in vitro (H2O2, TNF-α) models of renal tubular injury. There are advantages as well as limitations with both approaches. In vivo studies must deal with multiple cell types within an organ and also with complex physiological control within an animal. In contrast, in vitro studies with a pure population of one cell type such as HK-2 cells eliminate complex external physiological influences and allow us to study signaling cascades more directly. We acknowledge that H2O2 and TNF-α are just models of components of the complex pathophysiology occurring with IR. Moreover, these agents mimic limited components of the grander event of renal IR.
In summary, we demonstrated in this study that sevoflurane provided powerful protection against renal IR injury in mice and in cultured proximal tubules. However, mice deficient in TGF-β1 signaling (TGF-β1+/− mice or SMAD3−/− mice) were not protected against renal IR injury with sevoflurane. Clinical significance is high as inhalational anesthetics such as sevoflurane are the center piece of anesthetic regimen in the United States. Further elucidation of the signaling pathways of sevoflurane-mediated cytoprotective pathways may lead to effective therapies against renal IR injury and ARF.
GRANTS
This work was funded by R01-GM-067081.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 18: 442–445, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, Daley J. Preoperative renal risk stratification. Circulation 95: 878–884, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, Landolfo K. Acute renal failure following cardiac surgery. Nephrol Dial Transplant 14: 1158–1162, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci USA 92: 12215–12219, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405: 85–90, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol 2: 627–633, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol 174: 1393–1404, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF- beta1 secretion and the resolution of inflammation. J Clin Invest 109: 41–50, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones DR, Lee HT. Protecting the kidney during critical illness. Curr Opin Anaesthesiol 20: 106–112, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 291: F67–F78, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-β1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol 27: 416–424, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Lee HT, Kim M, Kim M, Kim N, Billings IV FT, D'Agati VD, Emala Sr CW. Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol 293: F713–F722, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Kim M, Song JH, Chen SWC, Gubitosa G, Emala CW. Sevoflurane mediated TGF-β1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiol In press. [DOI] [PubMed]
- 16.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology 101: 1313–1324, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol 284: F267–F273, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Liu F Receptor-regulated Smads in TGF-beta signaling. Front Biosci 8: s1280–s1303, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Prud'homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J Autoimmun 14: 23–42, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl 91: S56–S61, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Roberts AB TGF-beta signaling from receptors to the nucleus. Microbes Infect 1: 1265–1273, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Shull MM, Doetschman T. Transforming growth factor-beta 1 in reproduction and development. Mol Reprod Dev 39: 239–246, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Star RA Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Tang IY, Murray PT. Prevention of perioperative acute renal failure: what works? Best Pract Res Clin Anaesthesiol 18: 91–111, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 241: F403–F411, 1981. [DOI] [PubMed] [Google Scholar]
- 28.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc 11: 112–117, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Xiao YQ, Malcolm K, Worthen GS, Gardai S, Schiemann WP, Fadok VA, Bratton DL, Henson PM. Cross-talk between ERK and p38 MAPK mediates selective suppression of proinflammatory cytokines by transforming growth factor-beta. J Biol Chem 277: 14884–14893, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 18: 1280–1291, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]