Abstract
Transforming growth factor (TGF)-β1, once activated, binds to its receptors and mediates renal fibrosis via the downstream Smad signaling pathway. We reported here that mice overexpressing latent TGF-β1 in keratinocytes were protected against renal fibrosis in a model of obstructive kidney disease. In normal mice, both transgenic (Tg) and wild-type (WT) mice had normal renal histology and function, despite a 10-fold increase in plasma latent TGF-β1 in Tg mice. A severe renal fibrosis was developed in WT mice at 7 days after urinary obstruction. Unexpectedly, renal fibrosis was prevented in Tg mice, although levels of latent TGF-β1 in both circulation and renal tissues remained high. Compared with the WT mice, quantitative real-time PCR showed that upregulation of renal α-smooth muscle actin (SMA), collagen I, and collagen III mRNA was inhibited in Tg mice (60–70% reduced, all P < 0.01). These were further confirmed by immunohistochemistry with a marked inhibition of tubulointerstitial accumulation of α-SMA+ fibroblasts, collagen I, and collagen III matrix in Tg mice (all P < 0.001). Further studies showed that inhibition of renal fibrosis in Tg mice was associated with a significant reduction in renal TGF-β1 and CTGF (60% reduced, P < 0.05), an increase in renal Smad7, a suppression of TSP-1 (a critical factor for TGF-β1 activation), and an inhibition of Smad2/3 activation (all P < 0.001). In conclusion, latent TGF-β may play a protective role in renal fibrosis. Inhibition of renal TGF-β1 expression and activation, thereby blocking the downstream TGF-β signaling pathway, may be a critical mechanism by which latent TGF-β1 protects against renal fibrosis.
Keywords: TGF-β signaling, transgenic mice, Smads, UUO
transforming growth factor β1 (TGF-β1), a multifunctional cytokine with fibrogenic properties, has long been considered as a key mediator of renal fibrosis in both experimental and human kidney diseases (1, 2, 23). In the pathological conditions, TGF-β1 has diverse roles in anti-inflammation, while inducing fibrosis. This is illustrated by the finding that mice with targeted disruption of TGF-β1 gene develop autoimmune disease (16, 24). In contrast, mice with overexpression of a bioactive form of TGF-β1 in the liver develop severe renal damage with progressive renal fibrosis (11). Indeed, TGF-β stimulates extracellular matrix (ECM) deposition by increasing the synthesis of ECM proteins on the one hand, while inhibiting ECM degradation on the other (1). The important role of TGF-β in renal fibrosis is clearly demonstrated by the finding that renal scarring can be induced by overexpressing TGF-β1, but is inhibited by blocking TGF-β with a neutralizing TGF-β antibody, decorin, and antisense in a number of animal models (reviewed in Refs. 1, 2, 23).
TGF-β1 is produced and secreted as a latent complex, consisting of mature dimeric TGF-β1, a latency-associated peptide (LAP), and a latent TGF-β binding protein (LTBP). LAP binds to the NH2 terminal of TGF-β to prevent it from binding to its receptors, while LTBP-1 binds the LAP-TGF-β complex to prevent TGF-β from interacting with local matrix proteins (19). Activated TGF-β1 is released when an NH2-terminal LAP is cleaved from the mature TGF-β1 by pH, heat, protease, thrombospondin-1 (TSP-1), reactive oxygen species, and integrin αvβ6 (4, 10, 20). Once activated, TGF-β1 binds its receptors and then activates its downstream signaling mediators, called Smad2/3, to exert its biological activities (5). TGF-β is also able to induce its downstream inhibitor Smad7, which, in turn, balances TGF-β signaling by inhibiting Smad2/3 phosphorylation via its negative feedback mechanisms (9).
Although mice with overexpression of a bioactive form of TGF-β1 in the liver develop severe renal damage with progressive renal fibrosis (11), our recent finding shows that mice expressing a human latent TGF-β1 in skin keratinocytes exhibit a normal renal histology and function (22). These observations indicate a distinct role for active vs. latent TGF-β1 in renal fibrosis. Furthermore, we also found that mice that overexpress latent TGF-β1 in keratinocytes are protected against kidney from inflammatory injury in a mouse model of unilateral ureteral obstructive (UUO) kidney disease, despite a 10-fold increase in circulating levels of latent TGF-β1 (22). These contradictory findings suggest a complex role for TGF-β1 in renal inflammation and fibrosis.
While it is known that mice overexpressing latent TGF-β1 can prevent renal inflammation in a model of UUO (22), it remains unclear whether latent TGF-β1 is pathogenic or protective in renal fibrosis in UUO. To address this question, the present study examined renal fibrosis response in the same model of UUO induced in mice that overexpress latent TGF-β1 as previously described (22). Renal fibrosis including α-SMA+ myofibroblasts and collagen matrix accumulation and their mRNA expression was examined. Furthermore, the underlying mechanisms including expression of renal TGF-β and its downstream mediator CTGF, and activation of the TGF-β/Smad signaling pathway, were investigated.
MATERIALS AND METHODS
Obstructive kidney disease model.
Latent TGF-β1 transgenic (Tg) mice were generated from ICR background mice (14). A progressive UUO kidney disease model was induced in both wild-type (WT) and Tg mice (18–20 g body wt, 3 mo old) by left ureteral ligation (22). Groups of six to eight mice were euthanized on day 7 after the left ureteral ligation. In addition, groups of six normal WT and Tg mice were used as normal age-matched controls. Kidney tissues were collected for histology, immunohistochemistry, and real-time PCR analysis. The experimental procedures were approved by the Committee on the Use of Live Animals for Teaching and Research at the University of Hong Kong.
Real-time PCR.
Total kidney RNA was isolated using the RNeasy kit, following the manufacturer's instructions (Qiagen, Valencia, CA). The cDNA was synthesized and real-time PCR was run with the Opticon real-time PCR machine (MJ Research, Waltham, MA) using IQ SYBR green supermix reagent (Bio-Rad, Herculus, CA) (7). The specificity of real-time PCR was confirmed via routine agarose gel electrophoresis and Melting-curve analysis. Housekeeping gene GAPDH was used as an internal standard. The primers used in this study were α-smooth muscle actin (SMA): forward 5′-ACTGGGACGACATGGAAAAG-3′, reverse 5′-CATCTCCAGAGTCCAGCACA-3′; collagen I: forward 5′-GAGCGGAGAGTACTGGATCG-3′, reverse 5′-TACTCGAACGGGAATCCATC-3′; collagen III: forward 5′-TGGTCCTCAGGGTGTAAAGG-3′, reverse 5′-GTCCAGCATCACCTTTTGGT-3′; TGF-β forward 5′-CAACAATTCCTGGCGTTACCTTGG, reverse 5′-GAAAGCCCTGTATTCCGTCTCCTT; CTGF forward 5′-CTCCACCCGAGTTACCAATGACAA-3′, reverse 5′-CCAGAAAGCTCAAACTTGACAGGC-3′; GAPDH forward 5′-TGCTGAGTATGTCGTGGAGTCTA, reverse 5′-AGTGGGAGTTGCTGTTGAAATC.
Histology and immunohistochemistry.
Renal histology was examined in methyl Carnoy's fixed, paraffin-embedded tissue sections (4 μm) stained with hematoxylin and eosin or periodic acid-Schiff (PAS). Immunostaining was performed in paraffin sections using a microwave-based antigen retrieval technique (12). The antibodies used in this study included rabbit polyclonal antibodies to phosphorylated Smad2/3 (p-Smad2/3), Smad7, TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA), TSP-1 (Abcam, Cambridge, MA), collagen I and III (Southern Tech, Birmingham, AL), and α-SMA (Sigma, St. Louis, MO). After being developed with 3,3-diaminobenzidine, sections were counterstained with hematoxylin. An isotype-matched rabbit IgG (Sigma) was used as a negative control throughout the study.
To examine the relationship between expression of renal Smad7 and activation of TGF-β signaling, a microwave-based two color immunohistochemistry was used (12). Briefly, after being stained with Smad7 and developed with 3,3-diaminobenzidine to produce a color brown within the cytoplasm, sections were microwaved again for 10 min, which denatures bound IgG molecules on the sections to prevent antibody cross-reaction and inactivates the endogenous alkaline phosphatase. Sections then were labeled with rabbit anti-p-Smad2/3 using a three-layer mouse alkaline phosphatase-anti-alkaline phosphatase complex and developed with Fast Blue BB Base (Sigma), giving a blue product in the nucleus.
Quantitative analysis of immunostaining was carried out on coded slides (7, 22). Briefly, the number of positive cells for p-Smad2/3 was counted in 20 consecutive glomeruli and expressed as cells/glomerular cross-section (gcs), whereas positive cells in the tubulointerstitium were counted under high-power fields (×40) by means of a 0.0625-mm2 graticule fitted in the eyepiece of the microscope, and expressed as cells per millimeter squared. Accumulation of α-SMA+ cells, collagen I, collagen III, TGF-β1, and TSP-1 in the entire cortical tubulointerstitium (a cross-section of the kidney) was determined using the quantitative Image Analysis System (7, 22). Briefly, the examined area of the tubulointerstitium was outlined, positive staining patterns were identified, and the percent positive area in the examined tubulointerstitium was then measured. Data were expressed as percent positive area examined.
ELISA.
Since TSP-1 is a major mechanism of TGF-β1 activation, plasma levels of TSP-1 in both normal and diseased animals were measured using a TSP-1 Immunoassay kit (DTSP10, R&D Systems, Minneapolis, MN).
Statistical analyses.
Data obtained from this study were expressed as means ± SE. Statistical analyses were performed using one-way ANOVA from GraphPad Prism 3.0 (GraphPad Software, San Diego, CA).
RESULTS
Mice overexpressing latent TGF-β1 are protected against renal fibrosis in obstructive kidney disease.
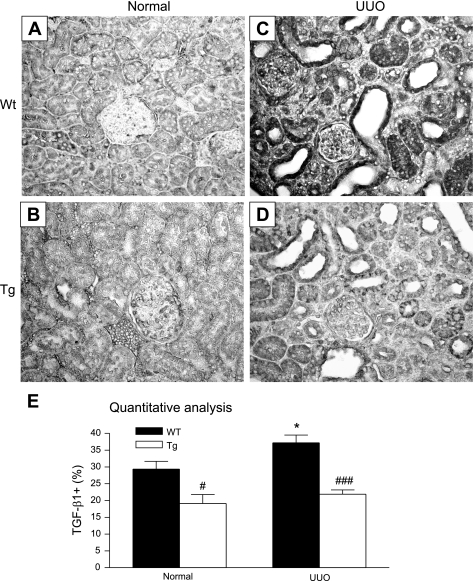
As shown previously, although TGF-β Tg mice exhibited a 10-fold increase in circulating latent TGF-β1 and a 2.5-fold elevation of latent TGF-β1 within the kidney, Tg mice exhibited a normal renal function and histology (22). This was further illustrated in Figs. 1, 2, 3, and 4 (A, B). Tg mice showed normal renal histology similar to the WT mice (Fig. 1, A and B). However, severe renal fibrosis as evident by morphology with abundant accumulation of elongated fibroblasts within the damaged tubulointerstitium was noted in the WT mice after UUO (Fig. 1C). In contrast, Tg mice exhibited a few fibroblasts and little ECM accumulation within the tubulointerstitium, although tubules remained highly dilated due to the high intralumen pressure induced by ureteral ligation (Fig. 1D). Immunohistochemically, both normal WT and Tg mice exhibited a few α-SMA+ myofibroblasts and collagen I and collagen III accumulation within the cortical tubulointerstitium (Figs. 2–4, A and B). However, in UUO kidney, severe renal fibrosis with a marked increase in accumulation of tubulointerstitial α-SMA+ myofibroblasts and collagen I and collagen III was developed in WT mice (Figs. 2–4C). Surprisingly, severe renal fibrosis was largely inhibited in Tg mice, showing a few α-SMA+ myofibroblasts and minimal collagen I and III accumulation within the UUO kidney (Figs. 2–4D). These findings were further confirmed by quantitative analysis as shown in Figs. 2–4E. Consistent with the immunohistochemical findings, real-time PCR revealed that a marked increase in mRNA expression of α-SMA, collagen I, and collagen III was found in the UUO kidney in WT mice, which was blocked in Tg mice (Fig. 5).
Fig. 1.
Histological features of kidneys of latent transforming growth factor (TGF)-β1 transgenic (Tg) mice and wild-type (WT) mice in normal and obstructive kidney. A and B: kidneys from both K5.TGFβ1wt Tg and WT mice show normal renal histology. C: representative obstructive kidney from a WT mouse with severe tubulointerstitial fibrosis as evident by abundant elongated fibroblasts and extracellular matrix accumulation at day 7 after ureteral ligation. D: representative obstructive kidney from a Tg mouse shows relatively normal histology at day 7 after the ureteral ligation. Tissue sections are stained with PAS. Magnification ×200. UUO, unilateral ureteral obstruction.
Fig. 2.
Immunohistochemistry shows that mice overexpressing latent TGF-β1 are protected against a marked accumulation of α-smooth muscle actin (SMA)+ myofibroblasts within the tubulointerstitium at day 7 after UUO. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), UUO kidney from a Tg mouse (D), and quantitative analysis (E). Note that both normal WT and Tg mice show a few α-SMA+ myofibroblasts (dark black) within the interstitium (A, B), but severe renal fibrosis with a prominent α-SMA+ myofibroblasts (dark black) within the tubulointerstitium is developed in WT mice with UUO (C), which is significantly inhibited in Tg mice (D). Sections are stained with the anti-α-SMA antibody as described in materials and methods. Each bar represents means ± SE for a group of 6 (normal) or 8 (UUO) mice. ***P < 0.001 compared with the normal control. ###P < 0.001 when compared with the WT UUO. Magnification ×200.
Fig. 3.
Immunohistochemistry shows that mice overexpressing latent TGF-β1 are protected against renal fibrosis as evident by a marked inhibition of collagen I accumulation within the tubulointerstitium at day 7 after UUO. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), UUO kidney from a Tg mouse (D), and quantitative analysis (E). Note that both normal WT and Tg mice show few collagen I (dark black) accumulation within the interstitium (A, B), but severe renal fibrosis with an abundant collagen I accumulation within the tubulointerstitium is developed in WT mice with UUO (C), which is significantly inhibited in Tg mice (D). Sections are stained with the anti-collagen I antibody as described in materials and methods. Each bar represents means ± SE for a group of 6 (normal) or 8 (UUO) mice. ***P < 0.001 compared with the normal control. ###P < 0.001 when compared with the WT UUO. Magnification ×200.
Fig. 4.
Immunohistochemistry shows that mice overexpressing latent TGF-β1 are protected against renal fibrosis as evident by a marked inhibition of collagen III accumulation within the tubulointerstitium at day 7 after UUO. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), UUO kidney from a Tg mouse (D), and quantitative analysis (E). Note that both normal WT and Tg mice show few collagen III (dark black) accumulation within the interstitium (A, B), but severe renal fibrosis with an abundant collagen I accumulation within the tubulointerstitium is developed in WT mice with UUO (C), which is significantly inhibited in Tg mice (D). Sections are stained with the anti-collagen III antibody as described in materials and methods. Each bar represents means ± SE for a group of 6 (normal) or 8 (UUO) mice. ***P < 0.001 compared with the normal control. ###P < 0.001 when compared with the WT UUO. Magnification ×200.
Fig. 5.
Real-time PCR shows that a marked upregulation of α-SMA, collagen I, and collagen III mRNA expression in WT mice with UUO at day 7 is prevented in latent TGF-β1 Tg mice. α-SMA mRNA expression (A), collagen I mRNA expression (B), collagen III mRNA expression (C). Each bar represents means ± SE for a group of 6 mice. **P < 0.01, ***P < 0.001 compared with the normal control. ##P < 0.01, ###P < 0.001 when compared with the WT UUO.
Inhibition of TGF-β and its downstream signaling pathway is a key mechanism by which TGF-β1 Tg mice are protected against renal fibrosis in UUO kidney disease.
We next investigated the mechanisms whereby TGF-β Tg mice were protected against renal fibrosis. We first examined the expression of renal TGF-β1. As shown in Fig. 6, real-time PCR demonstrated that a significant upregulation of renal TGF-β1 mRNA was found in the WT mice after UUO (Fig. 6A). In contrast, Tg mice exhibited a normal level of TGF-β1 mRNA in the UUO kidney. Similarly, immunohistochemistry showed that a marked upregulation of TGF-β1 protein was found in the diseased kidney of WT mice (Fig. 7, A, C, E), which was inhibited in Tg mice (Fig. 7, B, D, E). It is known that CTGF is a downstream mediator of TGF-β signaling. We then examined CTGF expression. Real-time PCR showed that WT mice exhibited a significant increase in renal CTGF mRNA expression within the UUO kidney, which was prevented in Tg mice (Fig. 6B).
Fig. 6.
Real-time PCR shows that a marked upregulation of TGF-β1 and CTGF mRNA expression in WT mice with UUO at day 7 is prevented in latent TGF-β1 Tg mice. TGF-β1 mRNA expression (A), CTGF mRNA expression (B). Each bar represents means ± SE for a group of 6 mice. **P < 0.01, ***P < 0.001 compared with the normal control. #P < 0.05, ##P < 0.01, when compared with the WT UUO.
Fig. 7.
Immunohistochemistry shows that upregulation of renal TGF-β1 protein is prevented in mice overexpressing latent TGF-β1 at day 7 after UUO. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), UUO kidney from a Tg mouse (D), and quantitative analysis (E). Note that renal TGF-β1 (dark black) is markedly upregulated with severe tubulointerstitial fibrosis in WT mice with UUO (C), which is significantly inhibited in Tg mice (D). Each bar represents means ± SE for a group of 6 (normal) or 8 (UUO) mice. *P < 0.05 compared with the normal control. #P < 0.05; ###P < 0.001 when compared with the WT UUO. Magnification ×200.
Increasing evidence shows that TGF-β1 acts by activating its downstream mediators, Smad2/3, to mediate renal fibrosis (2, 23). Indeed, compared with normal mice (Fig. 8, A and B), a marked activation of Smad2/3 as determined by phosphorylated Smad2/3 nuclear translocation was found in the diseased kidney of WT mice, particularly in the areas of severe tubulointerstitial fibrosis (Fig. 8C), which was inhibited in Tg mice (Fig. 8, D–F). Two-color immunohistochemistry further showed that a marked activation of Smad2/3 in the diseased kidney of WT mice was associated with a significant reduction of renal Smad7 (Fig. 9, A and C). In contrast, Tg mice exhibited a strong expression of renal Smad7 in both normal and diseased kidneys, which was associated with a marked inhibition of nuclear p-Smad2/3 (Fig. 9, B and C).
Fig. 8.
Immunohistochemistry shows that mice overexpressing latent TGF-β1 are protected against a marked activation of Smad2/3 within the fibrotic kidney at day 7 after UUO. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), UUO kidney from a Tg mouse (D), and quantitative analysis (E, F) in the glomerulus and tubulointerstitium. Note that both normal WT and Tg mice show a few phosphorylated Smad2/3 (p-Smad2/3) within the glomerulus and tubulointerstitium as evident by nuclear location (dark black; A and B), but a strong activation of p-Smad2/3 within areas of severe tubulointerstitial fibrosis is found in WT mice with UUO (C), which is significantly inhibited in Tg mice (D). Sections are stained with the anti-p-Smad2/3 Ab as described in materials and methods. Each bar represents means ± SE for a group of 6 (normal) or 8 (UUO) mice. *P < 0.05; ***P < 0.001 compared with the normal control. ###P < 0.001 when compared with the WT UUO. Magnification ×200.
Fig. 9.
Two-color immunohistochemistry shows that upregulation of renal Smad7 in Tg mice prevents activation of Smad2/3 at day 7 after UUO. Smad7 is labeled as brown, whereas phosphorylated Smad2/3 (p-Smad2/3) is stained as blue within the nucleus. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), and UUO kidney from a Tg mouse (D). Note that there is a strong activation of p-Smad2/3 (blue nuclei) with low expression level of Smad7 in the diseased kidney of WT mice (C). In contrast, renal Smad7 (brown) is markedly upregulated in Tg mice in both normal and UUO kidneys (B, D), which is associated with a significant inhibition of p-Smad2/3 nuclear translocation (blue nuclei). Magnification ×400.
Inhibition of TSP-1 expression may be a mechanism of prevention of renal TGF-β1 activation in mice with overexpression of latent TGF-β1.
Because only active TGF-β1 can bind to its receptors to initiate the downstream signaling pathway and because TSP-1 is the most important molecule mediating TGF-β activation (4, 20), we examined TSP-1 expression within the kidney by immunohistochemistry and systemic levels by ELISA. As shown in Fig. 10, normal kidneys from both WT and Tg mice exhibited a low, but constitutive expression of TSP-1 by glomerular cells, tubular epithelial cells, and interstitial cells (Fig. 10, A, B, E). In contrast, a marked increase in TSP-1 expression was found in the diseased kidney of WT mice, suggesting a strong activation of TGF-β1 locally within the diseased kidney, which was significantly inhibited in Tg mice (Fig. 10, C–E). ELISA analysis also demonstrated that there was a marked increase in plasma levels of TSP-1 in WT mice, while levels of TSP-1 in Tg mice remained normal (Fig. 10F).
Fig. 10.
TSP-1 is upregulated locally with the diseased kidney and increased systemically in plasma in WT mice, which is inhibited in Tg mice at day 7 after UUO. Normal kidney from a WT mouse (A), normal kidney from a Tg mouse (B), UUO kidney from a WT mouse (C), UUO kidney from a Tg mouse (D), quantitative analysis of TSP-1 immunostaining (E), and quantitative analysis (F) of plasma TSP-1 by ELISA. Note that renal TSP-1 (dark black) is markedly upregulated with severe tubulointerstitial fibrosis in WT mice with UUO, which is significantly inhibited in Tg mice. Each bar represents means ± SE for a group of 6 (normal) or 8 (UUO) mice. Arrows, TSP-1-positive cells. ***P < 0.05 compared with the normal control. ###P < 0.001 when compared with the WT UUO. Magnification ×200.
DISCUSSION
In addition to our previous report that latent TGF-β1 is able to protect the kidney from inflammation in a mouse model of UUO kidney disease (22), the present study added a new and significant finding that mice that overexpress latent TGF-β1 in skin keratinocytes were also capable of inhibiting progressive renal fibrosis. These observations provided a new role for latent TGF-β1 in anti-renal inflammation and fibrosis.
While mechanisms of latent TGF-β1 in anti-renal inflammation in a mouse model of UUO have been described previously (22), there were several mechanisms whereby latent TGF-β1 may exert its anti-fibrosis in UUO. First, inhibition of renal TGF-β1 expression and activation, thereby blocking TGF-β/Smad signaling, may be a critical mechanism of latent TGF-β1 in protection against renal fibrosis in obstructive kidney disease. It is well-documented that TGF-β is activated by a number of mechanisms including TSP-1 (4, 10, 20). In the present study, both renal and systemic TSP-1 expression was markedly increased in WT mice with UUO. In contrast, Tg mice had low expression levels of renal TSP-1 and normal levels of plasma TSP-1 during the disease course. This may largely account for a significant increase in active TGF-β1 in the UUO kidney in WT, but not in Tg mice (22). It is well accepted that active TGF-β1 binds its receptors and then activates its downstream mediators, Smad2 and Smad3, to exert its biological effects such as fibrosis (2, 5, 23). Thus, an increase in TSP-1 may contribute to high levels of active TGF-β1 within the diseased kidney in WT mice, which may result in activation on the downstream TGF-β/Smad signaling pathway, leading to progressive renal fibrosis. In contrast, inhibition of Smad2/3 activation and severe renal fibrosis in Tg mice with UUO may be associated with normal levels of TSP-1 and active TGF-β1. Thus, inhibition of TSP-1 expression, thereby reducing levels of active TGF-β1 and TGF-β/Smad signaling, may be one mechanism by which mice overexpressing latent TGF-β are protected against renal fibrosis in UUO. These findings suggest that latent and active TGF-β1 may work opposite to mediate renal fibrosis in obstructive kidney disease. This is supported by the findings that mice overexpressing a bioactive form of TGF-β1 in the liver develop progressive liver and renal fibrosis spontaneously (11) and that mutation in the gene encoding the LAP of TGF-β1 results in an increase in active TGF-β1 and causes Camurati-Engelmann disease (8). In addition, prevention of GVHD-associated skin fibrosis and liver damage in bioactive TGF-β1 Tg mice by administering the LAP demonstrates the counterregulating role of latent vs. active TGF-β1 in fibrosis (3, 25). This is further supported by the ability of additional LAPs to block TGF-β-induced fibronectin expression and PAI-1 promoter activity in vitro (3). In the present study, an increase in latent TGF-β1 resulted in inhibition of renal TGF-β/Smad signaling and its downstream fibrogenic mediator CTGF, thereby inhibiting renal fibrosis. This is consistent with our recent finding that mice overexpressing latent TGF-β1 are protected against renal fibrosis in an immunologically induced anti-GBM crescentic glomerulonephritis (7). Taken together, all these findings suggest a counterregulating role for latent TGF-β1 in renal fibrosis induced by active TGF-β1 and demonstrate the functional complexity of latent vs. active TGF-β1 in renal fibrosis and inflammation.
Second, although the counterregulating mechanisms between latent and active TGF-β1 remain largely unknown, induction of renal Smad7 could be a mechanism of anti-fibrosis and anti-inflammation by latent TGF-β1 in UUO. We previously showed that renal Smad7 is upregulated in TGF-β Tg mice with UUO and that upregulation of renal Smad7 is capable of inhibiting NF-κB-dependent renal inflammation by inducing a NF-κB inhibitor, IκBα, in this diseased model (22). In the present study, two-color immunohistochemistry clearly demonstrated that a marked Smad2/3 activation in the diseased kidney of WT mice was associated with a reduction in renal Smad7, while a strong upregulation of renal Smad7 in Tg mice resulted in a marked inhibition of p-Smad2/3 nuclear translocation, suggesting a critical role for Smad7 in inhibition of TGF-β signaling in renal fibrosis. The signaling mechanism that Smad7 inhibits Smad2/3-mediated renal fibrosis and NF-κB-dependent renal inflammation in response to latent TGF-β1 is further demonstrated in our recent study in an immunologically induced mouse model of anti-GBM glomerulonephritis (7). Although it remains unclear as to how latent TGF-β1 induces renal Smad7, it is possible that a persistent higher level of latent TGF-β1 in both plasma and kidney tissues may allow maintaining a low level of active TGF-β1, which may favor maintaining a TGF-β/Smad signaling at the physiological level, thereby steadily stimulating renal Smad7 expression that prevents overactivation of Smad2/3 and renal fibrosis. However, this could be lost when Smad2/3 became overactivated in response to higher levels of active TGF-β1 as seen in this and other models of renal fibrosis (6, 13). Normally, renal Smad7 is high, but it is significantly reduced in the fibrotic kidney associated with overactivation of Smad2/3 in various chronic kidney disease models (6, 13, 17), including this study. Thus, overexpression of renal Smad7 blocks TGF-β/Smad2/3-mediated renal fibrosis in vitro (15), in a rat model of UUO (20, 24), and in rat remnant kidney diseases (7). Taken together, upregulation of renal Smad7 could be a central mechanism by which mice overexpressing latent TGF-β1 are protected against activation of TGF-β/Smad signaling and Smad-dependent renal fibrosis in UUO.
Finally, inhibition of renal inflammation may also contribute to prevention of renal fibrosis. We previously showed that overexpression of renal Smad7 is able to inhibit renal inflammation, including macrophage and T cell infiltration and expression of IL-1β and TNF-α in a mouse model of UUO with high levels of latent TGF-β1 and in a rat model of remnant kidney disease (18, 22). Thus, reduced renal inflammation may in turn favor inhibition of renal fibrosis by blocking upregulation of fibrogenic growth factors such as TGF-β1 and CTGF in response to inflammatory stimulation within the UUO kidney.
In summary, in contrast to the previous finding that overexpression of an active hepatic TGF-β1 largely elevates circulating bioactive TGF-β1 and results in progressive renal fibrosis (11), mice that express a latent form of TGF-β1 in skin keratinocytes exhibited renoprotective effects on renal fibrosis in obstructive kidney disease. Inhibition of TSP-1 and upregulation of renal Smad7, thereby blocking renal TGF-β1 expression, activation, and TGF-β/Smad signaling, could be key mechanisms by which latent TGF-β protects against renal fibrosis.
GRANTS
This study was supported by grants from the Research Grant Council of Hong Kong (RGC GRF 759206 and 768207) to H. Y. Lan and the National Institutes of Health/National Cancer Institute (CA79998 and GM70966) to X. J. Wang.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Border WA, Noble NA. TGF-beta in kidney fibrosis: a target for gene therapy. Kidney Int 51: 1388–1396, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Bottinger EP TGF-beta in renal injury and disease. Semin Nephrol 27: 309–320, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bottinger EP, Factor VM, Tsang ML, Weatherbee JA, Kopp JB, Qian SW, Wakefield LM, Roberts AB, Thorgeirsson SS, Sporn MB. The recombinant proregion of transforming growth factor beta1 (latency-associated peptide) inhibits active transforming growth factor beta1 in transgenic mice. Proc Natl Acad Sci USA 93: 5877–5882, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauchard JH, Berton A, Godeau G, Hornebeck W, Bellon G. Activation of latent transforming growth factor beta 1 and inhibition of matrix metalloprotease activity by a thrombospondin-like tripeptide linked to elaidic acid. Biochem Pharmacol 67: 2013–2022, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol 166: 761–771, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang XR, Chung ACK, Zhou L, Wang XJ, Lan HY. Mice overexpressing TGF-β1 are protected against crescentic glomerulonephritis: protective role of latent TGF-β1. J Am Soc Nephrol 19: 233–242, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssens K, Gershoni-Baruch R, Guanabens N, Migone N, Ralston S, Bonduelle M, Lissens W, Van Maldergem L, Vanhoenacker F, Verbruggen L, Van Hul W. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat Genet 26: 273–275, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-beta receptor for degradation. Mol Cell 6: 1365–1375, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Khalil N TGFbeta from latent to active. Microbes Infect 1: 1255–1263, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Bottinger EP, Klotman PE, Thorgeirsson SS. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 74: 991–1003, 1996. [PubMed] [Google Scholar]
- 12.Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 43: 97–102, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Li AG, Wang D, Feng XH, Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J 23: 1770–1781, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol 13: 1464–1472, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26: 579–591, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Liu FY, Li XZ, Peng YM, Liu H, Liu YH. Arkadia-Smad7-mediated positive regulation of TGF-beta signaling in a rat model of tubulointerstitial fibrosis. Am J Nephrol 27: 176–183, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Ng YY, Hou CC, Wang W, Huang XR, Lan HY. Blockade of NF-kB activation and renal inflammation by ultrasound-mediated gene transfer of smad7 in rat remnant kidney. Kidney Int Suppl 94: S83–S91, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Oklu R, Hesketh R. The latent transforming growth factor beta binding protein (LTBP) family. Biochem J 352: 601–610, 2000. [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 274: 13586–13593, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Terada Y, Hanada S, Nakao A, Kuwahara M, Sasaki S, Marumo F. Gene transfer of Smad7 using electroporation of adenovirus prevents renal fibrosis in post-obstructed kidney. Kidney Int 61: 94–98, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 16: 1371–1383, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 10: 48–56, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood 87: 1439–1445, 1996. [PubMed] [Google Scholar]
- 25.Zhang Y, McCormick LL, Gilliam AC. Latency-associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol 121: 713–719, 2003. [DOI] [PubMed] [Google Scholar]