Abstract
The UT-A1 urea transporter plays an important role in the urine concentrating mechanism. Vasopressin (or cAMP) increases urea permeability in perfused terminal inner medullary collecting ducts and increases the abundance of phosphorylated UT-A1, suggesting regulation by phosphorylation. We performed a phosphopeptide analysis that strongly suggested that a PKA consensus site(s) in the central loop region of UT-A1 was/were phosphorylated. Serine 486 was most strongly identified, with other potential sites at serine 499 and threonine 524. Phosphomutation constructs of each residue were made and transiently transfected into LLC-PK1 cells to assay for UT-A1 phosphorylation. The basal level of UT-A1 phosphorylation was unaltered by mutation of these sites. We injected oocytes, assayed [14C]urea flux, and determined that mutation of these sites did not alter basal urea transport activity. Next, we tested the effect of stimulating cAMP production with forskolin. Forskolin increased wild-type UT-A1 and T524A phosphorylation in LLC-PK1 cells and increased urea flux in oocytes. In contrast, the S486A and S499A mutants demonstrated loss of forskolin-stimulated UT-A1 phosphorylation and reduced urea flux. In LLC-PK1 cells, we assessed biotinylated UT-A1. Wild-type UT-A1, S486A, and S499A accumulated in the membrane in response to forskolin. However, in the S486A/S499A double mutant, forskolin-stimulated UT-A1 membrane accumulation and urea flux were totally blocked. We conclude that the phosphorylation of UT-A1 on both serines 486 and 499 is important for activity and that this phosphorylation may be involved in UT-A1 membrane accumulation.
Keywords: protein kinase A consensus sites, protein kinase A, phosphorylation mutation, membrane trafficking, forskolin
intracellular camp levels within kidney cells are increased in response to a variety of hormonal and chemical stimuli, such as vasopressin (AVP) (8). AVP binds to the V2 receptor and activates the heterotrimeric G protein Gαs, resulting in an increased generation of cAMP by at least two adenylyl cyclase isoforms (2, 5, 13). Increased cAMP levels activate cAMP-dependent protein kinase A (PKA). While not all of the downstream targets of PKA have been elucidated in the inner medullary collecting duct (IMCD), PKA is known to phosphorylate the aquaporin-2 (AQP2) water channel (10) predominantly at two sites: S256 and S261. PKA phosphorylation at these sites is important for AVP-stimulated trafficking of AQP2 to the IMCD apical plasma membrane (6, 7), leading to increased levels of water reabsorption (12). Two additional potential phosphorylation sites have been reported, although their function is not known (7).
Increased AVP also results in the insertion of the UT-A1 urea transporter into the IMCD apical plasma membrane (9). While the mechanism for this action is unclear, it is likely that, similar to APQ2, movement of UT-A1 to the membrane is a result of PKA phosphorylation of the transporter. We previously demonstrated (15) that UT-A1 is phosphorylated by PKA, but the exact site of phosphorylation has not been determined. In this study, we demonstrate that UT-A1 is phosphorylated by PKA at two sites and that both of these sites must be phosphorylated for maximal AVP-regulated accumulation of UT-A1 into the cell membrane and increases in urea transporter activity.
METHODS
Phosphopeptide analysis.
Inner medullas from two Sprague-Dawley rats were metabolically labeled with [32P]orthophosphate as previously described (15). Nonradiolabeled IMCDs from six additional rats were treated in parallel. After stimulation with 10 μM forskolin for 30 min, UT-A1 was immunoprecipitated from IMCD lysates. The immunoprecipitated proteins were separated on a 25-cm × 25-cm SDS polyacrylamide gel. The gel was sectioned and the radiolabeled lane sliced to reveal the location of phosphorylated UT-A1. The section of the gel adjacent to the radiolabeled UT-A1 (containing phosphorylated but not radiolabeled UT-A1) was collected and submitted to 21st Century Biochemical (Marlboro, MA) for phosphopeptide analysis by tandem mass spectrometry (MS/MS).
Site-directed mutagenesis.
PKA consensus sites ([RK]-X-[ST]) (3) were mutated from rat UT-A1 with the QuickChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer's instructions. The serine (S) or threonine (T) residue was substituted with alanine (A) residue. The oligonucleotide 5′-CCCAGGCGTAAGGCCGTGTTCCATATCGAGTGG-3′ was used to mutate the first serine residue at S486. Oligonucleotide 5′-TCATCCATCCGGAGGAGGGGCAAAGTGTTTGGAAAAAG-3′ was used to mutate the second serine residue at S499. Oligonucleotide 5′-GTATCGGAAGCCCGCAGTGGAACTCTTGG-3′ was used to mutate threonine residue 524. A double mutant (S486A/S499A) construct was also generated. The constructs were verified by nucleotide sequence analysis (Macrogen, Rockville, MD).
Cell culture and transfections.
LLC-PK1 cells were maintained in DMEM containing 10% FBS, 5% l-glutamine, and 5% penicillin-streptomycin at 37°C and gassed with 5% CO2-95% air. Cells were grown on six-well plates. At 60% confluence, cells were transfected with UT-A1 (wild type), S486A, S499A, T524A, S486A/S499A, or vehicle, using Effectene (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Maximal protein expression occurred after 36 h.
Western blot analysis.
Proteins (20 μg/lane) were analyzed by Western blot as previously described (1, 9).
32P labeling of LLC-PK1 cells.
Transfected LLC-PK1 cells in six-well plates were metabolically labeled as previously described (15). After stimulation with 10 μM forskolin for 30 min, UT-A1 was immunoprecipitated from cell lysates. Proteins were analyzed by Western blot and autoradiography of the dried polyacrylamide gel.
Biotinylation of transfected LLC-PK1 cells.
Transfected LLC-PK1 cells were biotinylated as previously described (1, 9). Forskolin (10 μM) was added for 30 min at 37°C, and then samples were washed free of excess solution twice with PBS and three times with biotinylation buffer without biotin (mM: 215 NaCl, 4 KCl, 1.2 MgSO4, 2 CaCl2, 5.5 glucose, 10 triethanolamine, 2.5 Na2HPO4). Forskolin was added back during the incubation with biotinylation buffer containing 3 mg/ml biotinamidohexanoic acid 3-sulfo-N-hydroxysuccinimide ester (Sigma-Aldrich, St. Louis, MO) for 60 min at 4°C. With this protocol, biotin does not enter the cells (9). Cells were then washed free of unattached biotin by three washes with biotin quenching buffer (mM: 0.1 CaCl2, 1 MgCl2, 260 glycine in PBS), with the last wash incubated for 20 min at 4°C. Next, samples were washed three times with lysis buffer without detergent and the cells were solubilized for 1 h in lysis buffer containing 1% NP-40 (mM: 150 NaCl, 5 EDTA, 50 Tris). After centrifugation (14,000 g, 10 min, 4°C) to remove insoluble particulates, streptavidin beads were added to the supernatant fractions and allowed to absorb biotinylated proteins overnight at 4°C. After washing with high-salt and no-salt buffers, Laemmli SDS-PAGE sample buffer was added directly to the pellets, samples were boiled for 1 min, and the pool of biotinylated proteins was analyzed by Western blot.
cRNA injection and protein expression in Xenopus oocytes.
Oocytes were prepared for injection of UT-A1 cRNA as previously described (11). The full-length coding region of UT-A1 and each mutant (described above) was subcloned into the oocyte expression vector pGH19. After linearization, cRNAs were synthesized by T7 polymerase with the mMessage mMachine T7 Ultra kit (Ambion, Austin, TX) and injected into collagenase-treated oocytes. Two nanograms of each cRNA in 23 nl of water was injected into each oocyte. Injected oocytes were maintained in OR3 medium for 2–3 days at 18°C.
Urea assay.
Oocytes were preincubated with 1 ml of uptake solution 1 [mM: 200 mannitol, 2 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES buffer, 5 Tris·HCl (pH 7.4)]. PKA agonists were examined by preincubation of oocytes for 2 h in 500 μM 8-(4-chlorophenylthio)- adenosine-3′,5′-cyclic monophosphate (CPT-cAMP), 500 μM 3-isobutyl-1-methylxanthine (IBMX), and 50 μM forskolin. Cells were then incubated in a solution containing 2 μCi [14C]urea/ml and 1 mM cold urea. Cells were washed five times with ice-cold uptake solution containing 1 mM cold urea. Each individual cell was dissolved in 10% SDS, followed by scintillation counting.
To isolate protein, 20 oocytes were homogenized in 200 μl of homogenization buffer [mM: 20 Tris·HCl (pH 7.4), 5 MgCl2, 5 NaH2PO4, 2 EDTA, 80 sucrose] supplemented with protease inhibitors (Sigma-Aldrich). Homogenates were centrifuged twice at 100 g for 10 min to remove yolk proteins. Total lysate was prepared in Laemmli sample buffer (2 μl/oocyte) at 37°C for 1 h.
Statistics.
Data are presented as means ± SE; comparisons were made with one-way ANOVA and Fisher's least significant difference posttest. P < 0.05 was considered significant.
RESULTS
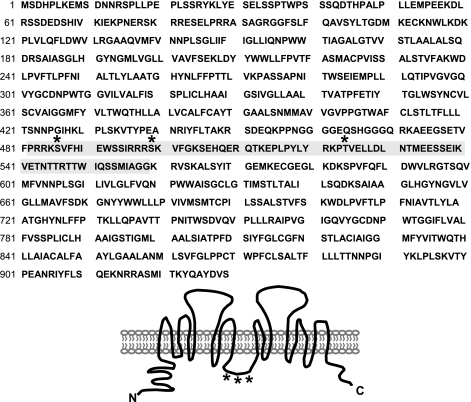
To determine potential AVP-sensitive phosphorylation sites, rat IMCD peptides were analyzed by HPLC followed by MS/MS analysis. The redundancy of peptide identities was considered a predictive indicator for in vivo phosphorylation sites. The shaded area in Fig. 1 indicates the sequence of the intracellular loop region where phosphorylation is likely. Within that area, three PKA consensus sites were detected: serine 486, serine 499, and threonine 524.
Fig. 1.
Tandem mass spectrometry (MS/MS) detects three protein kinase A (PKA) consensus sites in forskolin-stimulated UT-A1. UT-A1 was isolated from rat inner medulla treated with forskolin (10 μM) (methods). Potential vasopressin (AVP)-stimulated phosphorylation sites between residues 482 and 558 (highlighted in gray) were revealed. PKA consensus sites: S486, S499, and T524 (indicated by *) in the intracellular loop of UT-A1.
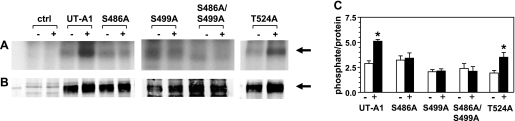
We mutated S486 and S499 as well as T524 to alanines and transfected them into LLC-PK1 cells. Given the strong potential for S486 and S499 to be phosphorylated as determined by our MS/MS analysis, a double mutation was also made (S486A/S499A). Wild type (unmutated) UT-A1 was transfected in parallel as a positive control. Figure 2 shows the autoradiogram (Fig. 2A) of the five transfected cells with and without forskolin stimulation. Although the absolute amount of UT-A1 produced in the transfected cells varied (Western blot, Fig. 2B), the percent stimulation by forskolin was able to be determined. The bar graph in Fig. 2C shows the results from six independent transfection experiments. There was a 50% increase in wild-type UT-A1 on stimulation. The S486A, S499A, and S486A/S499A mutants showed no increase in phosphorylation above basal levels in response to forskolin. The T524A mutant showed 57% stimulation, comparable to wild-type UT-A1.
Fig. 2.
S486 and S499 are targets for PKA phosphorylation. Shown are a representative autoradiogram (A) and a Western blot (B) of [32P]orthophosphate-labeled wild-type UT-A1 or UT-A1 phosphomutants immunoprecipitated from transiently transfected LLC-PK1 cells. Cells were treated with 10 μM forskolin (+) or vehicle (−), and untransfected LLC-PK1 cells were used as a negative control (ctrl). Densitometry was performed on Western blots and film with Licor and Image J, respectively, and phosphate-to-protein ratios were determined (C). Graph represents n = 6 independent transfection experiments. Data are means ± SE. *P < 0.05.
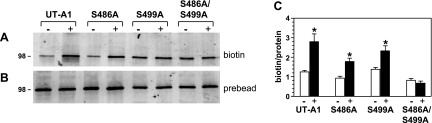
Next we determined whether the lack of phosphorylation of the mutants altered the ability of the S486A and S499A mutants to accumulate in the plasma membrane in response to forskolin. Figure 3A shows the membrane population of wild-type UT-A1, S486A, S499A, and S486A/S499A in transfected LLC-PK1 cells with and without forskolin (10 μM) treatment. The individual mutants (S486A and S499A) were inserted into the plasma membrane after forskolin treatment and assayed by biotinylation. However, the double mutant (S486A/S499A) was not moved to the membrane in response to forskolin stimulation. Figure 3B shows the Western blot of the total cell lysates probed for UT-A1. These protein values were used to normalize the UT-A1 in the membrane (biotinylated) so that the bar graph in Fig. 3C provides the amount of biotinylated UT-A1 per unit of total protein, combining the results from four independent transfection experiments. The accumulation of wild-type UT-A1 in the plasma membrane increased as previously reported (9).
Fig. 3.
Phosphorylation of both S486 and S499 is required for UT-A1 trafficking to the membrane. LLC-PK1 cells that were transiently transfected with UT-A1 and mutant constructs were treated with either vehicle control (−) or 10 μM forskolin (+) for 30 min before biotinylation was performed. Shown is a representative Western blot of biotinylated samples (A) and whole cell lysates (B). Densitometry was performed with Licor to determine the biotin-to-protein ratio (C). Graph represents n = 4 independent transfection experiments. Data are means ± SE. *P < 0.05.
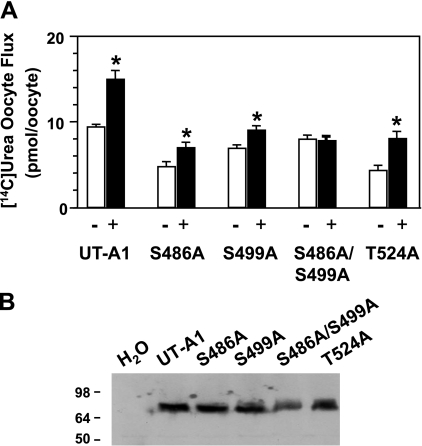
We used the oocyte system to monitor the transporter activity of wild-type UT-A1 and the phosphomutants. Figure 4A shows the average flux measurement by bar graph with SE bars. Forskolin increased urea flux of wild-type UT-A1 by 50%. The response to forskolin was reduced in the S486A and S499A mutants; however, there was a small but significant increase in urea flux. Only the S486A/S499A double mutant showed no response in flux after forskolin treatment. The T524A mutation resulted in a lower basal flux but had no effect on the ability of forskolin to stimulate urea transporter activity (Fig. 4). Figure 4B is a representative Western blot analysis of the oocyte proteins showing comparable productions of the transfected proteins.
Fig. 4.
PKA phosphorylation at sites S486 and S499 is needed for urea transport activity in Xenopus oocytes. Oocytes were injected with indicated cRNA or a mixture of cRNAs as described in methods. A: before urea flux was measured, oocytes were incubated in the absence (open bars) or presence (filled bars) of 500 μM 8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphate (CPT-cAMP), 500 μM 3-isobutyl-1-methylxanthine (IBMX), and 50 μM forskolin for 2 h. Each bar shows the mean ± SE obtained from 6 individual oocytes from 3 different experiments. *P < 0.05. B: representative Western blot analysis shows equal expression of wild-type UT-A1 and each of the UT-A1 mutant proteins generated by oocytes.
DISCUSSION
AVP activates the UT-A1 urea transporter (9), but the mechanism of this regulation has not been proven. It is assumed that regulation involves a PKA-mediated phosphorylation because the activation of adenylyl cyclase is the most common action ascribed to AVP. Early reports showed AVP stimulation of urea transport in isolated perfused IMCDs (12). This prompted a study of AVP stimulation of UT-A1 phosphorylation that characterized the time course and dose response (15). This report found that the phosphorylation occurred in a time frame (evident at 2 min, maximal at 5–10 min) that paralleled the increase in urea transport observed in perfused rat IMCDs (14). Although this study documented that UT-A1 was phosphorylated in a PKA-dependent manner, it did not specifically address whether UT-A1 was the actual substrate for PKA or if it was downstream from the observed PKA effect.
Recently, we showed (9) that UT-A1 accumulates in the plasma membrane in response to stimulation by AVP. Again, this is likely to be a PKA-mediated event. We also showed that in Madin-Darby canine kidney (MDCK) cells that stably express UT-A1 AVP increases urea transporter activity. This activity is partially blocked by inhibition of PKA with H-89, a PKA inhibitor (4). Together, these earlier studies indicate that UT-A1 is phosphorylated, possibly by PKA directly, and this is linked both to UT-A1 accumulation in the plasma membrane and an increase in urea transport activity.
To determine the mechanism by which UT-A1 is regulated, we investigated whether UT-A1 was phosphorylated at one or more PKA consensus sites. Our initial phosphopeptide analysis identified a number of peptides that had a high potential of being phosphorylated. Within the intracellular loop region of UT-A1, a span of amino acids between 482 and 558 showed the highest probability of containing the AVP-responsive phosphorylation site. This region contains three residues that conform to a PKA-consensus sequence: S486, S499, and T524. S486 was also identified in a proteomics approach by Hoffert et al. (7). These serines were mutated to alanines, along with a mutation of both S486 and S499 to create a double mutant. T524 was also mutated to alanine, although this residue did not have a high probability for phosphorylation. The lack of increase in radiolabeled phosphate incorporation into the S486A, S499A, and S486A/S499A constructs verifies that phosphorylation at these residues is cAMP sensitive. The failure of the S486A/S499A double mutant to accumulate in the plasma membrane suggests that phosphorylation of at least one of these residues, if not both, may be required for the movement of UT-A1 to, or insertion into, the plasma membrane. The fact that either the S486A or the S499A phosphomutant could be stimulated to accumulate in the membrane suggests that there may be a redundancy in the regulation. It is unclear at this time whether there is a primary and a secondary site of phosphorylation, or whether these two sites are interactive in some way.
Unfortunately, the LLC-PK1 cells that were used to assess the phosphorylation and biotinylation of the mutants and wild-type UT-A1 were not able to be used for evaluation of urea flux. However, injection of wild-type UT-A1 as well as each phosphomutated UT-A1 into oocytes allowed for successful urea flux measurements. Results suggest that there is a considerable loss of cAMP-stimulated urea flux on either single serine mutation, and a complete loss in the double mutant. The reduction in the degree of activation of the mutant transporters compared with the wild-type UT-A1 in the oocyte flux assays indicates that phosphorylation is responsible for the increased activity of the transporter in response to stimulation of cAMP. We conclude that the phosphorylation of UT-A1 at both S486 and S499 is important for activity and that this may play a role in accumulation of UT-A1 in the plasma membrane.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-62081, R01-DK-41707, and P01-DK-61521, American Heart Association Grant-in-Aid 0655280B and Postdoctoral Fellowship Grant, a Edward Noble-David Lowance, M.D. Research Fellowship from the National Kidney Foundation of Georgia, and the Cottrell Fellowship of Emory University.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Chabardes D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM. Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci 36: 275–328, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol 291: C600–C606, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Helies-Toussaint C, Aarab L, Gasc JM, Verbavatz JM, Chabardes D. Cellular localization of type 5 and type 6 ACs in collecting duct and regulation of cAMP synthesis. Am J Physiol Renal Physiol 279: F185–F194, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imbert M, Chabardes D, Montegut M, Clique A, Morel F. Vasopressin dependent adenylate cyclase in single segments of rabbit kidney tubule. Pflügers Arch 357: 173–186, 1975. [DOI] [PubMed] [Google Scholar]
- 9.Klein JD, Fröhlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680–2686, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J Biol Chem 270: 10384–10387, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Mistry AC, Mallick R, Fröhlich O, Klein JD, Rehm A, Chen G, Sands JM. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem 282: 30097–30106, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Morgan T, Sakai F, Berliner RW. In vitro permeability of medullary collecting ducts to water and urea. Am J Physiol 214: 574–581, 1968. [DOI] [PubMed] [Google Scholar]
- 13.Orloff J, Handler JS. The similarity of effects of vasopressin, adenosine-3′,5′-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest 41: 702–709, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall SM, Han JS, Chou CL, Knepper MA. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 262: F989–F998, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002. [DOI] [PubMed] [Google Scholar]