Abstract
Phosphorylation of serine 256 (S256) plays a critical role in vasopressin (VP)-mediated membrane accumulation of aquaporin-2 (AQP2). Recently, phosphorylation of serine 261 was also reported, raising the possibility that it has a role in AQP2 trafficking. We addressed this issue using transfected LLC-PK1 cells that express point mutations of AQP2 S261 and S256, mimicking the phosphorylated (S to D) or dephosphorylated (S to A) states of these residues. Both AQP2 (S261A) and AQP2 (S261D) were located in the perinuclear cytoplasm without stimulation but, like wild-type AQP2, they both accumulated on the plasma membrane after 20-min exposure to VP or forskolin. Following membrane accumulation, S261A, S261D, and wild-type AQP2 reinternalization was complete over a similar time frame, between 30 and 60 min after VP washout. Using various combinations of point mutations, we showed that the phosphorylation state of S256 is dominant with respect to AQP2 behavior; AQP2 membrane accumulation and internalization were not detectably affected by the phosphorylation state of S261. Finally, blocking AQP2 endocytosis by methyl-β-cyclodextrin caused membrane accumulation of AQP2 in cells expressing either a single S-A mutation or double mutations of S256 and S261, although as previously reported, the S256D mutation was always present at the cell surface. This suggests that constitutive recycling of AQP2 was not modified by the phosphorylation state of S261. Together, our data indicate that the phosphorylation state of AQP2 at S261 does not detectably affect regulated or constitutive trafficking of AQP2. The potential role of S261 phosphorylation/dephosphorylation in vasopressin action remains to be determined.
Keywords: vasopressin, cAMP
aquaporin-2 (AQP2) is expressed in kidney collecting duct principal cells and has a critical role in the urinary concentrating mechanism (4, 11, 19, 20). It is well known that phosphorylation of the serine 256 residue (S256) on the cytoplasmic COOH terminus by PKA is required for the functionally important vasopressin (VP)-induced membrane accumulation of AQP2 in VP target cells (6, 14, 16, 18, 25, 26). In addition to S256, other potential phosphorylation sites in the AQP2 COOH terminus, including serine 261 (S261), were identified recently (10). By using quantitative phosphoproteomic analysis of renal cells, it was found that in the presence of VP, monophosphorylated AQP2 at S256 and diphosphorylated AQP2 at S256/S261 were increased in abundance, whereas monophosphorylated AQP2 at S261 was decreased. These data raised the possibility that phosphorylation of AQP2 at both sites is involved in VP-dependent AQP2 trafficking. The dynamics of the phosphorylation of AQP2-S261 in the presence of VP was further studied by using phospho-specific antibodies against p-S261 and p-S256 (8). This study revealed a differential subcellular localization of phosphorylated S256 and phosphorylated S261 in the presence of VP and showed that phosphorylated p-S256 AQP2 increased whereas the level of phosphorylated S261 AQP2 decreased in freshly isolated rat inner medullary collecting ducts (IMCD) incubated with 1 nM [deamino-Cys(1),d-Arg(8)]vasopressin (dDAVP) for 30 min. This again suggests potentially distinct roles of phosphorylation of AQP2 at S256 and S261 in the regulation of AQP2 trafficking. However, the role of S261 phosphorylation/dephosphorylation in AQP2 trafficking remains to be determined. We address this issue in the present study using serine-to-alanine (S-A; mimicking dephosphorylation) and serine-to-glutamic acid (S-D; mimicking phosphorylation) point mutations in the S261 and S256 residues of AQP2, alone or in combination, and conclude that the phosphorylation state of the S256 residue is the critical factor that determines the fate and subcellular location of AQP2 in our transfected cell model.
MATERIALS AND METHODS
Antibodies and chemicals.
Monoclonal antibody against the c-myc tag was generated from the hybridoma cell line 9E10 that was purchased from American Type Culture Collection (ATCC). A polyclonal goat anti-AQP antibody raised against the extreme COOH terminus of AQP2 was purchased from Santa Cruz Biotechnology. VP ([Lys8]-vasopressin), forskolin, and methyl-β-cyclodextrin were purchased from Sigma-Aldrich.
Generation of AQP2 constructs carrying various point mutations.
cDNA encoding rat AQP2 tagged with a COOH-terminal c-myc epitope (pcDNAI-rAQP2/Neo) was used as a template to generate point mutations at S256 and/or S261. Point mutations at S261 of AQP2 were introduced into the pcDNAI-rAQP2 wild-type construct by site-directed mutagenesis using the following primers: AQP2-S261A forward: 5′-GGTGGAGCTCCACGCTCCTCAGAGCC-3′ and reverse: 5′-GGCTCTGAGGAGCGTGGAGCTCCACC-3′; and AQP2-S261D forward: 5′GTGGAGCTCCACGATCCTCAGAGCC-3′ and reverse: 5′AGGCAGGCTCTGAGGATCGTGGAGCTCCAC-3′. pcDNAI constructs containing AQP2-S256A and AQP2-S256D were generated previously and were used as templates for generating double mutation of S256 and S261. For double mutations, the following primers were used: AQP2-S256A/S261A forward: 5′GAAGTGCGGCGGCGGC AGGCTGTGGAGCTCCACGCTCCTCAGAG 3′ and reverse: AGGCAGGCTTGAGGAGCGTGGAGCTCCACAGCCTGCCGCCGCCG3′; AQP2-S256A/S261D forward: 5′GAAGTGCG GCGGCGGCAGGCTGTGGAGCTCCACGATCCTCAGAG 3′ and reverse: 5′ AGGCAGGCT CTGAGGATCGTGGAGCTCCACAGCCTGCCGCCGCCG 3′; AQP2-S256D/S261A forward: 5′GAAGTGCGGCGGCGGCAGGATGTGGAGCTCCACGCTCCTCAGAG 3′ and reverse: AGGCAGGCTCTGAGGAGCGTGGAGCTCCACATCCTGCCGCCGCCG 3′; and S256D/S261D forward: 5′GAAGTGCGGCGGCGGCAGGATGTGGAGCTCCACGATCCTCAGAG 3′ and reverse: 5′ AGGCAGGCTCTGAGGATGTGGAGCTCCACATCCTGCCGCCGCCG 3′. The codons corresponding to mutated amino acids are underlined. PcDNAI/Neo vectors with each mutated AQP2 construct were prepared for transfection into LLC-PK1 cells. Nucleotide sequences were verified twice for each construct using an automated DNA sequencer in the Massachusetts General Hospital Sequencing Core.
Transfection and establishment of stable cell lines.
LLC-PK1 cells stably expressing wild-type AQP2, AQP2–256A, or AQP2–256D with a c-myc tag at their COOH termini (referred to as 256A or 256D cells, respectively) were described previously (16). Following the same protocol, pcDNAI constructs containing single or double mutations of S256 and/or S261 were transfected into LLC-PK1 cells. Briefly, LLC-PK1 cells were grown on coverslips and were transfected with pcDNA1/Neo constructs using lipofectamine as previously described (13). Approximately 36–48 h after the transfection, some of these transiently transfected cells were directly studied for AQP2 trafficking, and some of the cells were cultured in the presence of G418 and allowed to grow as uncloned stable cells. Meanwhile, some cells were cloned using the limiting dilution method to obtain single clonal populations to confirm the data obtained in some mixed populations. Single-cell clones stably expressing AQP2-261A, AQP2-261D, AQP2-256A/261D, or AQP2-256D/261D (referred to as 261A, 261D, 256A/261D, or 256D/261D cells) were established following a standard protocol (16, 23). At least six clones from each transfection were assayed for VP-induced AQP2 trafficking. Single-cell cloning was not attempted for S256D/S261A or S256A/S261A because clear-cut results were obtained with mixed cell populations. The expression of each AQP2 mutant protein was analyzed by SDS-PAGE and immunoblot using anti-c-myc and anti-AQP2 antibody as described previously (17).
To be sure that the phenotype we observed for each mutant AQP2 represents the true phenotype rather than a sampling bias or clonal variation, we performed two more transient transfection experiments to verify our observed phenotype for each construct. We then repeated the trafficking study in uncloned populations of stable cells from each transfection to confirm the most representative/major phenotype of each construct in the uncloned population. Trafficking studies were conducted in both cloned populations as well as uncloned cells, and at least three independent experiments were performed on each cell line for each study.
AQP2 trafficking in transfected cells.
Cells were grown on coverslips in each culture dish for 2 or 3 days to reach near 90% confluence. Then the coverslips were treated with VP (10 nM), forskolin (1 μm), or methyl-β-cyclodextrin (10 mM), respectively, for 20 min. Coverslips were harvested and fixed with 4% paraformaldehyde in PBS at room temperature for 20 min. After washing three times with PBS, they were subjected to immunostaining as described below. Cells were permeabilized with 0.1% saponin in PBS at room temperature for 30 min. After blocking with 1% BSA in 0.1% saponin/PBS for 10 min, cells were incubated with anti-c-myc monoclonal antibodies (1:1 dilution) or anti-AQP2 antibodies (1:200 dilution) in PBS at room temperature for 2 h. After a wash with 0.1% saponin/PBS, cells were incubated with Cy3-conjugated donkey anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories) at 1:600 dilution in PBS for 1 h at room temperature. After final washes with PBS, cells were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and examined with a Nikon Eclipse 80i microscope equipped with epifluorescence optics and IP Lab Spectrum acquisition software (Scanalytics).
In the VP-washout experiment, after treatment with 10 nM VP for 20 min, cells were washed three times with prewarmed DMEM and then incubated in DMEM containing 10% FBS for 30 min, 1 h, and 2 h at 37°C, respectively. At various time points, coverslips were removed and cells were fixed and immunostained as above.
RESULTS AND DISCUSSION
Generation of cDNA constructs and expression of mutant AQP2 in stable cell lines.
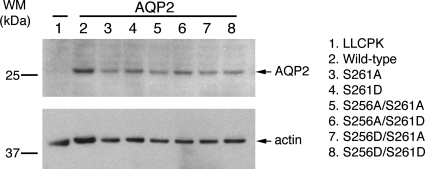
Single point mutations of S261 as well as double point mutations of S256 and S261, i.e., S261A, S261D, S256A/S261A, S256A/S261D, S256D/S261A, and S256D/S161D, were obtained by site-directed mutagenesis. Individual mutations were verified twice by DNA sequencing. A total of six constructs were transfected into LLC-PK1 cells. A clonal population was obtained from transfection with AQP2-S261A, S261D, S256A/261D, and S256D/S261D for further study. The level of expression of AQP2 in all transfected cell lines was confirmed by immunoblotting using an antibody against AQP2. The expression levels were comparable in all cell lines (Fig. 1).
Fig. 1.
Expression of mutant aquaporin-2 (AQP2) protein in LLC-PK1 cells. LLC-PK cells were transfected with various AQP2 constructs including S261A, S261D, S256A/S261A, S256A/S261D, S256D/S261A, and S256D/S261D. Cell lysates were obtained from stably transfected cells and subjected to SDS-PAGE and immunoblotting with anti-AQP2 antibody. Arrows indicate AQP2 and actin, respectively. Molecular weight markers (WM) are indicated on the left. The expression levels of the various AQP2 constructs were similar in all cell lines examined.
AQP2 trafficking in cells expressing mutated AQP2 at s256 and/or s261.
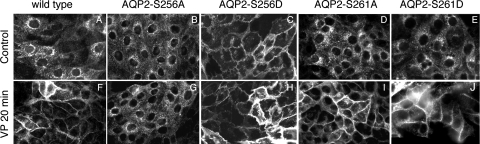
The trafficking of AQP2 was investigated in both transiently transfected cells and stably transfected cells with S261D, S261A, S256A/S261D, S256D/S261D, S256A/S261A, and S256D/S261A. Under basal, nonstimulated conditions, S261A and S261D mutants located mainly in the perinuclear region of the cytoplasm (Fig. 2, D and E). Upon stimulation with VP (or forskolin; data not shown) for 20 min, a dramatic membrane accumulation of AQP2 was seen in both cell lines (Fig. 2, I and J), similar to cells expressing wild-type AQP2 (Fig. 2, A and F) but unlike AQP2-S256A, which does not accumulate on the plasma membrane in response to VP (Fig. 2, B and G). This suggests that changing the phosphorylation state of AQP2 at S261 alone does not alter the trafficking/membrane accumulation of AQP2 that is mediated by an increase in intracellular cAMP.
Fig. 2.
Trafficking pattern of AQP2 in cells stably expressing AQP2-wild-type, AQP2-S256A, AQP2-S256D, AQP2-S261A, and AQP2-S261D in response to vasopressin (VP). In cells expressing wild-type AQP2, AQP2 is present in the perinuclear region under basal conditions and accumulated on the plasma membrane upon VP treatment (A and F), and AQP2-S256A remains perinuclear with or without VP stimulation (B and G). AQP2-S256D is mainly located on the plasma membrane under both basal and stimulated conditions (C and H), while AQP2-S261A and AQP2-S261D respond to VP by accumulating on the plasma membrane, similar to wild-type AQP2 (D, E, I, and J).
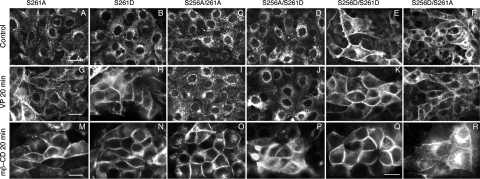
Based on the phosphoproteomic analysis by the Knepper group (8, 10), pS261 was estimated to be 24-fold more abundant than pS256 under basal conditions in collecting duct principal cells, whereas pS261 and pS256 are almost equally abundant after short-term dDAVP treatment (8, 9). This could be due to either dephosphorylation at S261 or phosphorylation at S256 in the presence of VP, resulting in a mixed population of dramatically increased pS256 and relatively reduced pS261 AQP2 molecules. It would be interesting to know whether phosphorylation of one site is required for the phosphorylation of the other site, i.e., whether the phosphorylation events are sequential or completely independent events. To examine the interaction between the phosphorylation or dephosphorylation of S256 and S261 in VP-induced AQP2 trafficking, we generated double-mutants at both S256 and S261. In the presence of simultaneous point mutations of S256 and S261, the cellular localization of AQP2 is determined mainly by the phosphorylation state of S256 and appears to be independent of the phosphorylation state of 261. S256A is always present in the cytoplasm (Fig. 2, B and G) and does not traffic in response to VP or forskolin irrespective of the phosphorylation state of S261 (Fig. 3, C, I, D, and J). Similarly, S256D is always present on the plasma membrane under basal or stimulated conditions (Fig. 2, C and H), irrespective of the phosphorylation state of S261 (Fig. 3, E, K, F, and L). Therefore, by studying various combinations of double mutations, our data suggest that the phosphorylation or dephosphorylation state at S261 does not alter the AQP2 trafficking pattern that is dictated by the phosphorylation state of S256. We conclude that phosphorylation or dephosphorylation at S256 plays a dominant role in this process. A summary of these mutations and the results is shown in Table 1.
Fig. 3.
VP stimulated AQP2 trafficking and constitutive recycling of AQP2 in stable cells expressing single and double mutations of AQP2 at S256 and S261. Cells expressing AQP2-S261A, S261D, S256A/S261A, S256A/S261D, S256D/S261A, and S256D/S261D were treated with VP (10 nM) and methyl-β-cyclodextrin (mβ-CD; 10 mM) for 20 min. Under unstimulated conditions, S256D/S261A and S256D/S261D accumulated on the plasma membrane (E and F) while AQP2-S261A, S261D, S256A/S261A, and S256A/S261D were located in the perinuclear cytoplasm (A–D). Upon stimulation with VP, S261A and S261D accumulated on the plasma membrane (G and H), similar to wild-type AQP2, while S256A/S261A and S256A/S261D remained in intracellular vesicles (I and J). S256D/S261A and S256D/S261D were located on the plasma membrane under all conditions (K and L). In all cell lines, a marked membrane accumulation was induced by treatment with methyl-β-cyclodextrin (from M–R). Results shown are representative of at least 3 independent experiments for each transfected cell line. B and H are duplicated images of Fig 2, E and F, and are included here for easy comparison. Bars = 20 μm.
Table 1.
Summary of AQP2 trafficking in S256 and S261 mutant cell lines with or without treatment of VP or methyl-β-cyclodextrin
|
Mutations |
Treatment
|
|||
|---|---|---|---|---|
| S256 | S261 | Control | VP | mβ−CD |
| –qSvelh | Spqslprgska | Cytoplasm | PM | PM |
| –qAvelh | Spqslprgska | Cytoplasm | Cytoplasm | PM |
| –qDvelh | Spqslprgska | PM | PM | PM |
| –qSvelh | Apqslprgska | Cytoplasm | PM | PM |
| –qSvelh | Dpqslprgska | Cytoplasm | PM | PM |
| –qAvelh | Apqslprgska | Cytoplasm | Cytoplasm | PM |
| –qAvelh | Dpqslprgska | Cytoplasm | Cytoplasm | PM |
| –qDvelh | Apqslprgska | PM | PM | PM |
| –qDvelh | Dpqslprgska | PM | PM | PM |
AQP2, aquaporin-2; VP, vasopressin; PM, plasma membrane; mβ−CD, methyl-β-cyclodextrin.
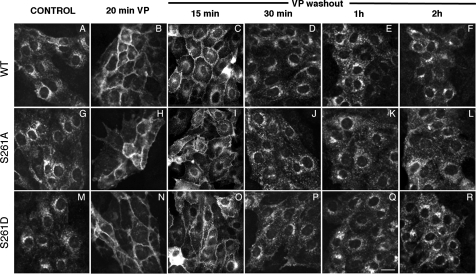
Our data also suggest that the phosphorylation of S256 is unlikely to be dependent on the phosphorylation or dephosphorylation state of S261. If they are indeed separate events, then what is the role (if any) of the phosphorylation and dephosphorylation of S261 in VP-mediated AQP2 trafficking? One possibility is that the rate of internalization of AQP2 after it has been inserted into the plasma membrane (via phosphorylation at S256 after stimulation by VP) is affected by S261 phosphorylation. To investigate this, a time course experiment after VP washout was performed. Our results show that the membrane retention of AQP2 in cells expressing S261A and S261D is indistinguishable from the wild-type AQP2 at the time points tested (Fig. 4). Fifteen minutes after VP washout, considerable plasma membrane staining was still apparent, but intracellular staining, especially in the perinuclear region of the cells, was clearly visible (Fig. 4, C, I, and O). The internalization process was complete between 30 and 60 min after VP washout. In all cases, plasma membrane AQP2 was lost over time, and antigenicity was returned to an intracellular location (presumably by endocytosis) in a similar pattern. These data indicate that the phosphorylation or dephosphorylation state of S261 does not noticeably alter the internalization of AQP2 after VP washout. As previously reported, all mutations in which S256 was replaced by glutamic acid remained concentrated at the cell surface under all conditions examined.
Fig. 4.
Distribution of wild-type AQP2, AQP2-S261A, and AQP-S261D during VP treatment and washout. Both AQP2-S261A, AQP2-S261D, and wild-type AQP2 (AQP2-WT) were present in the perinuclear region in the cytoplasm under control, nonstimulated conditions (A, G, and M). Upon treatment with VP for 20 min, AQP2-S261A, AQP-S261D, and AQP2-WT accumulated on the plasma membrane (B, H, and N). After washout of VP for 15 min, plasma membrane staining of AQP2 wild-type, S261A, and S261D remains but intracellular staining for AQP2 is more abundant than with no VP washout (compare B, H, and N with C, I, and O). After washout for 30 min, plasma membrane staining of AQP2 wild-type, S261A, and S261D was reduced, reflecting AQP2 internalization (D, J, and P). Between 1 and 2 h after washout, AQP2 wild-type, S261A, and S261D were located mainly in the perinuclear region of the cytoplasm (E, K, Q, F, L, and R). There was no obvious difference in the rate at which the plasma membrane staining of AQP2 was diminished over time after washout in these 3 cell lines. Bar = 20 μM.
As we have reported previously, AQP2 recycles constitutively between cytoplasmic vesicles and the plasma membrane (3, 7, 15, 16, 23). Does AQP2 still recycle constitutively in cells expressing AQP2 with S261A or S261D mutations? For this purpose, we used methyl-β-cyclodextrin, a nonspecific cholesterol-chelating agent, to block endocytosis as we have previously described (16). Simply by blocking endocytosis, AQP2 accumulates on the plasma membrane, revealing its constitutive recycling pattern. After blocking endocytosis, rapid membrane accumulation was observed in cells expressing S261A, S261D, S256A/S261A, and S256A/S261D, as well as cells expressing wild-type AQP2 protein (Fig. 3, M–R). Therefore, the phosphorylation and dephosphorylation state of S261 do not alter the constitutive recycling pathway of AQP2, since all variants tested accumulated at the plasma membrane after blockade of AQP2 internalization (summary in Table 1).
To summarize, our data indicate that the phosphorylation and dephosphorylation of S261 are unlikely to play a major role in the VP-induced membrane accumulation of AQP2, at least in our LLC-PK1 cell model. It does not alter the membrane accumulation of AQP2 in response to cAMP elevation, nor does it affect the membrane retention or internalization of AQP2 after washout of VP. Experiments using simultaneous mutations of S256 and S261 showed that the phosphorylation or dephosphorylation state of S261 does not alter the dominant effect driven by the phosphorylation and dephosphorylation state of S256 in VP-induced AQP2 trafficking. It remains possible that the effects that we have observed in our transfected cell model may not exactly mimic AQP2 trafficking in principal cells in vivo in this instance, although LLC-PK cells have proven to be a reliable predictor of the in vivo AQP2 signaling machinery in many previous studies (1, 2, 7, 12, 21–25). Also, it is possible that more subtle kinetic differences in the intracellular trafficking pathway of AQP2 occur that were not detectable using the present techniques. Nevertheless, we report here that the major cAMP-mediated event that characterizes vasopressin action in its target cells, and which leads to cell surface accumulation of AQP2, is not critically affected by the phosphorylation state of S261. The exact role of this newly discovered phosphorylation site of S261 on AQP2 trafficking remains, therefore, to be elucidated.
However, in addition to S261, two additional residues, S264 and S269, are also phosphorylated in response to VP treatment in vivo (8, 10). A very recent study addressed the phosphorylation status of S264 (pS264) during VP-mediated AQP2 trafficking in the renal medulla (5). Acute VP treatment in vivo increased the abundance of pS264-AQP2 in collecting ducts in parallel with a redistribution of pS264-AQP2 from intracellular vesicles to both apical and basolateral membranes of principal cells. The importance of S264 in this process remains to be examined by in vitro mutagenesis studies of the type that we describe here for S261. Nonetheless, the recent discovery of multiple differentially phosphorylated residues on the AQP2 COOH terminus, including S261, S264, and S269, in response to VP treatment in vivo suggests that they may play a complex regulatory role in AQP2 trafficking and compartmentalization in vivo.
GRANTS
H. J. Lu is supported by an National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant KO8 DK-075940. R. Bouley is supported by a Young Investigator Award from the National Kidney Foundation. U. Hasler is supported by a Swiss Fondation Suisse de Bourse en Médicine et Biologie Fellowship and an Executive Committee on Research Fellowship from Massachusetts General Hospital. This work is also supported by NIDDK Grant DK-38452 to D. Brown. The Microscopy Core facility of the Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (DK-57521) and the Center for the Study of Inflammatory Bowel Disease (DK-43341).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amlal H, Sheriff S, Soleimani M. Upregulation of collecting duct aquaporin-2 by metabolic acidosis: role of vasopressin. Am J Physiol Cell Physiol 286: C1019–C1030, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Deen PM, van Balkom BW, Kamsteeg EJ. Routing of the aquaporin-2 water channel in health and disease. Eur J Cell Biol 79: 523–530, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamsteeg EJ, Deen PM, van Os CH. Defective processing and trafficking of water channels in nephrogenic diabetes insipidus. Exp Nephrol 8: 326–331, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA 104: 16696–16701, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsura T, Ausiello DA, Brown D. Direct demonstration of aquaporin-2 water channel recycling in stably transfected LLC-PK1 epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 270: F548–F553, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997. [PubMed] [Google Scholar]
- 15.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA 92: 7212–7216, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Nejsum LN, Zelenina M, Aperia A, Frokiaer J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frokiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 10: 647–663, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Russo LM, McKee M, Brown D. Methyl-β-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Sabolic I, Katsura T, Verbavatz JM, Brown D. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol 143: 165–175, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Valenti G, Frigeri A, Ronco PM, D'Ettorre C, Svelto M. Expression and functional analysis of water channels in a stably AQP2-transfected human collecting duct cell line. J Biol Chem 271: 24365–24370, 1996. [DOI] [PubMed] [Google Scholar]
- 25.van Balkom BW, Graat MP, van Raak M, Hofman E, van der Sluijs P, Deen PM. Role of cytoplasmic termini in sorting and shuttling of the aquaporin-2 water channel. Am J Physiol Cell Physiol 286: C372–C379, 2004. [DOI] [PubMed] [Google Scholar]
- 26.van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002. [DOI] [PubMed] [Google Scholar]