Abstract
A mutation in the human FXYD2 polypeptide (Na-K-ATPase γ subunit) that changes a conserved transmembrane glycine to arginine is linked to dominant renal hypomagnesemia. Xenopus laevis oocytes injected with wild-type FXYD2 or the mutant G41R cRNAs expressed large nonselective ion currents. However, in contrast to the wild-type FXYD2 currents, inward rectifying cation currents were induced by hyperpolarization pulses in oocytes expressing the G41R mutant. Injection of EDTA into the oocyte removed inward rectification in the oocytes expressing the mutant, but did not alter the nonlinear current-voltage relationship of the wild-type FXYD2 pseudo-steady-state currents. Extracellular divalent ions, Ca2+ and Ba2+, and trivalent cations, La3+, blocked both the wild-type and mutant FXYD2 currents. Site-directed mutagenesis of G41 demonstrated that a positive charge at this site is required for the inward rectification. When the wild-type FXYD2 was expressed in Madin-Darby canine kidney cells, the cells in the presence of a large apical-to-basolateral Mg2+ gradient and at negative potentials had an increase in transepithelial current compared with cells expressing the G41R mutant or control transfected cells. Moreover, this current was inhibited by extracellular Ba2+ at the basolateral surface. These results suggest that FXYD2 can mediate basolateral extrusion of magnesium from cultured renal epithelial cells and provide new insights into the understanding of the possible physiological roles of FXYD2 wild-type and mutant proteins.
Keywords: ion currents, mutant proteins, inward rectification, Na-K-ATPase
fxyd2 (Na-K-ATPase γ subunit) is a small, hydrophobic protein of 10 kDa, which was originally identified in purified preparations of the Na-K-ATPase (11). Although it is not required for Na-K-ATPase function, FXYD2 can influence the characteristics of the Na-K-ATPase αβ heteromer. When associated with the Na-K-ATPase, the subunit can modify the voltage dependence of K+ activation (4) and influence the apparent affinity of the enzyme for Na+, K+, and ATP (2, 12, 38). FXYD2 belongs to a family of homologous single transmembrane proteins named after an invariant extracellular, FXYD motif (36). In mammals the family consists of seven members numbered according to the order of their initial sequencing: phospholemman (PLM; FXYD1), Na-K-ATPase γ subunit (FXYD2), mammary tumor protein of 8 kDa (Mat-8; FXYD3), corticosteroid hormone-induced factor (CHIF; FXYD4), related to ion channel (RIC; FXYD5), phosphohippolin (PHP; FXYD6), and FXYD7 originally identified in the databases. All family members have been shown to interact with and modify the activity of the Na-K-ATPase in a tissue- and isoform-specific manner (10, 12). Although the FXYD proteins influence the kinetic parameters of the Na-K-ATPase to only a modest extent, these effects may have important physiological consequences in cation homeostasis. Because each of these proteins has a different tissue distribution and regulatory role in Na-K-ATPase function, FXYD proteins may also serve as tissue-specific modulators of the Na-K-ATPase to adjust its kinetic properties to the needs of a specific tissue, cell type, or physiological state.
In addition to their ability to influence Na-K-ATPase activity, there is strong evidence that FXYD family members can also regulate other transporters (35) and either influence ion channels or form channels themselves. For example, PLM induces a taurine-selective ion channel, Mat-8 a hyperpolarization-activated Cl− current (22, 25), and CHIF evokes slowly activating, depolarization-induced K+ currents (3). FXYD2 induces large nonselective currents in Xenopus laevis oocytes (21, 33). It is not clear whether these proteins activate endogenous currents or form the actual ion pathway (39). Support for the latter comes from reconstitution studies with recombinant PLM. In planar lipid bilayers, recombinant PLM forms a taurine-selective ion channel with properties similar to the current observed in oocytes expressing PLM, suggesting that channel activity is intrinsic to the protein (22). In the absence of taurine, PLM may function as a nonselective channel (7). Studies with hyposmotically stimulated human embryonic kidney (HEK) cells overexpressing PLM (23) or cerebellar astrocytes with reduced PLM expression (24) suggest PLM may participate in the osmodependent taurine leak pathway. In contrast to these studies suggesting activity inherent to the polypeptide, others indicate that X. laevis oocytes have endogenous channels with properties similar to those induced by the FXYD proteins (18). Influenza B virus NB protein, an unrelated single membrane spanning protein, activates an endogenous oocyte conductance by shifting its voltage dependence to less hyperpolarized potentials (34). Consequently, expression of membrane proteins in X. laevis oocytes may induce or modify endogenous currents (34), and it remains unclear whether the FXYD proteins are themselves sufficient for channel activity, or whether they require unidentified, endogenous protein partners.
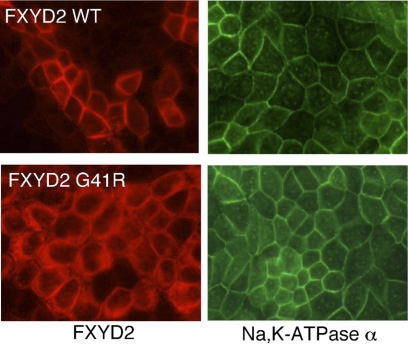
Immunohistochemistry of rat kidney sections shows that FXYD2 is highly expressed in the basolateral membrane of the thick ascending limb of the loop of Henle and the distal convoluted tubule (DCT) (2, 27). Although a distinct functional role for FXYD2 is unclear, recent results suggest an important role in renal function. A human mutation in the gene coding the polypeptide results in renal hypomagnesemia associated with hypocalciuria (20). The conversion of a conserved glycine within the transmembrane domain to arginine (G41R) caused misrouting of FXYD2 from the plasma membrane to an intracellular compartment in transfected COS cells. The hypothesis of Meij et al. (20) is that this misrouting of FXYD2 results in the diminution of Na-K-ATPase activity at the plasma membrane with consequent hypomagnesemia. However, expression of the G41R mutant in HeLa cells (28), or polarized Madin-Darby canine kidney (MDCK) cells (see Fig. 6), does not retard the trafficking of the Na-K-ATPase to the cell surface. Therefore, the involvement of FXYD2 could be more complex and dynamic in the pathophysiology of autosomal dominant renal Mg2+ loss.
Fig. 6.
Expression of FXYD2 WT and the G41R mutant in polarized Madin-Darby canine kidney (MDCK) cells. Left: FXYD polypeptide. Right: Na-K-ATPase α subunit.
The aim of the present study was to further examine the cation channel activities of FXYD2 and the G41R mutation in X. laevis oocytes and to identify the electrophysiological properties of whole cell currents induced by FXYD2 and the G41R mutant. We report here that the G41R mutant generated whole cell ion currents with a novel Mg2+-dependent gating on inward rectification. In addition, substitution of Gly41 with other residues demonstrates that a positive charge at the site is required for this inward rectification. Moreover, when wild-type FXYD2 is expressed in MDCK cells, the cells in the presence of a large apical-to-basolateral Mg2+ gradient exhibit an increased transepithelial current. This current is significantly reduced in MDCK cells expressing the G41R mutant. The results demonstrate that the FXYD2 G41R mutant induces a channel in X. laevis oocytes and MDCK cells that is distinct from the wild-type FXYD2 channels.
MATERIALS AND METHODS
Wild-type FXYD2 and G41R expression in X. laevis oocytes.
cDNAs encoding FXYD2 were subcloned into pXOV-60. This vector is a derivative of pSP64 (Promega, Madison, WI) and contains promoter elements for X. laevis globin that promote high levels of expression in X. laevis oocytes. cRNAs were transcribed in vitro using SP6 RNA polymerase and capping from linearized cDNA (mMessage mMachine RNA transcription kit, Ambion, Austin, TX). Stage V-VI X. laevis oocytes were isolated by partial ovariectomy under tricaine anesthesia and then defolliculated by treatment with 1 mg/ml collagenase (Type 1A, Sigma) in 0 mM Ca2+ ND96 for 1 h. From 2 to 24 h after defolliculation, oocytes were pressure injected with ∼50–80 nl of cRNA (0.1–2 μg/μl). Oocytes were maintained at room temperature in ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM Na-HEPES, pH 7.5) containing 2 mM Ca2+ and supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) for 1–2 days before recording. Amino acid substitutions at G41 were introduced using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
Two-electrode voltage clamp.
FXYD2 currents were measured using the two-electrode voltage clamp technique in a small chamber (100 μl) mounted on the stage of a binocular microscope (SMZ-1, Nikon Instruments). The chamber was connected through agar bridges to the current-sensing headstage of the voltage clamp amplifier (OC-725C Oocyte Clamp, Warner Instruments) and rapidly perfused with a laminar flow of bathing solution supplied by one of five reservoirs connected to a manifold at the inlet to the chamber. The exchange rate for the external solution surrounding the oocyte was less than 1∼2 s. Experiments were performed at room temperature (20°C). Intracellular electrodes were filled with 3 M KCl and had tip resistances from 0.5 to 2 MΩ. Data were digitized on-line and stored on a computer, or were digitized at 2 kHz onto videotape (Neuro-corder DR-890, Neuro-Data Instruments) for off-line analysis. Currents were recorded either in ND96 solution without Ca2+, with additions as indicated, or in KD98 (98 mM KCl, 1 mM MgCl2, K-HEPES, pH 7.5). Intracellular injections were made using a pneumatic pressure ejection device (PV800; WPI, Sarasota, FL). All experiments were done at room temperature.
Isolation of oocyte membranes.
Plasma membranes from X. laevis oocytes were prepared as described before (14). Approximately 200 oocytes were homogenized in 1.5 ml of ice-cold buffer containing 150 mM NaCl, 25 mM Tris·HCl, pH 7.4, 10 mM Mg acetate, and 10% (wt/vol) sucrose using a glass-glass homogenizer. The homogenate was centrifuged at 500 g for 5 min. The supernatant was removed and the pellet was resuspended in 1.5 ml of homogenization buffer and rehomogenized. The homogenate was centrifuged as before and the supernatant was recovered and combined with the first. The combined supernatants were then overlaid on top of a discontinuous sucrose gradient composed of 20% (wt/vol) sucrose in 50 mM NaCl, 25 mM Tris·HCl, pH 7.4, 10 mM Mg acetate (4.5 ml), and 50% sucrose in the same solution (5 ml) and centrifuged at 30,000 g for 1 h in a swinging bucket rotor. Membranes at the interfaces between 20 and 50% sucrose (enriched in intracellular membranes and designated heavy membranes) and 10 and 20% sucrose (enriched in plasma membranes and designated light membranes) were carefully removed. Membranes were diluted threefold in homogenization buffer and centrifuged at 100,000 g for 30 min. The membrane pellet was resuspended in homogenization buffer and subjected to SDS-PAGE and immunoblotting as described previously (21).
Cell culture and Ussing chamber experiments.
Type I MDCK epithelial cells were cultured in MDCK media DMEM containing 10% FBS, 800 μg/ml geneticin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 mg/ml Fungizone (all from Sigma, St. Louis, MO) at 37°C in a humidified atmosphere of 5% CO2. Cells were transfected with the wild-type or G41R cDNAs subcloned into pcDNA3.1(−) (Invitrogen, Carlsbad, CA) (19). As a control, MDCK cells were also transfected with the empty vector. To grow MDCK cells as polarized epithelia, cells were seeded on permeable filter supports (culture plate inserts, 0.4-μm pore size, 6.5-mm diameter, Corning) at a density of 2.3 × 105 cells per 0.33 cm2. Every other day after seeding, the MDCK media were changed and transepithelial resistance was measured using an epithelial voltohmmeter (EVOM; Warner Instruments, Hamden, CT). By day 8, the transepithelial resistance for the cell monolayer approached 3,500 Ω (3,500 ± 450, n = 35). For electrical measurements, cell monolayers were mounted in an Ussing chamber (0.75-cm window radius, 1.76-cm2 exposed epithelial area; Warner Instruments) maintained at 37°C. To measure the transepithelial electrical currents in the wild-type or mutant G41R FXYD2-transfected MDCK cells, the transepithelial voltage was clamped with a stepwise voltage clamp protocol (identical to the protocol used in the oocytes), and the corresponding currents were recorded. For the first set of experiments, the apical and basolateral media contained 140 mM NaCl, 5 mM KCl, 0.36 mM K2PO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, and 10 mM HEPES, pH 7.4. For other experiments, the apical medium contained 116 mM MgCl2, 40 mM sucrose, and 10 mM HEPES, pH 7.4, and the basolateral medium contained 140 mM choline Cl, 10 mM MgCl2, and 10 mM HEPES, pH 7.4. Osmolalities of the apical and basolateral solutions were ∼300 mosmol/kgH2O as determined by a vapor pressure osmometer (Wescor, Logan, UT).
Immunofluorescence.
MDCK cells were plated at low density on collagen-coated coverslips and grown to confluence. Once confluent the MDCK cells were washed once with 150 mM NaCl, 25 mM HEPES, pH 7.4 (HBS) and fixed/permeabilized in 100% methanol at −20°C for 10 min. The coverslips were washed three times with 4°C HBS. Nonspecific sites were blocked for 1 h at 37°C in HBS containing 5% normal goat serum (NGS). Primary antibodies against the α subunit of the Na-K-ATPase (5α; diluted 1:100) and γ (γ969; diluted 1:50; preparation of antibody described in Ref. 21) were diluted in HBS containing 1% NGS and applied to cells overnight at 4°C. Following three washes in HBS, FITC- or Cy3-conjugated secondary antibodies (Molecular Probes, Eugene, OR) diluted 1:1,000 in HBS containing 0.1% NGS were applied for 1 h at room temperature. Samples were washed three times in HBS and mounted in ProLong Antifade (Molecular Probes). Samples were viewed with a Zeiss Axioskop microscope (×40 objective). Images were captured digitally with a Zeiss Axioskop using Axiovision 2.0 software.
RESULTS
Expression of wild-type FXYD2 and the G41R mutant generates distinct ion currents in X. laevis oocytes.
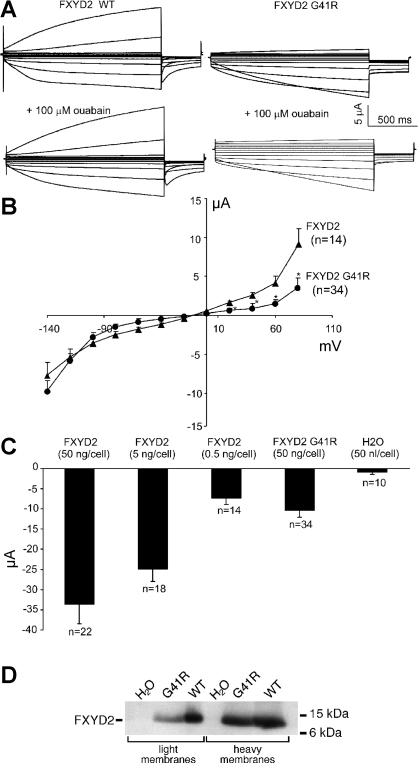
X. laevis oocytes were injected with FXYD2 and FXYD2 G41R mutant mRNAs (cRNA). All oocyte currents evoked by both depolarization and hyperpolarization were examined 1–3 days postinjection and unless noted, all experiments were performed using the voltage protocol as described previously (33). Whole cell currents were recorded in response to voltage steps between −140 and +80 mV from a holding potential of −10 mV in ND96 solution with a pulse duration of 2 s, with a 50-s interval between each pulse to give sufficient time for recovery from activation. For oocytes injected with wild-type cRNA, hyperpolarization to −140 mV evoked a slowly activated inward current that attained an amplitude of −35 to −40 μA within a 2-s activating pulse; depolarization also elicited a slowly activated outward current that reached an amplitude of 37 to 48 μA at +80 mV (Fig. 1A). These currents did not inactivate, even when activating pulses lasting longer than 1 min were applied. As shown in Fig. 1B the current-voltage relationship (I-V) of the wild-type-induced currents is nearly symmetrical between depolarization and hyperpolarization voltage steps, suggesting that the channels are nonselective. Using an inverted protocol of the voltage pulses, starting instead with +80 mV down to −140 mV, resulted in nearly identical I-V plots as from the original voltage protocol (data not shown). In addition, the I-V plots recorded in high [Na] (ND96) or high [K] (KD98) solutions were identical (data not shown).
Fig. 1.
Expression of nonselective ionic currents in Xenopus laevis oocytes expressing wild-type (WT) and G41R FXYD2 polypeptides. A: 2 microelectrode currents in response to voltage steps from −10 mV to voltages between −140 and +80 mV, in 20-mV increments, from 2 oocytes that had been injected with the FXYD2 WT and FXYD2 G41R cRNAs, respectively, in ND96 solutions. B: “steady-state” current and voltage relationships for the WT and G41R-induced currents. *P < 0.01, G41R compared with FXYD2 WT. C: mean inward currents at −140 mV from oocytes injected with WT and G41R cRNA or H2O as indicated. Currents in ND96 solution were measured 1∼2 days after injection of cRNAs. Bars show means ± SE [number of oocytes (n) indicated]. D: immunoblot of membrane fractions prepared from oocytes expressing FXYD2 WT (5 ng cRNA injected/cell) or the G41R mutant (50 ng cRNA injected/cell). Membranes enriched in plasma membranes (light membranes) or intracellular membranes (heavy membranes) were immunoblotted using an anti-FXYD2 antibody.
Expression of the G41R mutation also gave rise to large inward currents (5 to 16 μA; Fig. 1A). However, the G41R mutant outward currents at +80, +60, +40, and +20 mV were smaller compared with the wild-type currents, indicating that the G41R-induced channels exhibit significant inward rectification (Fig. 1A). In addition, ouabain, a Na-K-ATPase inhibitor, did not significantly affect the wild-type or mutant FXYD2 currents (Fig. 1A). The FXYD2 G41R mutant required the injection of nearly 100 times more cRNA than the wild-type to obtain comparable conductance levels (Fig. 1C). As shown before in insect and mammalian cells, the majority of G41R protein is retained within intracellular compartments (20, 28), therefore it is likely that only a small percentage of the expressed mutant protein is delivered to the oocyte plasma membrane. Moreover, when wild-type FXYD2 is expressed in oocytes in the absence of the Na-K-ATPase α and β subunits, the majority of FXYD2 polypeptide is found in intracellular compartments (4). To better characterize G41R mutant localization in oocytes, we isolated plasma membranes from oocytes expressing wild-type or G41R FXYD2 polypeptides. As shown in Fig. 1D, both wild-type and mutant FXYD2 polypeptides are found in the light membrane fractions that are enriched in plasma membranes (14). Proportionally, more wild-type FXYD2 polypeptides are present in the plasma membrane fraction than the G41R mutant. However, it is clear that both the wild-type FXYD2 and G41R polypeptides are delivered to the plasma membrane where they can induce channel activity.
Mg2+-dependent gating of the FXYD2 G41R mutant-induced channels.
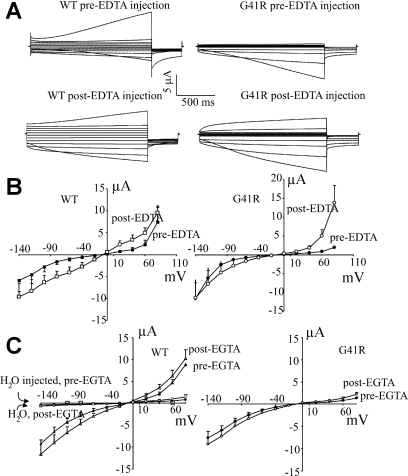
We assessed the possibility that the inward rectification of the G41R mutant was dependent on intracellular Mg2+ by manipulating the intracellular Mg2+ through a fluid microinjection electrode. To determine the influence of intracellular Mg2+ on the G41R currents, we injected 25 nl of 200 mM Tris-EDTA (pH 7.3) into the oocyte cytoplasm. Intracellular delivery of EDTA at this concentration should result in a final EDTA concentration of ∼5 mM, sufficient to chelate the majority of intracellular free Mg2+. In oocytes expressing the wild-type FXYD2, injection of EDTA caused a small increase in conductance at all voltage steps (Fig. 2A). However, in oocytes expressing the G41R mutant, intracellular EDTA resulted in a significant enhancement of the “steady-state” outward currents in the absence of changes in the inward currents (Fig. 2A). After EDTA injection and presumably a reduction in intracellular free Mg2+, the G41R-induced currents become nonrectifying. As shown in Fig. 2B, the I-V plots after EDTA injection are similar for both wild-type and mutant FXYD2 polypeptides. H2O injection did not affect either the wild-type or G41R currents. In addition, injection of 25 nl of 200 mM Tris-EGTA (pH 7.3) did not significantly modify the FXYD2-induced currents (Fig. 2C).
Fig. 2.
Effects of EDTA injection on the FXYD2 WT and G41R-induced currents in X. laevis oocytes. A: representative currents for each of the FXYD2 WT currents and the G41R currents, before and after injection of 25 nl of 200 mM Tris-EDTA (pH 7.3). B: steady-state current-voltage (I-V) relationships for the FXYD2 WT and the G41R currents, before and after EDTA injection. Bars show means ± SE (WT, n = 7; G41R, n = 8). *P < 0.01, G41R compared with WT. C: pseudo steady-state I-V relationships for the FXYD2 WT and the G41R currents, before and after injection of 25 nl of 200 mM Tris-EGTA (pH 7.3).
Wild-type and G41R mutant channels display similar sensitivities to extracellular divalent cations and trivalent cations.
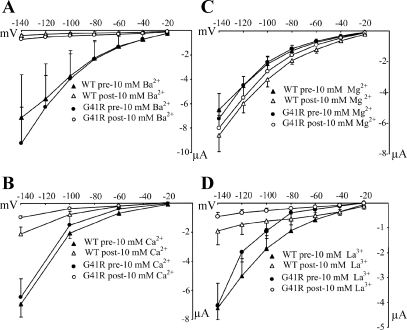
As previously shown, the wild-type FXYD2 conductances are inhibited by extracellular Ba2+ and Ca2+ (33). Given the inward rectification property of the G41R-activated conductance, we examined the influence of extracellular divalent cations on the FXYD2-induced currents. As shown in Fig. 3, 10 mM Ba2+ (Fig. 3A) and 10 mM Ca2+ (Fig. 3B) inhibited both the wild-type and G41R conductances. However, neither the wild-type nor G41R FXYD2 conductances were inhibited by 10 mM extracellular Mg2+ (Fig. 3C). Interestingly, in the presence of 10 mM extracellular La3+, a potent Mg2+ channel blocker, both the wild-type and G41R conductances are inhibited.
Fig. 3.
Effects of external divalent or trivalent cations on both FXYD2 WT and G41R-induced currents. The I-V relationships of the WT and mutant currents: A: 10 mM Ba2+ (WT, n = 6; G41R, n = 8); B: 10 mM Ca2+ (WT, n = 6; G41R, n = 6); C: 10 mM Mg2+ (WT, n = 10; G41R, n = 12); or D: 10 mM La3+ (WT, n = 8; G41R, n = 8). Divalent extracellular and trivalent cations had similar inhibitory effects on both WT and G41R currents. Bars show means ± SE. In all cases, filled and unfilled triangles represent FXYD2WT before and after cation treatment, respectively; filled and unfilled circles represent FXYD2G41R before and after cation treatment.
Ion selectivity of both the wild-type and G41R-induced ion channel activities.
As previously noted (20, 30), the FXYD2-activated currents (IFXYD2) appear to be nonselective. Replacement of Cl− by gluconate− did not qualitatively alter the currents, nor shift the reversal potential, suggesting that the FXYD2 current is not a Cl− current. Replacement of Na+ by choline+ did not significantly change the amplitude or time dependence of IFXYD2, indicating a high permeability to cations. We next determined whether the G41R mutant channel activity observed in oocytes is also selective for cations. Vrev of the wild-type and G41R-activated channels is not significantly changed either by an increase in the [K+] or by a reduction in the [Cl−] (data not shown). In contrast, in both the wild-type and G41R-activated channels, Vrev was significantly shifted to more negative (wild-type: 2.4 ± 2.7 to −10.2 ± 1.9 mV, n = 14; G41R: 4.3 ± 3.4 to −13.4 ± 2.4 mV, n = 16) by substitution of extracellular Na+ with the large cation N-methyl-d-glucosamine (NMDG) in the Na+-free solution, indicating these channels are highly permeable to cations.
A positive charge at residue 41 is required for inward rectification.
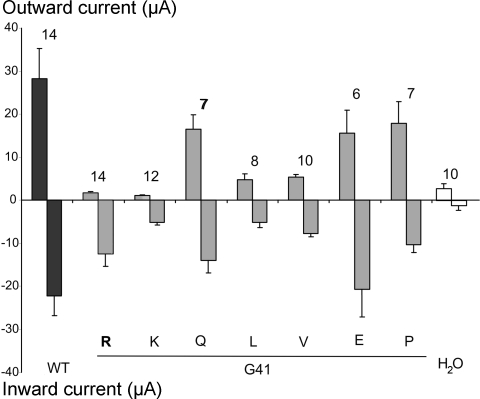
To determine the structural basis for inward rectification of the G41R current, we used site-directed mutagenesis to introduce other amino acid residues at G41. As shown in Fig. 4, substitution of G41 by a bulky hydrophobic residue (L or V) resulted in FXYD2 channel activity. However, G41L or V activity were both significantly reduced compared with the wild-type activity. In contrast, changing G41 to glutamine or proline, or to a negatively charged glutamate, did not significantly alter channel activity. However, substitution of G41 to the positively charged lysine again resulted in a channel that exhibited significant inward rectification.
Fig. 4.
Ion channel properties induced by WT and G41 FXYD2 mutants in oocytes. Using the step voltage clamp protocol, the peak outward current was determined at the end of the depolarizing pulse to +80 mV, and the peak inward was measured at the end of hyperpolarizating pulse to −140 mV. Bars show means ± SE. The numbers (n) are listed on the top for each group. Substitutions of G41 are indicated at the bottom of the figure. For all constructs, ∼50 ng cRNA was injected into each oocyte.
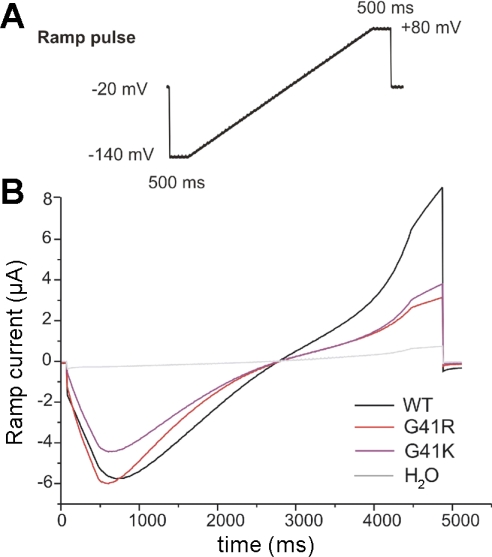
To further characterize FXYD2 (and the mutants) conductance, a different voltage protocol using a brief ramp from −140 to +80 mV was used (Fig. 5A). As shown in Fig. 5B, the wild-type FXYD2 conductance responding to the ramp voltage is significantly different than the G41R or G41K currents. The outward IFXYD2 is clearly larger than the outward G41R or G41K currents. Thus it appears that inward rectification requires a positive charge at residue 41 of the polypeptide.
Fig. 5.
Different voltage protocol approach was used with a brief ramp protocol from −140 to +80 mV. IFXYD2WT traces responding to ramp voltage are different than the G41R currents or G41K traces. The outward IFXYD2WT is clearly larger than the outward IFXYD2G41R or IFXYD2G41K traces. Each ramp trace represents average data from original ramp recording from each cell (WT, n = 10; G41R, n = 12; G41K, n = 6; H2O-injected control, n = 7).
Effect of expression of wild-type FXYD2 and G41R on the electrical properties of MDCK cells.
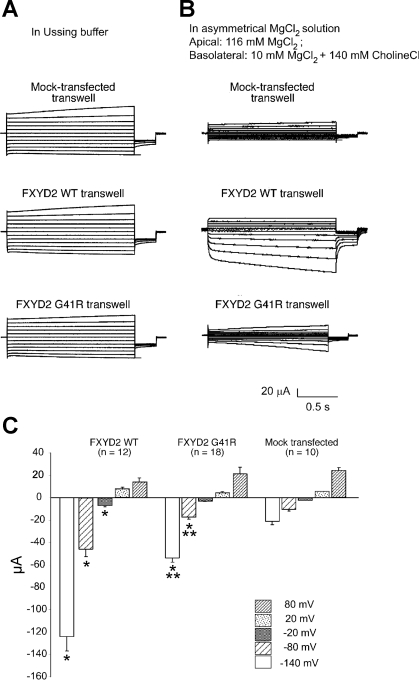
To determine whether FXYD2 expression influences the electrical properties of MDCK cells, cells were transfected with the wild-type or G41R FXYD2 cDNAs. As shown in Fig. 6, when expressed in MDCK cells the wild-type FXYD2 polypeptides are delivered to the basolateral membrane surface. In contrast, the G41R mutation causes the retention of the polypeptide within intracellular compartments. Although there is substantial localization of the polypeptide to the plasma membrane, the majority of the FXYD2 mutant polypeptide is within the cytoplasm. In contrast, the Na-K-ATPase α subunit is localized to the basolateral plasma membrane. It is apparent that the expression of the G41R mutant does not influence the trafficking of the Na-K-ATPase to the cell surface. This result is consistent with previous studies demonstrating that the expression of G41R in HeLa cells does not retard the trafficking of the Na-K-ATPase to the plasma membrane (28). To test whether FXYD2 expression influences the electrical properties of MDCK cells, cells were grown on permeable supports and subjected to analysis using an Ussing chamber. After 8 days in culture and with Ussing buffer (140 mM NaCl, 5 mM KCl, 0.36 mM K2PO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, and 10 mM HEPES, pH 7.4) at both the apical and basolateral surfaces, the transepithelial potential and resistance were not significantly different between the control (cells transfected with the empty expression vector, pcDNA), wild-type, and G41R-transfected MDCK cells (Table 1). In the same buffer the transepithelial voltage was clamped with a stepwise voltage clamp protocol (identical to the protocol used in the oocytes) and the corresponding currents were recorded. As shown in Fig. 7A, under these conditions there were no significant differences between the control, wild-type, and G41R-transfected MDCK cells. Apparently FXYD2 channel activity is obscured by the endogenously expressed channels present in the MDCK cells. However, when the cells were exposed to a large Mg2+ concentration gradient (116 mM apical, 10 mM basolateral), the wild-type FXYD2 MDCK cells, at negative potentials, had an increase in transepithelial current compared with the control transfected cells (Fig. 7B). On the other hand, the G41R-transfected MDCK cells had a slight increase in transepithelial current at the negative potentials compared with the control transfected cells. However, this current was substantially reduced compared with the wild-type FXYD2 currents. These results are summarized in Fig. 7C.
Table 1.
MDCK transepithelial potential and resistance in Ussing buffer
|
Mock Transfected Cells (n = 12) |
FXYD2 WT Transfected Cells (n = 12)
|
FXYD2 G41R Transfected Cells (n = 11)
|
|||
|---|---|---|---|---|---|
| Em, mV | Rm, kΩ/cm2 | Em, mV | Rm, kΩ/cm2 | Em, mV | Rm, kΩ/cm2 |
| −2.1 ± 0.6 | 1.54±0.36 | −1.8±0.8 | 1.32±0.19 | −2.6±0.9 | 1.20±0.08 |
MDCK, Madin-Darby canine kidney cells; WT, wild type.
Fig. 7.
Transepithelial current measurements in control, FXYD2 WT, or G41R-expressing MDCK cells. A: apical and basolateral membranes were exposed to the identical physiological solution containing 140 mM NaCl, 5 mM KCl, 0.36 mM K2PO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, and 10 mM HEPES, pH 7.4 (Ussing buffer). Representative transepithelial currents in response to voltage steps from −10 mV to voltages between −140 and +60 mV, in 20-mV increments for the mock transfected (control), WT, and G41R MDCK cells are shown. B: representative current traces from control, WT, and G41R MDCK cells with 116 mM Mg2+ at the apical membrane and 10 mM Mg2+ at the basolateral membrane. C: comparison of the transepithelial currents at different voltages from the control, WT, and G41R MDCK cells when exposed to a large transepithelial Mg2+ concentration gradient {apical [Mg2+], 116 mM; basolateral [Mg2+], 10 mM}. *Values that are significantly different from the control value and **values statistically different from WT values (P < 0.01).
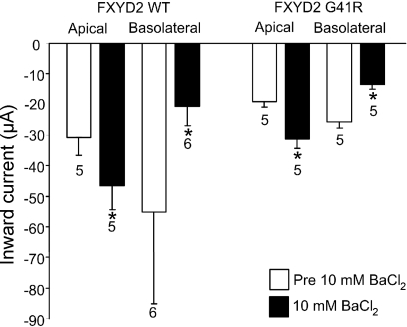
To further characterize the FXYD2-induced currents in MDCK cells, we tested whether the transepithelial currents were inhibited by extracellular Ba2+. In X. laevis oocytes, extracellular Ba2+ inhibits the FXYD2-induced currents (Fig. 3). As shown in Fig. 8, 10 mM extracellular Ba2+ at the basolateral surface inhibited Mg2+ currents in MDCK cells expressing either the wild-type FXYD2 or G41R mutant. In contrast, 10 mM extracellular Ba2+ at the apical surface increased the transepithelial current. The mechanism by which apical Ba2+ increases the transepithelial current is unknown.
Fig. 8.
Transepithelial current measurements in FXYD2 WT or G41R-expressing MDCK cells. The transepithelial currents at −80 mV from WT and G41R MDCK cells when exposed to a large transepithelial Mg2+ gradient and in the absence (open bars) or presence (filled bars) of 10 mM Ba2+ at the apical or basolateral surface. *Values that are significantly different from the values in the absence of Ba2+ (P < 0.05).
DISCUSSION
Electrophysiological properties of the FXYD2-activated channels and the G41R mutant-activated conductance.
When expressed in X. laevis oocytes, FXYD2 activates large nonselective ion channels (21). The FXYD2 currents exhibit unique activation kinetics. The voltage jump to the first test potential −140 mV initially induced a small conductance (instantaneously after the voltage step), followed by a progressive increase in conductance. Following activation, the deactivation kinetics at the holding potential of −10 mV were extremely slow (>5 min for full recovery), consequently with a subsequent interpulse interval of 30 s, the conductance was already partially activated at the holding potential. Therefore, an instantaneous current, followed by the time-dependent development of more complete activation, was seen during subsequent hyperpolarizing test pulses. Thus it seems that the shape of the I-V relationship with this protocol might be dependent on the time at which the current amplitudes are evaluated, and the order in which the voltage pulses are applied. To evaluate the original voltage clamping protocol, inverted voltage pulses, starting from +80 mV down to −140 mV, were also examined. Identical I-V plots from the two protocols demonstrated that the activated currents were not dependent on the order of the pulse protocol.
External divalent cations Ba2+, Ca2+, and the trivalent ion lanthanide inhibited the FXYD2 channels in a voltage-independent manner. La3+ ions are considered sensitive and potent blockers for Drosophila TRP, TRPL, human TRP1, TRP3 (26), and native Ca2+ channels (15) and ICRAC (5). External Mg2+ did not inhibit the channels induced by the wild-type or the mutant G41R channel. The FXYD2 channel activities in oocytes are independent of exogenously expressed Na-K-ATPase α and β subunits (21) and extracellular ouabain does not alter the FXYD2 current amplitude or kinetics (Fig. 1). In Sf-9 insect cells, a cell line that does not express the Na-K-ATPase at discernable levels, confocal microscopy demonstrated that FXYD2 could be delivered to the plasma membrane independent of the other Na-K-ATPase subunits (21). The present report demonstrates that the G41R mutant, a mutation causing human renal hypomagnesemia, induces a voltage-activated ion channel in X. laevis oocytes exhibiting novel Mg2+-dependent inward rectification. To identify the mechanism of the G41R inward rectification, we injected EDTA into the cytoplasm of oocytes expressing either the wild-type or G41R FXYD2 polypeptides. Cytoplasmic EDTA removed inward rectification in cells expressing the G41R mutation, while it did not significantly influence wild-type currents. In contrast, cytoplasmic EGTA did not significantly alter the wild-type of G41R-induced currents.
It is somewhat unexpected that conversion of an intramembrane glycine residue to a positively charged arginine residue should result in Mg2+-dependent inward rectification. Changing G41 to various residues indicates that a positive charge at position 41 within the transmembrane electrical field is required for the inward rectification properties of the FXYD2 mutant. A positive charge at this site may drive conformational changes in the channel that facilitates the binding of Mg2+ to sites in the pore or at intracellular sites causing Mg2+ to block the permeation of other cations in a voltage-dependent manner. The elucidation of the exact structural details leading to the Mg2+-dependent inward rectification awaits further study.
Although it is clear that the FXYD family members influence Na-K-ATPase activity (13), there is strong evidence that members of the family can also influence the activity of other transporters or ion channels (1, 12). When reconstituted into planar lipid bilayers, recombinant PLM initiates ionic conductances similar to those observed in oocytes expressing PLM, suggesting activity intrinsic to the protein (7). A recent report suggests that CHIF directly modulates the voltage sensitivity of the KCNQ channels, although there is no evidence for actual colocalization of CHIF and KCNQ1 channels in native tissues (16). The present study demonstrates that an inward rectifying cation channel was formed in the oocyte membrane by expression of a mutant FXYD2 that is associated with hereditary renal hypomagnesemia. In MDCK cells, wild-type FXYD2 leads to an increase in transepithelial current when there is a large Mg2+ concentration gradient across the epithelium. This transepithelial current is significantly reduced in MDCK cells expressing the G41R mutant. Similar to the oocyte currents, extracellular Ba2+, when present at the basolateral surface, inhibited the FXYD2-induced currents. These findings suggest that FXYD2 forms a cation channel within the basolateral membrane of MDCK cells that can mediate Mg2+ efflux.
Role of cation channels in renal hypomagnesemia.
Like calcium, magnesium plays an important role in many physiological and biochemical processes. Homeostasis of Mg2+ is precisely regulated and depends on the balance between intestinal absorption and renal excretion. Generally, management of total body Mg2+ resides mainly in the nephron segments of the kidney. From the glomerular filtrate, the proximal tubule reabsorbs 10%, the thick ascending limb of the loop of Henle absorbs 70–80%, and the DCT reabsorbs 10% of the filtered magnesium. Beyond the DCT, there is little Mg2+ reabsorption, therefore, the DCT plays an important role in regulating the final urinary excretion of Mg2+ (29). Current understanding of the mechanisms involved in Mg2+ transport across the DCT suggests that an apical Mg2+ channel and a basolateral Mg2+ extrusion system are involved in transepithelial Mg2+ transport (8). More recently, members of the transient receptor potential (TRP) channel family have been identified as Mg2+-permeable channels that play important roles in magnesium homeostasis. TRP channel melastatin 7, TRPM7, is ubiquitously expressed and has been characterized as an ion channel that is permeable to a variety of divalent cations, including Zn2+, Ca2+, and Mg2+. Under physiological conditions, TPRM7 facilitates the influx of primarily Mg2+ and thus plays a crucial role in cellular Mg2+ homeostasis (32). A closely related family member, TRPM6 is a Mg2+-permeable channel predominantly expressed along the apical membrane of the DCT, the small intestine, and colon. Mutations in TRPM6 cause autosomal recessive hypomagnesemia with secondary hypocalemia (HSH) (31, 40). A missense mutation, P1071R, in the putative pore-forming region of TPRM6 causes the dominant negative inhibition in TRPM6/7 heteromeric channel complexes (9). The functional defect in the putative pore of TRPM6/7 channel appears to be responsible for the impaired body Mg2+ homeostasis. Although the mechanisms for the apical transport of Mg2+ are relatively well characterized, the exact mechanisms for active Mg2+ efflux at the basolateral membrane are unknown. Moreover, the function of FXYD2 in magnesium transport and the role that the FXYD2 G41R mutation plays in hypomagnesium are not clear. Surprisingly, the FXYD2 knockout mouse has a phenotype indistinguishable from the wild-type mouse (17). These mice do not exhibit hypomagnesemia and have normal Mg2+ urine concentrations. This would suggest that in mice FXYD2 does not play a direct role in magnesium transport or that other pathways can compensate for the loss of FXYD2.
Using peptides derived from the transmembrane (TM) domain, Therien and Deber (37) found that the FXYD2 TM region oligomerizes in the mild detergent perfluorooctanoate. This association is blocked in peptides that contain the G41R or the more conservative G41L substitutions. This is consistent with the observation that glycine, with its small size, is often found at the interface of TM helices where it maximizes van der Waals forces (30). These results suggest that native FXYD2 polypeptides exist as oligomers and that the G41R mutation may abrogate this association. More recently, it was found using X. laevis oocytes that the complete wild-type FXYD2 oligomerizes (6). However, in contrast to studies using the TM domain, the FXYD2 G41R polypeptides also self-oligomerize and oligomerize with wild-type FXYD2. When associated with the wild-type FXYD2, the G41R mutant reduces the trafficking of wild-type FXYD2 to the plasma membrane (6). Thus the dominant nature of the G41R mutation may be mediated through its association with wild-type FXYD2. This dominant nature of the disorder is supported by the observation that individuals with a 11q23.3-ter deletion, a deletion that includes the FXYD2 gene, have normal serum Mg2+ levels suggesting that the presence of the FXYD2 G41R mutant polypeptide rather than hyploinsufficiency causes the hypomagnesemia (20).
Understanding the etiology of the FXYD2-mediated hypomagnesemia will require a more thorough characterization of the functional properties of the protein. Physiological stress studies of the FXYD2-deficient mouse along with the creation of FXYD2 G41R mutant mice may provide important insights into the physiological function of native and mutant FXYD2 proteins. In addition, elucidating the distinct functional properties of the FXYD2 G41R polypeptide may provide valuable clues to understanding the elements of the FXYD2-mediated hypomagnesemia.
GRANTS
This work was supported by National Institutes of Health Grants GM-39746 and DK-064704 to R. W. Mercer.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song JL, Wang JF, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem 280: 19875–19882, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na+ and K+ affinity of the renal Na,K-ATPase. J Biol Chem 274: 33183–33185, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Attali B, Latter H, Rachamim N, Garty H. A corticosteroid-induced gene expressing an “IsK-like” K+ channel activity in Xenopus oocytes. Proc Natl Acad Sci USA 92: 6092–6096, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beguin P, Wang X, Firsov D, Puoti A, Claeys D, Horisberger JD, Geering K. The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J 16: 4250–4260, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland LM, Brown TA, Dinglendine R. Gadolinium block of calcium channels: influence of bicarbonate. Brain Res 563: 142–150, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Cairo ER, Friedrich T, Swarts HG, Knoers NV, Bindels RJ, Monnens LA, Willems PH, De Pont JJ, Koenderink JB. Impaired routing of wild-type FXYD2 after oligomerisation with FXYD2–G41R might explain the dominant nature of renal hypomagnesemia. Biochim Biophys Acta 1778: 398–404, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZH, Jones LR, Moorman JR. Ion currents through mutant phospholemman channel molecules. Receptors Channels 6: 435–447, 1999. [PubMed] [Google Scholar]
- 8.Chubanov V, Gudermann T, Schlingmann KP. Essential role for TRPM6 in epithelial magnesium transport and body magnesium homeostasis. Pflügers Arch 451: 228–234, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chubanov V, Schlingmann KP, Wäring J, Heinzinger J, Kaske S, Waldegger S, Schnitzler MM, Gudermann T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J Biol Chem 282: 7656–7667, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Delprat B, Schaer D, Roy S, Wang J, Puel JL, Geering K. FXYD6 is a novel regulator of Na,K-ATPase expressed in the inner ear. J Biol Chem 282: 7450–7456, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Forbush B, Kaplan JH, Hoffman JF. Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na,K-ATPase. Biochemistry 17: 3667–3676, 1978. [DOI] [PubMed] [Google Scholar]
- 12.Garty H, Karlish SJD. Role of FXYD proteins in ion transport. Annu Rev Physiol 68: 431–459, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Geering K FXYD proteins: new regulators of Na-K-ATPase. J Bioenerg Biochem 37: 387–392, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Hill WG, Southern NM, MacIver B, Potter E, Apodaca G, Smith CP, Zeidel ML. Isolation and characterization of the Xenopus oocyte plasma membrane: a new method for studying activity of water and solute transporters. Am J Physiol Renal Physiol 289: F217–F224, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol 465: 359–386, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jespersen T, Grunnet M, Rasmussen HB, Jørgensen NB, Jensen HS, Angelo K, Olesen SP, Klærke DA. The corticosteroid hormone induced factor: a new modulator of KCNQ1 channels? Biochem Biophys Res Commun 341: 979–988, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Jones DH, Li TY, Arystarkhova E, Barr KJ, Wetzel RK, Peng J, Markham K, Sweadner KJ, Fong GH, Kidder GM. Na,K-ATPase from mice lacking the gamma subunit (FXYD2) exhibits altered Na+ affinity and decreased thermal stability. J Biol Chem 280: 19003–19011, 2005. [DOI] [PubMed]
- 18.Kowdley GC, Ackerman SJ, John JE, Jones LR, Moorman JR. Hyperpolarization-activated chloride currents in Xenopus oocytes. J Gen Physiol 103: 217–230, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansbery KL, Burcea LC, Mendenhall ML, Mercer RW. Cytoplasmic targeting signals mediate the delivery of phospholemman to the plasma membrane. Am J Physiol Cell Physiol 290: C633–C650, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Meij IC, Koenderink JB, Bokhoven HV, Assink KFH, Groenestege WT, Joep J, de Pont HHM, Bindels RJM, Monnens LAH, van den Heuvel LPWJ, Knoers NVAM. Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase γ-subunit. Nat Genet 26: 265–266, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Minor NT, Sha Q, Nichols CG, Mercer RW. The gamma subunit of the Na,K-ATPase induces cation channel activity. Proc Natl Acad Sci USA 95: 6521–6525, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorman JR, Ackerman SJ, Kowdley GC, Griffin MP, Mounsey JP, Chen Z, Cala SE, O'Brian JJ, Szabo G, Jones LR. Unitary anion currents through phospholemman channel molecules. Nature 377: 737–740, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Morales-Mulia M, Pasantes-Morales H, Moran J. Volume sensitive efflux of taurine in HEK293 cells overexpressing phospholemman. Biochim Biophys Acta 1496: 252–260, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Moran J, Morales-Mulia M, Pasantes-Morales H. Reduction of phospholemman expression decreases osmosensitive taurine efflux in astrocytes. Biochim Biophys Acta 1538: 313–320, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Morrison BW, Moorman JR, Kowdley GC, Kobayashi YM, Jones LR, Leder P. Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. J Biol Chem 270: 2176–2182, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem 273: 10279–10287, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Pu HX, Cluzeaud F, Goldshleger R, Karlish SJD, Farman N, Blostein R. Functional role and immunological localization of the γa and γb forms of the Na,K-ATPase subunit. J Biol Chem 276: 20370–20378, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Pu HX, Scanzano R, Blostein R. Distinct regulatory effects of the Na,K-ATPase γ subunit. J Biol Chem 277: 20270–20276, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Quamme GA, Rouffignac C. Epithelial magnesium transport and regulation by the kidney. Front Biosci 5: 694–711, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Russ WP, Engelman DM. The GXXXG motif: a framework for transmembrane helix-helix association. J Mol Biol 296: 911–919, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Schlingmann KP, Weber S, Peters M, Nejsum LN, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen N, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114: 191–200, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Sha Q, Lansbery KL, Distefano D, Mercer RW, Nichols CG. The nature of the conductance induced by the Na,K-ATPase γ subunit in Xenopus oocytes. J Physiol 535: 407–417, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimbo K, Brassard DL, Lamb RA, Pinto LH. Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophys J 69: 1819–1829, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song JL, Zhang XQ, Carl LL, Qureshi A, Rothblum LI, Cheung JY. Overexpression of phospholemman alters contractility and [Ca2+](i) transients in adult rat myocytes. Am J Physiol Heart Circ Physiol 283: H576–H583, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Sweadner KJ, Rael K. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68: 41–56, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Therien AG, Deber CM. Oligomerization of a peptide derived from the transmembrane region of the sodium pump gamma subunit: effect of the pathological mutation G41R. J Mol Biol 322: 583–590, 2002. [DOI] [PubMed]
- 38.Therien AG, Karlish SJD, Blostein R. Expression and functional role of the γ subunit of the Na,K-ATPase in mammalian cells. J Biol Chem 274: 12252–12256, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Tzounopoulos T, Maylie J, Adelman JP. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys J 69: 904–908, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31: 171–174, 2002. [DOI] [PubMed] [Google Scholar]