Abstract
Clinical and experimental studies have provided evidence suggesting that statins exert renoprotective effects. To investigate the mechanisms by which statins may exert renoprotection, we utilized the hypertensive Dahl salt-sensitive (DS) rat model, which manifests cardiovascular and renal injury linked to increased angiotensin II-dependent activation of NADPH oxidase and decreased nitric oxide (NO) bioavailability. DS rats given high salt diet (4% NaCl) for 10 wk exhibited hypertension [systolic blood pressure (SBP) 200 ± 8 vs. 150 ± 2 mmHg in normal salt diet (0.5% NaCl), P < 0.05], glomerulosclerosis, and proteinuria (158%). This was associated with increased renal oxidative stress demonstrated by urinary 8-F2α-isoprostane excretion and NADPH oxidase activity, increased protein expression of transforming growth factor (TGF)-β (63%) and fibronectin (181%), increased mRNA expression of the proinflammatory molecules monocyte chemoattractant protein-1 (MCP-1) and lectin-like oxidized LDL receptor-1 (LOX-1), as well as downregulation of endothelial NO synthase (eNOS) activity (−44%) and protein expression. Return to normal salt had no effect on SBP or any of the measured parameters. Atorvastatin (30 mg·kg−1·day−1) significantly attenuated proteinuria and glomerulosclerosis and normalized renal oxidative stress, TGF-β1, fibronectin, MCP-1 and LOX-1 expression, and eNOS activity and expression. Atorvastatin-treated rats showed a modest reduction in SBP that remained in the hypertensive range (174 ± 8 mmHg). Atorvastatin combined with removal of high salt normalized SBP and proteinuria. These findings suggest that statins mitigate hypertensive renal injury by restoring the balance among NO, TGF-β1, and oxidative stress and explain the added renoprotective effects observed in clinical studies using statins in addition to inhibitors of the renin-angiotensin system.
Keywords: end-organ injury, transforming growth factor, hypertension
hypertension is a leading risk factor for the development and progression of chronic kidney disease (CKD) and cardiovascular disease (CVD). Hypertensive renal injury manifests as proteinuria, glomerulosclerosis, vascular hypertrophy, tubulointerstitial inflammation, and fibrosis (11, 15). It has been demonstrated that transforming growth factor (TGF)-β1, oxidative stress, and proinflammatory cytokines, including monocyte chemoattractant protein (MCP)-1 and lectin-like oxidized LDL receptor-1 (LOX-1), play an important role in hypertensive, as well as diabetic, nephropathy (8, 11, 20).
The 3-hydroxy-3-methylgutaryl (HMG)-CoA reductase inhibitors (statins) are potent inhibitors of cholesterol biosynthesis. These agents slow the progression and promote the regression of atherosclerosis, resulting in an improvement of cardiovascular outcomes in humans with elevated as well as normal serum cholesterol levels (1, 2, 26). Accumulating evidence indicates that, in addition to blood pressure normalization and inhibition of the renin-angiotensin system (RAS), statin therapy may also contribute to slowing the progression of CKD (3, 7, 23, 27, 37).
Besides lowering cholesterol levels, statins exert beneficial, lipid-independent or “pleiotropic” effects upon CVD, including restoration/normalization of endothelial function, upregulation of nitric oxide (NO), and reduction in oxidative stress and vascular inflammation (9, 13, 33, 42). Furthermore, experimental studies have indicated that statins may reduce renal injury via diminishing the proliferation of mesangial and vascular smooth muscle cells, suppressing mesangial matrix expansion, and inhibiting macrophage infiltration and the production of cytokines and chemokines such as MCP-1 (7, 14, 16, 36). However, despite substantial experimental work, the clinical evidence supporting the benefit of statins in the progression of CKD is limited (3, 7, 27).
Normalization of blood pressure prevents or arrests progression of CKD (7). RAS inhibition controls systemic and intraglomerular pressure and inhibits intrarenal angiotensin (ANG) II-dependent upregulation of TGF-β1 and oxidative stress, which contribute to progression of renal injury. Recent clinical studies provided evidence suggesting that statins have additive or synergistic salutary effects to those afforded by RAS inhibition (7). Dahl salt-sensitive (DS) rats, a paradigm of salt-sensitive hypertension in humans (5, 6), manifest cardiovascular and renal injury linked to decreased NO bioavailability and increased ANG II activation of NADPH oxidase, which result in vascular oxidative stress and upregulation of vascular proinflammatory gene expression (12, 40). We recently demonstrated that treatment of hypertensive DS rats with atorvastatin exerted a significant cardiovascular protective effect (42). In addition, we detected a significant reduction in proteinuria, a marker of renal injury (42). In the present study, we investigated the mechanisms by which statins may contribute to renoprotection utilizing the hypertensive DS rat model.
METHODS
Animals and experimental protocols.
The animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee at the Miami Veterans Affairs Medical Center approved the studies. Six-week-old DS male rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and maintained under controlled conditions of light, temperature, and humidity. After 2-wk accommodation to the new environment, the rats were divided into five groups: NS, fed 0.5% NaCl diet for 10 wk (n = 8); HS, fed 4% NaCl diet for 10 wk (n = 8); HS+AT, fed 4% NaCl diet plus atorvastatin (30 mg·kg−1·day−1 by gavage, n = 7) for 10 wk; HS/NS, fed 4% NaCl diet for 6 wk followed by 4 wk of 0.5% NaCl diet (n = 6); HS/NS+AT, fed 4% NaCl diet for 6 wk followed by 4 wk of 0.5% NaCl diet plus atorvastatin (30 mg·kg−1·day−1 by gavage, n = 6). Systolic blood pressure (SBP) was measured in conscious rats by the tail-cuff method. Twenty-four-hour urine excretion was collected in individual metabolic cages. Urine protein concentration was determined with the use of a Bio-Rad protein assay kit and expressed in milligrams per 24 h. At the end of the study, the rats were killed by decapitation and kidneys were harvested.
NADPH oxidase activity.
Renal NADPH oxidase activity was determined as previously described (44). In brief, kidney tissue was homogenized in 50 mmol/l phosphate buffer. The homogenate was subjected to low centrifugation (1,000 g) for 10 min to remove debris. Twenty-microliter aliquots of supernatant were added into 50 mmol/l phosphate assay buffer (pH 7.4) containing 1.0 mmol/l EGTA and 5 μmol/l lucigenin; the reaction was trigged by addition of 100 μmol/l NADPH as substrate and superoxide anion (O2−) production was determined by lucigenin-enhanced chemiluminescence. NADPH oxidase-derived O2− was confirmed using flavoprotein inhibitor diphenyleneiodinium (DPI), which reduced production of O2− over 95% in the homogenate. The data were expressed as counts per minute per milligram of protein.
Determination of urine free 8-F2α isoprostane levels.
Purification and enzyme immunoassay procedures for measurement of urine free 8-F2α isoprostanes were performed by EIA using a commercial kit (Cayman Chemical) and following the manufacturer's instructions, as previously described (43). Concentrations were estimated from a standard curve and normalized by urine creatinine.
Measurement of NO synthase activity.
NO synthase (NOS) catalytic activity in the kidney was measured by conversion of [14C]l-arginine to [14C]l-citrulline as previously described (13, 42). Briefly, renal tissue was homogenized in 3.5 volumes of ice-cold buffer solution containing 50 mmol/l Tris·HCl (pH 7.4), 0.1% mercaptoethanol, 0.1 mmol/l EDTA, 0.1 mmol/l EGTA, 2 μmol/l leupeptin, 1 μmol/l pepstatin A, 1 mmol/l PMSF, and 1% Triton X-100 and centrifuged at 20,000 g for 45 min. Sixty microliters of the supernatant were added to 200 μl of assay buffer containing 50 mmol/l KH2PO4, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 50 mmol/l valine, 1 mmol/l l-citrulline, 20 μmol/l l-arginine, 1 mmol/l dithiothreitol, 2 mmol/l NADPH, 3 μmol/l BH4, 3 μmol/l FAD, 3 μmol/l FMN, and 0.5 μCi/ml [14C]l-arginine HCl. The mixture of supernatant and assay buffer solution was incubated for 30 min at 37°C in the presence or absence of either EGTA (1 mmol/l) or EGTA (1 mmol/l) plus l-nitro arginine (1 mmol/l) to determine the level of the Ca2+-dependent (cNOS) and -independent (iNOS) activities. After incubation, the reaction was stopped by adding 500 μl of ice-cold solution containing 20 mmol/l HEPES, 2 mmol/l EDTA, and 2 mmol/l EGTA. The incubated mixture was loaded onto 1-ml columns of Dowex resin (Na form). Columns were then eluted with 500 μl of distilled water. The amounts of [14C]l-citrulline were determined by a liquid scintillation counter. NOS activity was expressed as nanomoles of [14C]citrulline formed per minute per gram of protein.
Western blot.
Protein expression of TGF-β1, fibronectin, and NOS3 (eNOS) was determined by Western blot analysis. Briefly, after homogenization, protein content of the different samples was determined by Bio-Rad. Fifty micrograms of protein were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Transferred proteins were incubated overnight with specific polyclonal antibodies against TGF-β1 (1:250 dilution, Sigma, St. Louis, MO), fibronectin (1:2,000 dilution, R&D Systems, Mineapolis, MN), or eNOS (1:1,000 dilution, Cell Signaling, Danvers, MA). After being washed, the blots were incubated with the appropriate secondary antibody and the signal was detected by luminol chemiluminescence followed by exposure to an autoradiography film. The data were normalized by β-actin and expressed as fold increase vs. NS group.
Real-time PCR.
Primers and probes for rat MCP-1 and LOX-1 were designed by using Primer Express Software [Applied Biosystems (ABI)]. Real-time PCR was performed in a 50-μl reaction mixture containing an appropriately diluted cDNA solution, 0.1 μmol/l of each primer, 0.2 μmol/l of probe, and PCR Master Mix assay (ABI) under the following conditions: at 50°C for 2 min, at 95°C for 10 min, and 40 cycles at 95°C for 15 s and at 60°C for 1 min. A housekeeping gene (GADPH) was determined as an internal control. The data were expressed as fold increase vs. NS group.
Renal histology.
After fixation in 4% paraformaldehyde, the tissues were processed to paraffin, sectioned at 4 μm, and stained with periodic acid-Schiff (PAS). For assessment of glomerulosclerosis, PAS-stained sections were examined using a light microscope. The glomerular lesions were evaluated by use of a semiquantitative scoring method described previously (25). Briefly, 30 glomeruli per section were randomly selected and the degree of glomerular damage was assessed using a semiquantitative scoring method: grade 0, normal glomeruli; grade 1, sclerotic area up to 25% (minimal sclerosis); grade 2, sclerotic area 25 to 50% (moderate sclerosis); grade 3, sclerotic area 50 to 75% (moderate-severe sclerosis); grade 4, sclerotic area 75 to 100% (severe sclerosis). The glomerulosclerosis index (GSI) was calculated using the following formula: GSI = (1 × n1) + (2 × n2) + (3 × n3) + (4 × n4)/n0 + n1 + n2 + n3 + n4, where nx is the number of glomeruli in each grade of glomerulosclerosis. Slide examination, structural grading of lesions, and representative photomicrographs were taken in a strictly blinded fashion without knowledge of the experimental group.
Data analysis.
The results were expressed as means ± SE. Statistical analyses were performed by ANOVA with Bonferonni's correction for multiple comparisons, followed by Scheffé's test. Significance was assumed at P < 0.05.
RESULTS
SBP and urine protein excretion.
As previously shown, high salt intake for 10 wk significantly increased SBP (200 ± 8 vs. 150 ± 2 mmHg in NS; P < 0.05) and urine protein excretion in DS rats (Fig. 1). In rats receiving a high salt diet, atorvastatin (HS+AT) significantly mitigated the increase in SBP (174 ± 8 mmHg) and proteinuria. The return to a normal salt diet (HS/NS) alone after 6 wk of high salt diet did not reduce SBP (205 ± 7 mmHg) or urine protein excretion. However, after 6 wk of high salt diet, the return to normal salt diet and the addition of atorvastatin (HS/NS+AT) normalized SBP (152 ± 2 mmHg) and urine protein excretion, compared with control DS rats on normal salt diet (Fig. 1).
Fig. 1.
Systolic blood pressure (SBP; A) measured by tail-cuff method and urine protein excretion (B) in atorvastatin-treated hypertensive Dahl salt-sensitive (DS) rats. High salt diet (HS) for 10 wk significantly increased SBP and urinary protein excretion. Atorvastatin in the presence of high salt diet (HS+AT) reduced SBP and proteinuria. Return to a normal salt diet (HS/NS) did not reduce SBP or proteinuria. Combination of atorvastatin with removal of high salt diet (HS/NS+AT) normalized SBP and proteinuria. The data are expressed as means ± SE. *P < 0.05 vs. HS, HS/NS; †P < 0.05 vs. NS, HS/NS+AT; n = 6–8.
Renal oxidative stress.
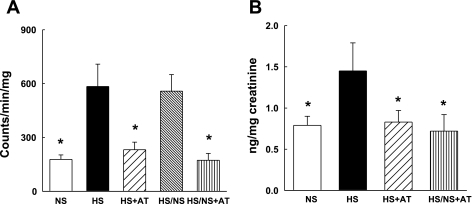
We previously showed that increased superoxide anion (O2−) generation is linked to ANG II activation of NADPH oxidase in the vasculature of hypertensive DS rats. High salt diet significantly increased renal NADPH oxidase activity and urinary excretion of 8-isoprostane compared with DS rats on normal salt diet (Fig. 2). Return to a normal salt diet after 6 wk of high salt (HS/NS) failed to significantly reduce NADPH oxidase activity. Atorvastatin prevented the increase in both renal NADPH oxidase activity and urinary excretion of 8-isoprostane in DS rats maintained on a high salt diet. Furthermore, atorvastatin in combination with a return to normal salt diet normalized these markers of renal oxidative stress (Fig. 2).
Fig. 2.
Renal NADPH oxidase activity (A) and urinary free 8-isoprostane excretion (B) in atorvastatin-treated DS rats. High salt diet significantly increased renal NADPH oxidase activity and urinary 8-isorpostane excretion. Atorvastatin in the presence of high salt diet or combination of atorvastatin with removal of high salt diet normalized renal NADPH oxidase activity and urinary 8-isoprostane excretion. Return to a normal salt diet did not reduce renal NADPH oxidase activity. *P < 0.05 vs. HS or HS/NS; n = 6–7.
Renal cNOS activity and NOS3 protein expression.
Similar to what we showed previously in the vasculature, cNOS activity in the kidney was significantly lower in hypertensive high-salt rats than in normal-salt rats (0.88 ± 0.11 vs. 1.51 ± 0.16 nmol·min−1·g−1 protein in NS; P < 0.05; Fig. 3A). The return to a normal salt diet after 6 wk of high salt diet did not improve cNOS activity (0.90 ± 0.2 nmol·min−1·g−1 protein; Fig. 3). Atorvastatin prevented the decrease in cNOS activity in DS rats maintained on high salt (HS + AT, 1.65 ± 0.34 nmol·min−1·g−1 protein) and normalized cNOS activity in DS rats switched to a normal salt diet (HS/NS + AT, 1.60 ± 0.2 nmol·min−1·g−1 protein; Fig. 3). cNOS consists of NOS3 and NOS1 (nNOS). To identify whether statin treatment upregulates renal NOS3, we determined NOS3 protein expression by Western blot. Consistent with the cNOS activity findings, protein expression of NOS3 was significantly lower in hypertensive high-salt rats than normal-salt rats. The return to a normal salt diet alone after 6 wk of high salt diet did not increase NOS3 expression. Atorvastatin prevented the decrease in NOS3 protein expression in DS rats maintained on a high salt diet and normalized NOS3 expression in DS rats switched to a normal salt diet (Fig. 3B).
Fig. 3.
Renal cNOS activity (A; n = 6–7) and NOS3 protein expression (B; n = 4) in the atorvastatin-treated DS rats. High salt diet significantly decreased renal cNOS activity and NOS3 protein expression. Atorvastatin in the presence of high salt diet or combination of atorvastatin with removal of high salt diet prevented the fall in aortic cNOS activity and NOS3 expression. Return to a normal salt diet alone did not affect renal cNOS activity and NOS3 expression. *P < 0.05 vs. HS, HS/NS.
TGF-β1 and fibronectin protein expression.
Protein expression of TGF-β1 and fibronectin in the renal cortex of DS rats on high salt diet was significantly increased compared with those on normal salt diet (Fig. 4). The return to a normal salt diet after 6 wk of high salt diet did not prevent the increase in TGF-β1 and fibronectin. Atorvastatin prevented the increase in TGF-β1 and fibronectin in hypertensive DS rats maintained on high salt (HS + AT) and normalized the expression of these profibrotic molecules in DS rats switched to normal salt (HS/NS + AT; Fig. 4).
Fig. 4.
Protein expression of transforming growth factor (TGF)-β1 (A) and fibronectin (B), determined by Western blot in the kidney cortex of atorvastatin-treated DS rats. High salt diet significantly increased protein expression of TGF-β1 and fibronectin. Return to a normal salt diet did not affect protein expression of TGF-β1 and fibronectin. Atorvastatin in the presence of high salt diet or combination of atorvastatin with removal of high salt diet normalized protein expression of TGF-β1 and fibronectin. Data were normalized by β-actin and expressed as fold increase vs. NS group. *P < 0.05 vs. HS, HS/NS; n = 4.
MCP-1 and LOX-1 mRNA expression.
MCP-1 and LOX-1 mRNA expression in the kidney cortex of DS rats on high salt diet, determined by real-time PCR, was significantly increased compared with those on normal salt diet (Fig. 5). Atorvastatin prevented the increase in MCP-1 and LOX-1 mRNA expression in hypertensive DS rats (HS + AT) and normalized the expression of these proinflammatory molecules in DS rats switched to normal salt (HS/NS + AT; Fig. 5). Switch to a normal salt diet after 6 wk of high salt diet alone did not affect MCP-1 and LOX-1 mRNA expression.
Fig. 5.
mRNA expression of LOX-1 (left) and monocyte chemoattractant protein-1 (MCP-1; right), determined by real-time PCR, in the renal cortex of atorvastatin-treated DS rats. High salt diet significantly increased mRNA expression of LOX-1 and MCP-1. Return to a normal salt diet alone did not prevent increase in mRNA expression of LOX-1 and MCP-1. Atorvastatin in the presence of high salt diet or combination of atorvastatin with removal of high salt diet normalized mRNA expression of LOX-1 and MCP-1. Data were normalized by housekeeper gene GADPH and expressed as fold increase vs. NS group. *P < 0.05 vs. HS; HS/NS; n = 4.
Renal histology.
The DS rats on high salt (HS) diet manifested mesangial extracellular matrix (ECM) expansion, capillary wall thickening, and diffuse segmental glomerulosclerosis, with a glomerulosclerosis index score of 2.1 ± 0.21 vs. 1.0 ± 0.19 in the DS rats on normal salt (NS) diet (P < 0.01; n = 7; Fig. 6). Atorvastatin significantly ameliorated glomerular injury in hypertensive DS rats (HS + AT, GIS 1.2 ± 0.16; P < 0.01 vs. HS; n = 7; Fig. 6) and in DS rats switched to normal salt (HS/NS + AT, 1.3 ± 0.28; P < 0.05 vs. HS; n = 6; Fig. 6).
Fig. 6.
Renal histology. For assessment of glomerular injury, PAS-stained sections (×40) were examined using a light microscope. A: DS rats fed normal salt (0.5% NaCl; NS). B: DS rats fed high salt (4% NaCl; HS). C: DS rats fed high salt and atorvastatin (HS+AT). D: DS rats on high salt switched to normal salt in combination with atorvastatin (HS/NS+AT). Each image represents 6–7 samples.
DISCUSSION
The major findings of the present study are that in a model of salt-sensitive hypertension, atorvastatin produced both preventive and therapeutic renal effects. Atorvastatin, given for 10 wk in combination with high salt diet or initiated concomitantly with a switch to normal salt after 6 wk of high salt, significantly reduced blood pressure, proteinuria, and glomerular injury, increased renal cNOS activity, inhibited renal oxidative stress, and decreased the expression of the profibrotic molecules TGF-β1 and fibronectin and the proinflammatory molecules LOX-1 and MCP-1.
Statins have been reported to ameliorate glomerular injury and preserve renal function in several animal models of hypertension, including the spontaneously hypertensive rat, ANG II-induced hypertensive rat, and hypertensive DS rats (13, 16, 24, 34, 36). However, the mechanisms underlying the renoprotective effect of statins have not been fully elucidated. The balance between NO and oxidative stress plays a critical role in the maintenance of cardiovascular and renal homeostasis (28, 41). In the kidney, NO induces renal arterial vasodilation, inhibits sodium reabsorption, and reduces mesangial cell proliferation and ECM synthesis in response to injury (12, 45). Reactive oxygen species (ROS), such as O2−, not only diminish NO bioavailability but also produce peroxynitrite, a prooxidant molecule with detrimental biologic effects on vascular and renal systems (46). We previously showed that renal and vascular injury in the hypertensive DS rat is mechanistically associated with reduction in renal and vascular NO bioavailability (12). Similar to what we previously showed in the vasculature of DS rats, in the present study atorvastatin treatment upregulated renal cNOS activity and NOS3 expression and concomitantly reduced oxidative stress, which may importantly contribute to renal protection.
Emerging evidence suggests that proinflammatory mechanisms may contribute to the pathogenesis of hypertensive nephropathy (31). Hypertensive DS rats manifest glomerular sclerosis associated with inflammatory cell infiltration and increased MCP-1 and LOX-1 expression (17, 20, 38). Reducing renal inflammatory cell infiltration by immunosuppressive therapy has been shown to attenuate nephrosclerosis and lower blood pressure in these animals (31). MCP-1 is critical for macrophage cell recruitment and infiltration into the kidney (17, 38). Increased LOX-1 is closely associated with glomerulosclerosis in hypertensive DS rats (20). We previously showed in this animal model that upregulation of vascular MCP-1 and LOX-1 results from ANG II activation of NADPH oxidase (40, 41). Indeed, inhibition of NADPH oxidase activation, by either an ANG II type 1 receptor blocker or the specific NADPH oxidase inhibitor gp91ds-tat, prevented the increase in vascular MCP-1 and LOX-1 expression in hypertensive DS rats (40, 41). In the present study, atorvastatin prevented the increase in renal MCP-1 and LOX-1 expression as well as renal oxidative stress, suggesting that statins may exert anti-inflammatory effects in the kidney through their antioxidant effects.
In DS rats, glomerulosclerosis is associated with increased deposition of ECM proteins and abnormal expansion of the mesangium (4). TGF-β1 is a fibrogenic cytokine that stimulates ECM accumulation in the mesangium by increased synthesis and reduced degradation of matrix proteins (4). In hypertensive DS rats, increased protein expression of TGF-β1 was located in the glomerular sclerotic areas (29, 30), and inhibition of TGF-β1 with a neutralizing antibody or gene-silencing reduced the severity of glomerulosclerosis and proteinuria, suggesting a critical role of TGF-β1 in hypertensive renal injury (8, 19). Here, we show that atorvastatin inhibited TGF-β1 and its downstream effector molecule fibronectin, associated with upregulation of NOS3 and inhibition of renal oxidative stress. It has been demonstrated that TGF-β1 is upregulated by ANG II, oxidative stress, and increased glomerular pressure and inhibited by NO (39). Therefore, upregulation of NO and inhibition of oxidative stress by statins may contribute, at least in part, to the inhibition of TGF-β1.
Of note, blood pressure was slightly but significantly reduced in the atorvastatin-treated DS rats maintained on high salt. With this small reduction in blood pressure, which remained in the hypertensive range, there was normalization of renal NO, oxidative stress, TGF-β1, LOX-1, and MCP-1. Normalization of blood pressure and protein excretion was achieved by switching from high salt to normal salt in combination with atorvastatin, whereas switching to normal salt alone did not lower blood pressure or proteinuria or improve markers of oxidative stress and fibrosis. The reduction in blood pressure by atorvastatin may be a result of improvement in endothelial function, upregulation of NOS3, and reduction of ROS (18, 32, 35, 42). Thus, these studies suggest that removal of salt in combination with an agent that restores the balance between ROS and NO is effective in reducing/normalizing blood pressure in salt-sensitive hypertension.
Our studies do not exclude that reduction in blood pressure per se and improvement in renal artery endothelial function may contribute to the renoprotective effect of statins. However, several studies in hypertensive DS rats have demonstrated that reduction of blood pressure by hydralazine produced only a minimal beneficial renal effect (17, 21–23). On the other hand, the angiotensin-converting enzyme inhibitor benazepril, the aldosterone receptor blocker eplerenone, and the antioxidant Tempol, which have antioxidant and anti-inflammatory effects in addition to antihypertensive effects, produced a significant reduction in proteinuria and renal histological injury despite a similar reduction in blood pressure. Therefore, we surmise that the renoprotective effects of statins are largely mediated by their pleiotropic effects on the glomerular endothelium and mesangium (7, 10).
In summary, the balance among NO, TGF-β1, and oxidative stress is critical for the maintenance of renal homeostasis. Statins restore renal NO production and inhibit renal TGF-β1, oxidative stress, and proinflammatory gene expression. These pleiotropic effects of statins mitigate renal injury and are a potential additional therapeutic tool for preventing or arresting the progression of renal disease.
GRANTS
This work was supported by a University of Miami Stanley Glaser Research Foundation grant and American Heart Association National Scientist Development Award to M.-S. Zhou, by funds from the Veterans Affairs Administration to L. Raij and E. A. Jaimes, and by National Institutes of Health (NIH) Grant NIH/National Institute of Diabetes and Digestive and Kidney Diseases DK-069372 to E. A. Jaimes.
Acknowledgments
We appreciate the excellent technical assistance of Run-Xia Tian. We are grateful to Pfizer for generously providing the atorvastatin used in these studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anonymous. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339: 1349–1357, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S.). Lancet 344: 1383–1389, 1994. [PubMed] [Google Scholar]
- 3.Agarwal R Effects of statins on renal function. Am J Cardiol 97: 748–755, 2006. [DOI] [PubMed] [Google Scholar]
- 4.August P, Suthanthiran M. Transforming growth factor beta and progression of renal disease. Kidney Int Suppl S99–S104, 2003. [DOI] [PubMed]
- 5.Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension 23: 195–199, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bigazzi R, Bianchi S, Baldari G, Campese VM. Clustering of cardiovascular risk factors in salt-sensitive patients with essential hypertension: role of insulin. Am J Hypertens 9: 24–32, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Campese VM, Nadim MK, Epstein M. Are 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors renoprotective? J Am Soc Nephrol 16, Suppl 1: S11–S17, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Davignon J Beneficial cardiovascular pleiotropic effects of statins. Circulation 109: III39–III43, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Gianella A, Nobili E, Abbate M, Zoja C, Gelosa P, Mussoni L, Bellosta S, Canavesi M, Rottoli D, Guerrini U, Brioschi M, Banfi C, Tremoli E, Remuzzi G, Sironi L. Rosuvastatin treatment prevents progressive kidney inflammation and fibrosis in stroke-prone rats. Am J Pathol 170: 1165–1177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartner A, Veelken R, Wittmann M, Cordasic N, Hilgers KF. Effects of diabetes and hypertension on macrophage infiltration and matrix expansion in the rat kidney. BMC Nephrol 6: 6, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 96: 2407–2413, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest 101: 2711–2719, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heusinger-Ribeiro J, Fischer B, Goppelt-Struebe M. Differential effects of simvastatin on mesangial cells. Kidney Int 66: 187–195, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hill GS, Heudes D, Jacquot C, Gauthier E, Bariety J. Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int 69: 823–831, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kido M, Ando K, Oba S, Fujita T. Renoprotective effect of pravastatin in salt-loaded Dahl salt-sensitive rats. Hypertens Res 28: 1009–1015, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, Mita S, Nakano S, Tsubokou Y, Matsuoka H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertension 45: 538–544, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Koh KK, Quon MJ, Waclawiw MA. Are statins effective for simultaneously treating dyslipidemias and hypertension? Atherosclerosis 196: 1–8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda H, Fukuda N, Ueno T, Tahira Y, Ayame H, Zhang W, Bando T, Sugiyama H, Saito S, Matsumoto K, Mugishima H, Serie K. Development of gene silencing pyrrole-imidazole polyamide targeting the TGF-beta1 promoter for treatment of progressive renal diseases. J Am Soc Nephrol 17: 422–432, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Nagase M, Kaname S, Nagase T, Wang G, Ando K, Sawamura T, Fujita T. Expression of LOX-1, an oxidized low-density lipoprotein receptor, in experimental hypertensive glomerulosclerosis. J Am Soc Nephrol 11: 1826–1836, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47: 1084–1093, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol 15: 306–315, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int 65: 972–981, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JK, Muller DN, Mervaala EM, Dechend R, Fiebeler A, Schmidt F, Bieringer M, Schafer O, Lindschau C, Schneider W, Ganten D, Luft FC, Haller H. Cerivastatin prevents angiotensin II-induced renal injury independent of blood pressure- and cholesterol-lowering effects. Kidney Int 58: 1420–1430, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335: 1001–1009, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 17: 2006–2016, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Schulman IH, Zhou MS, Raij L. Interaction between nitric oxide and angiotensin II in the endothelium: role in atherosclerosis and hypertension. J Hypertens Suppl 24: S45–S50, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Siegel AK, Kossmehl P, Planert M, Schulz A, Wehland M, Stoll M, Bruijn JA, de Heer E, Kreutz R. Genetic linkage of albuminuria and renal injury in Dahl salt-sensitive rats on a high-salt diet: comparison with spontaneously hypertensive rats. Physiol Genomics 18: 218–225, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki K, Okuda S, Nakayama M, Yanagida T, Fujishima M. Transforming growth factor-beta 1 in hypertensive renal injury in Dahl salt-sensitive rats. J Am Soc Nephrol 7: 2578–2589, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 292: H1018–H1025, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Vecchione C, Brandes RP. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res 91: 173–179, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 22: 300–305, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto E, Yamashita T, Tanaka T, Kataoka K, Tokutomi Y, Lai ZF, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S. Pravastatin enhances beneficial effects of olmesartan on vascular injury of salt-sensitive hypertensive rats, via pleiotropic effects. Arterioscler Thromb Vasc Biol 27: 556–563, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita T, Kawashima S, Miwa Y, Ozaki M, Namiki M, Hirase T, Inoue N, Hirata K, Yokoyama M. A 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor reduces hypertensive nephrosclerosis in stroke-prone spontaneously hypertensive rats. J Hypertens 20: 2465–2473, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Yao K, Sato H, Ina Y, Suzuki K, Ohno T, Shirakura S. Renoprotective effects of benidipine in combination with angiotensin II type 1 receptor blocker in hypertensive Dahl rats. Hypertens Res 26: 635–641, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Yasui N, Kajimoto K, Sumiya T, Okuda T, Iwai N. The monocyte chemotactic protein-1 gene may contribute to hypertension in Dahl salt-sensitive rats. Hypertens Res 30: 185–193, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ying WZ, Sanders PW. The interrelationship between TGF-β1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285: F902–F908, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension 42: 945–951, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension 47: 81–86, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Zhou MS, Jaimes EA, Raij L. Atorvastatin prevents end-organ injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension 44: 186–190, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am J Hypertens 17: 167–171, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Zhou MS, Jaimes EA, Raij L. Vascular but not cardiac remodeling is associated with superoxide production in angiotensin II hypertension. J Hypertens 23: 1737–1743, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, hypertension. Semin Nephrol 24: 366–378, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]