Abstract
The molecular pathogenesis of diabetic nephropathy (DN), the leading cause of end-stage renal disease worldwide, is complex and not fully understood. Transforming growth factor-β (TGF-β1) plays a critical role in many fibrotic disorders, including DN. In this study, we report protein kinase B (PKB/Akt) activation as a downstream event contributing to the pathophysiology of DN. We investigated the potential of PKB/Akt to mediate the profibrotic bioactions of TGF-β1 in kidney. Treatment of normal rat kidney epithelial cells (NRK52E) with TGF-β1 resulted in activation of phosphatidylinositol 3-kinase (PI3K) and PKB/Akt as evidenced by increased Ser473 phosphorylation and GSK-3β phosphorylation. TGF-β1 also stimulated increased Smad3 phosphorylation in these cells, a response that was insensitive to inhibition of PI3K or PKB/Akt. NRK52E cells displayed a loss of zona occludins 1 and E-cadherin and a gain in vimentin and α-smooth muscle actin expression, consistent with the fibrotic actions of TGF-β1. These effects were blocked with inhibitors of PI3K and PKB/Akt. Furthermore, overexpression of PTEN, the lipid phosphatase regulator of PKB/Akt activation, inhibited TGF-β1-induced PKB/Akt activation. Interestingly, in the Goto-Kakizaki rat model of type 2 diabetes, we also detected increased phosphorylation of PKB/Akt and its downstream target, GSK-3β, in the tubules, relative to that in control Wistar rats. Elevated Smad3 phosphorylation was also detected in kidney extracts from Goto-Kakizaki rats with chronic diabetes. Together, these data suggest that TGF-β1-mediated PKB/Akt activation may be important in renal fibrosis during diabetic nephropathy.
Keywords: diabetic nephropathy, transforming growth factor-β1
diabetes mellitus currently affects between 6 and 10% of the world's population and is threatening to reach epidemic proportions worldwide. Diabetic patients are at increased risk of developing vascular complications affecting the heart, eye, and kidney. Diabetic kidney disease (nephropathy) affects between 25 and 40% of all diabetic patients and is currently the leading cause of renal failure worldwide (36). The increasing numbers of patients presenting with diabetic nephropathy (DN) are likely to have a major socioeconomic impact.
Tubulointersitial fibrosis is thought to be the final common pathway in most cases of end-stage renal disease (31). This scarring of the kidney compromises the delicate balance of fluid and electrolyte transport across the epithelial layer of both proximal and distal tubules. Contributory factors that drive renal fibrosis include increased numbers of fibroblasts recruited from the circulation, increased proliferation and activation of resident renal fibroblasts, and a phenotypic conversion of renal epithelial cells to an activated myofibroblast phenotype [epithelial-mesenchymal transition (EMT)] (22). Increased numbers of myofibroblasts in the diabetic kidney leads to increased extracellular matrix secretion, which contributes to glomerulosclerosis and a decline in renal function. Transforming growth factor-β (TGF-β1) is the primary cytokine driving fibrosis in kidney and other organs susceptible to fibrotic injury such as lung and colon (38, 39, 46). TGF-β1 engages type II and type I (Alk5) receptors at the plasma membrane, triggering phosphorylation of Smad2/3, which then form dimers with Smad4 that accumulate in the nucleus, driving transcriptional events that mediate the TGF-β1 response (45). TGF-β1 also activates non-Smad-dependent signaling events that may contribute to fibrosis. Roles for p38 MAPK, protein kinase B (PKB)/Akt, integrin-linked kinase (ILK), RhoA, and β-catenin have been described downstream of TGF-β1 in various cell types including mammary epithelial cells, transformed mammary epithelia, and keratinocytes (2, 3, 5, 12, 19, 25).
EMT is thought to contribute up to 35% of fibroblasts found during renal fibrosis (22). EMT is characterized by decreased expression of epithelial markers such as zona occludins 1 (ZO-1) and E-cadherin, together with increased expression of collagen type I, α-smooth muscle actin (α-SMA), and matrix metalloproteinases (22, 49). The primary pathogenic driver of EMT during renal fibrosis is thought to be TGF-β1, which can stimulate each phase of EMT (26). Apart from TGF-β1, other extrinsic mediators can drive EMT, including epidermal growth factor (EGF) (42), advanced glycation end-products (AGEs) (32), connective tissue growth factor (CTGF) (7), and fibroblast growth factor-2 (FGF-2) (44). Other factors such as hepatocyte growth factor (HGF) and bone morphogenic factor-7 (BMP-7) can inhibit EMT, and these factors have been shown to reduce renal fibrosis in mouse models of kidney damage (43, 48). The complexity of signal transduction pathways implicated in EMT signaling suggests that EMT is a dynamic, coordinated process that may require the integration of many signaling pathways at different stages (26).
In this report, we identify a key role for PKB/Akt in TGF-β1-mediated EMT in normal rat kidney epithelial cells (NRK52E). TGF-β1 activated both phosphatidylinositol 3-kinase (PI3K) and PKB/Akt, and inhibition of either PI3K or PKB/Akt activity attenuated TGF-β1-mediated EMT in these cells. Furthermore, increased levels of TGF-β1 signaling, together with elevated levels of PKB/Akt activity, were detected in the kidney tubules of a diabetic rat with incipient DN, suggesting that this signaling cascade may be involved in DN-associated EMT in vivo. These data therefore position PKB/Akt as a key mediator of epithelial cell damage during renal fibrosis.
MATERIALS AND METHODS
Cell culture.
NRK52E cells (ECACC, Salisbury, UK) were cultured in Dulbecco's modified Eagle's medium (DMEM D-6546; Sigma) supplemented with 10% (vol/vol) fetal calf serum (GIBCO), 1% (vol/vol) nonessential amino acids (Sigma), 2 mM l-glutamine (Sigma), 50 U/ml penicillin (GIBCO), and 50 μg/ml streptomycin (GIBCO) at 37°C in 95% air-5% CO2. For TGF-β1 treatments, NRK52E cells (2 × 105) cultured in six-well plates were made quiescent by culturing in medium containing 0.1% (vol/vol) fetal calf serum for 24 h before treatment with 10 ng/ml TGF-β1. Pharmacological inhibitors were added before TGF-β1 as follows: LY-294002 (Calbiochem,) 20 μM for 30 min; Akt inhibitor II (Calbiochem), 40 μM for 30 min.
For EMT morphology experiments, NRK52E were plated at 1 × 105 cells/well in two-well glass slides (Lab-Tek) 24 h before TGF-β1 treatment. The cells were then cultured in KI medium [DMEM supplemented with 10× ITS (insulin-transferrin-selenium solution; Sigma), 36 μg/ml hydrocortisone, 10 ng/ml EGF, and 10 ng/ml TGF-β1]. Medium was changed every 2 days for experiments of longer duration.
Cell transfection.
NRK52E cells were plated at 2 × 105 cells/well in six-well plates 24 h before transfection. Cells were transfected with 1 μg of pCGN empty vector, pCGN-HA-PTEN wild type, or pCGN-HA-PTEN C124S lipid phosphatase mutant (generous gifts from Prof. Nicholas Tonks, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) using OptiMEM (GIBCO) and Fugene6 (Roche) at a 3:1 lipid-DNA ratio. Cells were incubated with DNA-lipid complexes for 24 h, after which transfection medium was removed and replaced with serum-free medium containing 10 ng/ml TGF-β1. After 60 min, cells were lysed and probed as described in Protein extraction and Western blotting. Transfected cells were identified based on immunoblotting by using antibodies to a hemagglutinin (HA) isotope tag located at the NH2-terminus of PTEN wild type or C124S mutant.
Protein extraction and Western blotting.
For immunoblotting, kidney tissues and cells were lysed in radioimmunoprecipitation assay buffer containing 50 mM Tris·HCl, pH 7.4, 1% (vol/vol) Nonidet P-40, 0.25% (wt/vol) sodium deoxycholate, 150 mM NaCl, and 1 mM EDTA, supplemented with fresh 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail (Sigma), 1 mM NaF, 40 mM β-glycerophosphate, 2 μM microcystin, and 1 mM sodium vanadate. Cell lysates were incubated on ice for 20 min, vortexed every 5 min, and centrifuged at 20,000 g for 15 min at 4°C to pellet the cell debris. Protein extract (25 μg) estimated using the modified Bradford assay (6) was then resolved using 10% (vol/vol) SDS-PAGE. Proteins were then transferred onto polyvinylidene difluoride membrane (Immobilon P; Millipore) for 75 min at 110 V and incubated in blocking buffer TBS-T (10 mM Tris·HCl, pH 7.4, 150 mM NaCl, and 0.1% Tween 20) supplemented with 3% (wt/vol) skim milk for 60 min. Membranes were then washed in TBS-T for 10 min and incubated with the following antibodies: polyclonal pAkt Ser473 (1:1,000; Cell Signaling), pGSK-3β Ser9 (1:1,000; Cell Signaling), affinity-purified total PKB/Akt (1:1,000; a generous gift from Dr. Brian Hemmings, Friedrich Miescher Institute, Basel, Switzerland), vimentin (1:1,000; Abcam), pSmad3 (1:1,000; Cell Signaling), total Smad3 (1:1,000; Cell Signaling), monoclonal E-cadherin (1:500; BD Biosciences), α-smooth muscle actin (1:500; Sigma), β-actin (1:25,000; Sigma), and HA (1:1,000; Covance) diluted in 3% milk in TBS-T overnight at 4°C. After three 10-min washes in TBS-T, the membranes were incubated with anti-rabbit IgG or anti-mouse IgG horseradish peroxidase-linked secondary antibodies (Cell Signaling) at 1:5,000 in TBS-T containing 3% milk for 60 min. Reactive bands were revealed using enhanced chemiluminescence reagents (Santa Cruz Biotechnology) and X-ray film.
EMT morphology and immunocytochemistry.
NRK52E cells (1 × 105 cells/well) were cultured on two-well chamber slides (Lab-Tek) for 24 h before treatment with 10 ng/ml TGF-β1. Cells were washed with 1× PBS and changed to KI medium as described earlier. In EMT morphology experiments, cells were grown for 6 days in the presence or absence of 10 ng/ml TGF-β1. For pharmacological inhibition studies, cells were exposed to DMSO (vehicle), 10 μM LY-294002, or 20 μM Akt inhibitor II for 72 h in the presence or absence of 10 ng/ml TGF-β1. Cells were fixed with 4% (wt/vol) paraformaldehyde for 15 min at room temperature, permeabilized with 0.1% (vol/vol) Triton X-100 for 5 min at room temperature, and then washed three times in 1× PBS. Cells were blocked with 5% (vol/vol) normal goat serum in 1× PBS for 60 min and incubated with primary monoclonal ZO-1 antibody (1:100; Zymed Laboratories) or polyclonal rabbit vimentin antibody (1:100; Abcam) diluted with ChemMate antibody diluent (DakoCytomation) overnight at 4°C. Cells were washed three times in PBS and then incubated with TRITC-labeled anti-mouse or anti-rabbit secondary antibodies (Molecular Probes) at 1:500 for 60 min in the dark. Cells were counterstained with 1 μg/ml Hoechst 33258 (Sigma) to visualize nuclei and were mounted using Anti-fade mounting medium (Molecular Probes). Stained cells were visualized using an Axioplan 2 Zeiss microscope at ×400 magnification. Images were captured using Axiovision LE Rel 4.5 software.
Quantitative estimation of pAkt/ pGSK-3β by electrochemiluminescence.
Quantitative levels of pAkt Ser473 and pGSK-3β Ser9 in protein lysates were measured using Meso Scale Discovery (MSD) Multi-Spot electrochemiluminescence technology (www.mesoscale.com). Briefly, cells were lysed in lysis buffer provided with the MSD kit containing Tris buffer supplemented with protease inhibitors. Cell lysates were incubated on ice for 30 min and centrifuged at 20,000 g for 15 min at 4°C to pellet the cell debris. MSD Multi-Spot pAkt Ser473/total Akt or MSD Multi-Spot pGSK-3β Ser9/total GSK-3β 96-well plates were blocked with 150 μl of blocking buffer for 60 min at room temperature (RT) and washed four times in Tris wash buffer. The plates were incubated with 25 μl of pAkt Ser473/total Akt or 12.5 μl of pGSK-3β/total GSK-3β protein lysate on the plate shaker at RT. The wells were then incubated with 25 μl of detection antibody per well for 60 min on the shaker. Plates were then washed with Tris wash buffer four times after each step, and 150 μl of read buffer was added. The plates were read using a MSD Sector Imager 2400. The mean signal values of samples (quadruplicates) were normalized to total protein and statistically analyzed using Microsoft Excel.
Animal models and tissue/sample preparation.
Both 11- to 12-wk and 9- to 10-mo-old male Goto-Kakizaki (GK) rats were purchased from M and B Taconic (Ry, Denmark). Age-matched control Wistar rats were obtained from Harlan (Bichester, UK). All animal handling was performed by licensed technicians in the University College Dublin Biomedical Facility in accordance with the appropriate governmental and institutional ethical and legal guidelines. Rats were euthanized with 1 ml of Euthatal (phenobarbital sodium), followed by cervical dislocation. Kidneys were carefully dissected out and cut longitudinally for histology and protein preparation. One half was placed in 10% formalin solution and paraffin embedded; the other half was snap-frozen in liquid N2. Rat kidney sections were placed into OCT compound, and fresh frozen kidney sections (5 μm) were obtained using a microtome. The sections were then fixed with acetone for 10 min. Four rats per group were used in these experiments.
Histology and immunohistochemistry.
Rat kidney coronal sections (5 μm thick) were prepared using a microtome and stained with Masson's Trichrome for collagen or periodic acid-Schiff for extracellular matrix using an automated slide stainer (Tissue-Tek DRS 2000; Sakura Finetek Europe, Zoeterwoude, The Netherlands). Sections were examined using a phase-contrast microscope at ×400 magnification to visualize the structural components.
Frozen kidney sections (5 μm) were blocked with 5% (vol/vol) goat serum for 1 h at RT. Fresh frozen sections were then incubated with primary antibodies against E-cadherin (monoclonal; BD Biosciences) at 1:100 dilution for 2 h at RT, followed by incubation with FITC-coupled secondary goat anti-mouse antibody at 1:500 dilution (Oregon green 488; Molecular Probes) for 1 h in the dark.
Deparaffinized kidney sections (5 μm) were heated in sodium citrate buffer, pH 6.0, for antigen unmasking and incubated with 5% (vol/vol) goat serum (Sigma) for 1 h, followed by incubation with primary antibody against vimentin (rabbit polyclonal; Abcam) at 1:100 dilution overnight at 4°C. Reactive proteins were visualized using FITC-coupled secondary goat anti-rabbit antibody (Oregon green 488; Molecular Probes) at 1:400 dilution. Sections were counterstained with 1 μg/ml Hoechst 33258 (Sigma) to visualize nuclei and mounted using DAKO fluorescence mounting medium (DakoCytomation). Stained cells were visualized using an Axioplan 2 Zeiss microscope at ×400 magnification. Images were captured using Axiovision LE Rel 4.5 software.
For phospho-PKB/Akt and pGSK-3β analysis, paraffin-embedded kidney sections (5 μm) were deparaffinized using a standard protocol. Deparaffinized kidney sections were heated in sodium citrate buffer, pH 6.0, and blocked with 3% (wt/vol) H2O2 for 10 min, followed by a 5-min wash in 1× PBS. Sections were then blocked with 5% (vol/vol) goat serum, followed by incubation with immunohistochemistry-specific rabbit polyclonal antibody against residue pSer473 of PKB/Akt (Cell Signaling) or residue pSer9 of GSK-3β (Cell Signaling) at 1:100 dilution overnight at 4°C. Tissue sections were then exposed to FITC-coupled secondary anti-rabbit (Oregon Green 488; Molecular Probes) for 1 h in the dark. The tissues were then incubated with ABC reagent (Vecta Stain kit) for 30 min, followed by a 5-min wash in 1× PBS. Tissues were finally incubated in diaminobenzidine (Sigma) for 2 min and counterstained with hematoxylin for 2 min. The tissues were rehydrated, mounted on a slide, examined using an Axio Imager M1 fluorescent microscope at ×400 magnification, and analyzed using Axiovision LE Rel 4.5 software.
Statistical analysis.
All experiments were carried out in triplicate unless otherwise stated, at least three times, and results are plotted as means ± SE unless otherwise stated. Statistical significance was determined using a paired two-tailed t-test. P values <0.05 were considered significant.
RESULTS
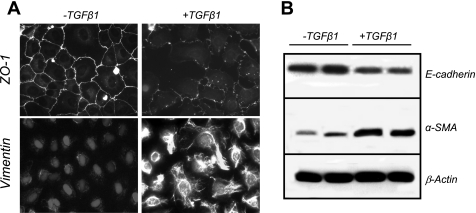
NRK-52E normal rat tubular epithelial cells were used to examine TGF-β1-induced EMT. As previously reported for other renal tubular epithelial cells (40, 41, 49), decreased expression of ZO-1 (epithelial marker) and increased expression of vimentin (mesenchymal marker) were observed in cells treated with TGF-β1 (Fig. 1A). Similar decreased expression of E-cadherin (epithelial protein; Fig. 1B, top) and increased expression of α-SMA (mesenchymal protein; Fig. 1B, middle) were observed. Thus TGF-β1 induces EMT-like changes in NRK52E cells.
Fig. 1.
Transforming growth factor-β1 (TGF-β1) induces tubular epithelial-mesenchymal transition (EMT) in NRK52E normal rat kidney epithelial cells. A: NRK52E proximal tubule epithelial cells were grown on glass coverslips in the presence of vehicle (left) or 10 ng/ml TGF-β1 (right) for 6 days. Cells were fixed using 4% (wt/vol) paraformaldehyde and incubated with primary antibodies against zona occludins 1 (ZO-1) to visualize cell junctions or vimentin to visualize cytoskeleton as described in materials and methods. Immunoreactivity was visualized using TRITC-labeled secondary antibodies, and nuclei were visualized using 4,6-diamidino-2-phenylindole (DAPI)/Hoechst 33258 at ×200 magnification. B: Western blot analysis of extracts (25 μg protein/lane) from NRK52E cells incubated in the presence or absence of 10 ng/ml TGF-β1. Lysates were probed for E-cadherin, α-smooth muscle actin (α-SMA), or β-actin overnight.
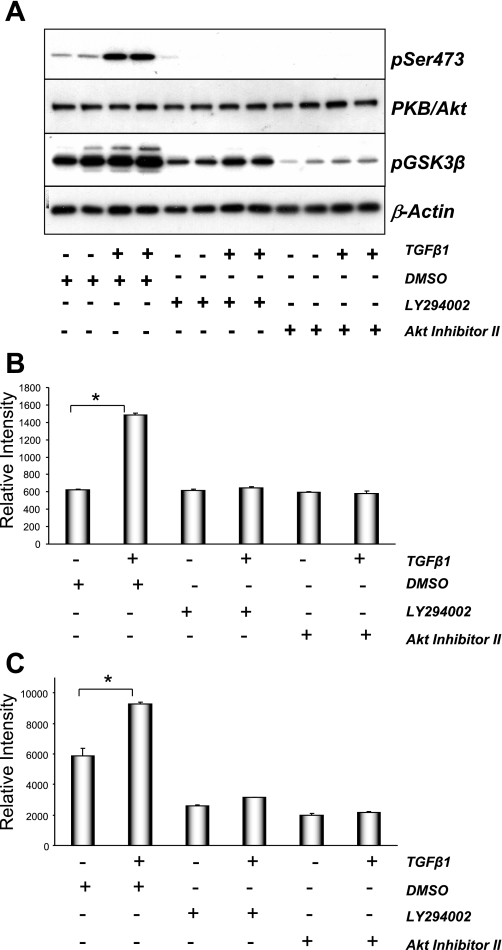
Many intracellular signaling molecules have been implicated in TGF-β1-induced EMT, including Smad3, ILK, and p38 MAPK. PI3K→PKB/Akt signaling have been suggested to play a role in TGF-β1 signaling in mammary and lens epithelial cells (3). To assess whether TGF-β1 activated PI3K, we used mouse embryo fibroblasts stably expressing GFP-Akt-PH, the plasma membrane binding of which is indicative of phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] formation due to PI3K activation. Treatment of mouse embryonic fibroblast (MEF) cells with TGF-β1 induced the translocation of GFP-Akt-PH to the plasma membrane (see Supplemental Fig. 1A). (Supplemental data for this article is available online at the American Journal of Physiology-Renal Physiology website.) TGF-β1 also stimulated PtdIns(3,4,5)P3 formation in NRK52E kidney epithelial cells, suggesting that TGF-β1 triggers PI3K activation in these cells (Supplemental Fig. 1B). Increased phosphorylation of the downstream target of PI3K PKB/Akt was also observed in NRK52E cells upon TGF-β1 treatment (Fig. 2 A). Phosphorylation of a downstream target of PKB/Akt, GSK-3β, was also elevated upon TGF-β1 stimulation. Pretreatment of cells with an inhibitor of either PI3K (LY-294002) or PKB/Akt (Akt inhibitor II) significantly reduced the phosphorylation of both PKB/Akt and GSK-3β in the absence or presence of TGF-β1. These data were quantified using electrochemiluminescence, revealing similar patterns of TGF-β1-induced activation and inhibition (Fig. 2B). Together, these data suggest that TGF-β1 activates PKB/Akt in renal epithelial cells in a PI3K-dependent manner.
Fig. 2.
TGF-β1 induced phosphatidylinositol 3-kinase (PI3K)→PKB/Akt signaling in NRK52E cells. A: serum-starved NRK52E cells were pretreated with DMSO, 20 μM LY-294002, or 40 μM Akt inhibitor II for 30 min. Cells were then treated with vehicle or 10 ng/ml TGF-β1 for 60 min. Protein lysate (25 μg) was probed using antibodies against activated PKB/Akt (pSer473), total Akt protein (PKB/Akt), phosphorylated GSK-3β (pSer9), or β-actin. Starved NRK52E cells were processed and proteins extracts quantified for pAkt (B) and pGSK-3β Ser9 (C) using Meso Scale Discovery (MSD) electrochemiluminescence technology. Values for pAkt and pGSK-3β were normalized to their respective total protein levels. Data are means ± SE; n = 4. *P < 0.0005; paired t-test.
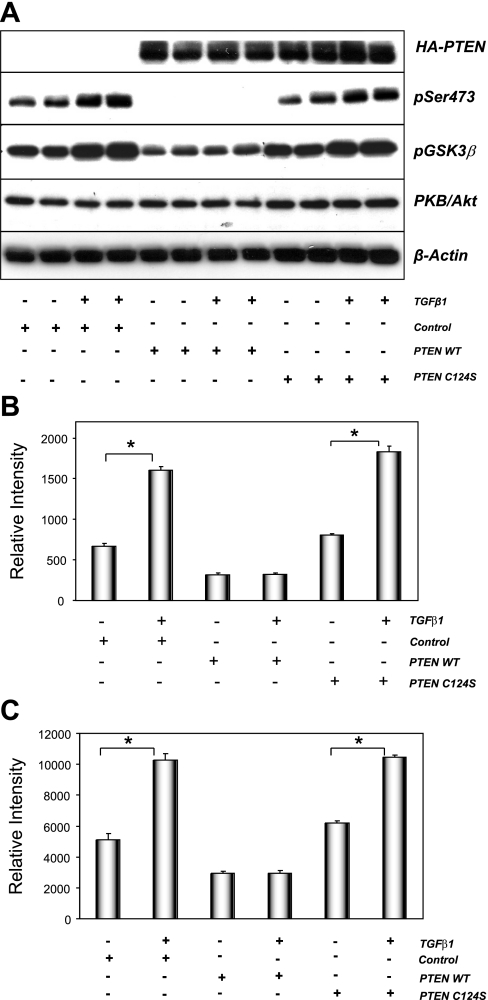
Pharmacological inhibitors such as LY-294002 may cross-react with other protein kinases (14). To further support our observations, plasmids expressing PTEN, a PtdIns(3,4,5)P3 lipid phosphatase that limits PKB/Akt activity in cells (29), or a mutant form of PTEN with no lipid phosphatase activity (PTEN C124S) were utilized. As previously observed, TGF-β1 induced the activation of PKB/Akt in cells transfected with control plasmid (pCGN; Fig. 3A). Transfection of wild-type PTEN abolished PKB/Akt phosphorylation and significantly reduced GSK-3β phosphorylation on Ser9. In contrast, expression of the lipid phosphatase mutant C124S PTEN did not affect PKB/Akt or GSK-3β phosphorylation (Fig. 3A). Again, these data were supported by quantified electrochemiluminescence analysis showing similar results (Fig. 3, B and C). Together, these data suggest that reductions in the level of PtdIns(3,4,5)P3 limit the degree of PKB/Akt activation in the absence or presence of TGF-β1 and that TGF-β1-mediated activation of PKB/Akt is a PI3K-dependent event in renal epithelial cells.
Fig. 3.
Wild-type but not mutant PTEN expression blocks TGF-β-mediated PKB/Akt activation in NRK52E cells. A: NRK52E cells were transfected with hemagglutinin (HA)-tagged pCGN empty vector (control), pCGN-PTEN wild type (PTEN WT), or pCGN-PTEN C124S phosphatase inactive mutant (PTEN C124S). Transfected cells were then cultured in the presence of vehicle or 10 ng/ml TGF-β1 for 24 h. Protein extracts (25 μg) were probed for HA-PTEN or HA-PTEN C124S, phosphorylated PKB/Akt and GSK-3β, total PKB/Akt, and β-actin as described. NRK52E cells were processed and proteins extracts quantified for pSer473 (B) and pGSK-3β Ser9 (C) using MSD electrochemiluminescence technology. Values represent the ratio of phosphoprotein to total protein (see materials and methods). Data are means ± SE; n = 4. *P < 0.0005; paired t-test.
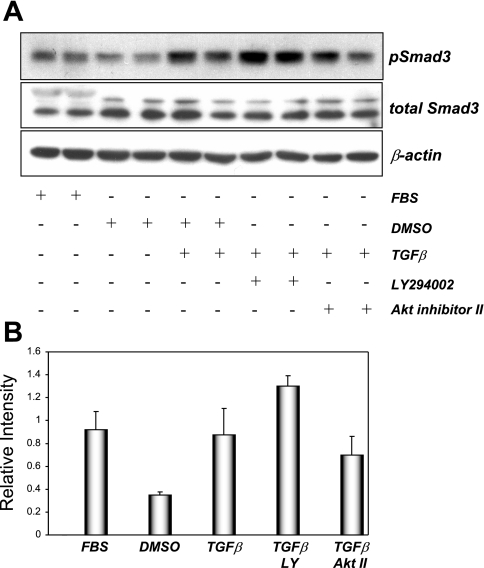
The canonical signaling pathway downstream of TGF-β1 involves the phosphorylation of Smad 2/3, which then dimerize with Smad4 and translocate to the nucleus to regulate gene transcription (28). In glomerular mesangial cells, TGF-β1-mediated Smad3 phosphorylation and subsequent promoter activation were dependent on PI3K activity (37). In NRK52E cells, TGF-β1 increased Smad3 phosphorylation within 60 min (Fig. 4). In contrast to results observed in mesangial cells, TGF-β1-stimulated Smad3 phosphorylation was not diminished in the presence of inhibitors of PI3K or PKB/Akt (Fig. 4), suggesting that TGF-β1-induced Smad3 phosphorylation occurs independently of the activity of this kinase pathway in NRK52E cells.
Fig. 4.
TGF-β1-stimulated Smad3 phosphorylation in NRK52E cells is insensitive to PI3K inhibition. Serum-starved NRK52E cells were treated with DMSO, 20 μM LY-294002, or 20 μM Akt inhibitor II for 30 min before treatment with vehicle or 10 ng/ml TGF-β1 for 60 min at 37°C. Nonstarved cells (FBS) were also included in this experiment. Protein extracts (25 μg) were probed using antibodies raised against pSmad3, total Smad3, or β-actin as loading control. Values are representative of 3 experiments carried out in duplicate. Band intensities were calculated using Scion Image software. Data are means ± SD.
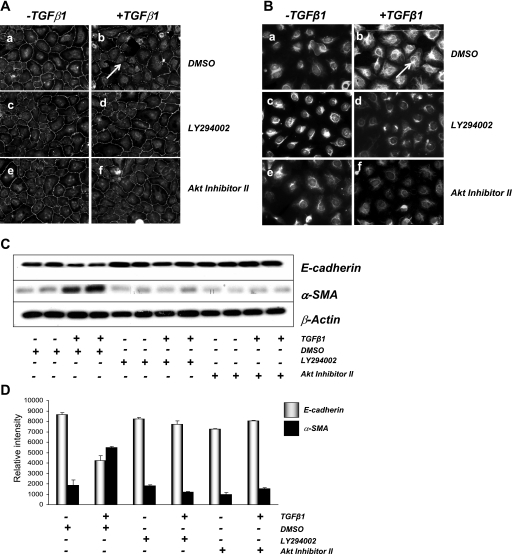
The role of PI3K→PKB/Akt signaling in TGF-β1-induced EMT in renal epithelial cells was then examined. As previously observed, exposure of NRK52E cells to TGF-β1 decreased ZO-1 levels in the tight junctions between cells and increased cytoskeletal vimentin expression (Fig. 5, A and B, top). Coincubation of cells with LY-294002 or Akt inhibitor II significantly reduced TGF-β1-induced changes in epithelial (ZO-1) and mesenchymal (vimentin) marker expression (Fig. 5, A and B, middle and bottom). These observations were supported by Western blot analysis, using changes in additional epithelial (E-cadherin) and mesenchymal (α-SMA) proteins as readouts of TGF-β1-induced EMT (Fig. 5, C and D). Both TGF-β1-induced decreases in E-cadherin expression and increases in α-SMA expression were inhibited by LY-294002 and Akt inhibitor II. Together, these data suggest that TGF-β1-induced EMT in NRK52E renal tubular cells is a PI3K→PKB/Akt-dependent process.
Fig. 5.
TGF-β1-mediated phosphorylation of PKB/Akt and GSK-3β is important for EMT in rat kidney cells. NRK52E cells were treated with DMSO, LY-294002 or Akt inhibitor II for 30 min before treatment with 10 ng/ml TGF-β1 for 72 h. Cells were stained for epithelial marker ZO-1 (A) and mesenchymal marker vimentin (B). Cells were fixed using 4% (wt/vol) paraformaldehyde, and immunoreactivity was visualized using TRITC-labeled secondary antibodies at ×200 magnification using fluorescence microscopy. C: NRK52E cells were exposed to DMSO, 20 μM LY-294002, and 40 μM Akt inhibitor II in the presence of vehicle or 10 ng/ml TGF-β1 for 72 h. Protein extracts (25 μg) were probed using antibodies against E-cadherin, α-SMA, or β-actin. D: immunoblots were analyzed using densitometry (Scion Image software). The ratios of E-cadherin and α-SMA to β-actin were calculated. This experiment was carried out in triplicate with similar results. Data are means ± SD.
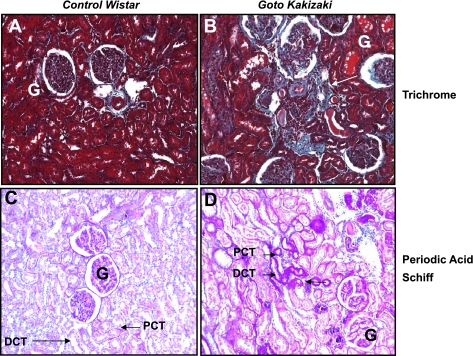
EMT is thought to play a significant role in tubulointerstitial fibrosis in many cases of end-stage renal disease (32, 34, 41, 47). We used the GK non-obese, nonhypertensive rat model of type 2 diabetes (16) to explore in vivo mechanisms of TGF-β1 signaling and EMT. These rats developed spontaneous type 2 diabetes at 11–12 wk of age with elevated random blood glucose evident (control Wistar, 8.6 ± 2.1 mM; Goto-Kakizaki, 13.1 ± 1.9 mM, P < 0.02; Kattla et al., unpublished observations). As previously reported (20, 23), GK diabetic rats displayed evidence of mild to moderate tubulointerstitial fibrosis at 9–10 mo of age compared with Wistar controls (Fig. 6). Deposition of collagen was evident in the tubulointerstitial space in GK but not control Wistar rats, suggestive of renal fibrosis (Fig. 6, A and B). Similarly, increased extracellular matrix production suggestive of increased glomerular basement membrane thickening was also evident in diabetic compared with control rat kidneys (Fig. 6, C and D).
Fig. 6.
Diabetic Goto-Kakizaki (GK) rats display mild to moderate tubulointerstitial fibrosis at 9–10 mo of age. Kidney sections (5 μm) from nondiabetic control Wistar (A and C) or diabetic GK rats (B and D) (n = 4) were stained with Masson's Trichrome for collagen (A and B) or periodic acid-Schiff for extracellular matrix proteins (C and D) as described in materials and methods and examined at ×400 magnification. The locations of proximal convoluted tubule (PCT) and distal convoluted tubule (DCT) are indicated. Arrows pinpoint moderate tubulointerstitial fibrosis with collagen deposition (B) and thickening of the tubular basement membrane (D) in diabetic GK but not in control Wistar rats. Glomeruli are also marked (G).
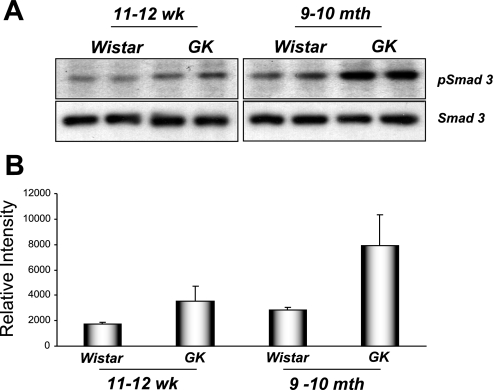
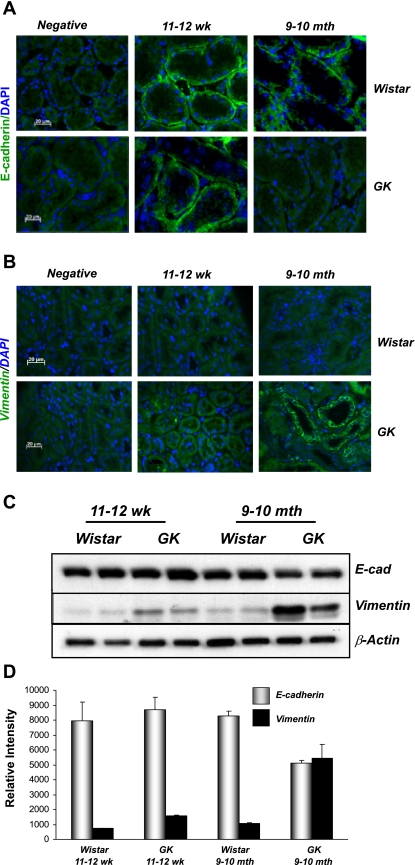
EMT is thought to be a significant component of renal fibrosis. We detected elevated levels of TGF-β1 mRNA in extracts from 9- to 10-mo-old diabetic GK but not control rat kidneys (data not shown). Protein lysates from kidneys of diabetic GK rats at 9–10 mo displayed elevated levels of phospho-Smad3 compared with controls, with more modest increases observed at 11–12 wk (Fig. 7). Together, these data suggested that amplified TGF-β signaling was present in kidneys of diabetic rats at more advanced stages of disease. We hypothesized that elevated levels of TGF-β1 present in diabetic GK rats at 9–10 mo of age would have consequences for epithelial cell integrity in the renal tubules. Kidney sections from control Wistar and diabetic GK rats were prepared and stained using epithelial (E-cadherin) and mesenchymal (vimentin) cell markers. No changes in either E-cadherin or vimentin staining were detected in either control or hyperglycemic rat kidneys at 11–12 wk; i.e., at the time of onset of diabetes (data not shown). In contrast, 9- to 10-mo-old diabetic GK rats displayed patchy loss of E-cadherin staining in the kidney tubules with concomitant increases in vimentin levels detected compared with nondiabetic Wistar controls (Fig. 8, A and B). Western blotting of whole kidney lysates also revealed reduced E-cadherin and increased vimentin levels in diabetic compared with control rat kidneys (Fig. 8, C and D). Similar to the above-described findings, no significant changes in E-cadherin or vimentin staining were observed at the time of onset of diabetes (11–12 wk), suggesting that these effects occurred as a result of long-term hyperglycemia.
Fig. 7.
Evidence of elevated TGF-β1 signaling in diabetic rata kidneys. Active TGF-β1 signaling was examined by immunoblotting for Smad3, a phosphorylated downstream target of TGF-β1. A: 25-μg protein extracts from 11- to 12-wk and 9- to 10-mo-old control and GK rat kidneys were probed for pSmad3 and total Smad3 using standard immunoblotting techniques. B: densitometry using Scion Image software was performed, and the intensity of pSmad3 bands were normalized to total Smad3 expression.
Fig. 8.
Evidence of EMT in GK diabetic rats. Fresh frozen sections (5 μm) from control Wistar or GK rats ages 11–12 wk or 9–10 mo were incubated with primary antibodies against E-cadherin (A) or vimentin (B) as described in materials and methods. Immunoreactivity (green) was visualized using FITC-labeled anti-mouse secondary antibodies, and nuclei were visualized using DAPI/Hoechst 33258 (blue). A control in which the primary antibody was replaced with blocking buffer was included in each experiment (negative). Sections were visualized at ×400 magnification. C and D: protein lysates from control Wistar or GK rats were probed for EMT markers. Protein extracts (25 μg) were probed using antibodies against E-cadherin, α-SMA, and β-actin (C). Band intensities were quantified using Scion Image software. The intensity ratios of E-cadherin and α-SMA to β-actin are shown (D). Data are means ± SD.
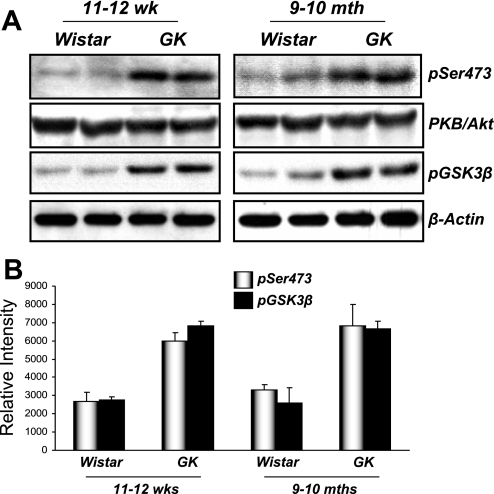
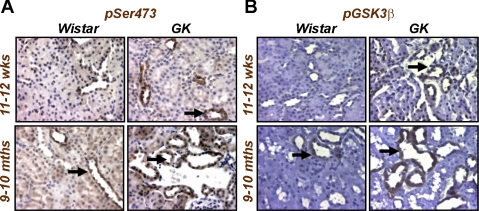
Our data suggested that TGF-β1-induced EMT in renal tubular cells in vitro required PI3K→PKB/Akt signal transduction (Fig. 5). To examine whether elevated PKB/Akt activity was present in the fibrotic kidney in vivo, we performed Western blotting of kidney lysates from control Wistar or diabetic GK rats using antibodies against phospho-PKB/Akt and the phosphorylated (inactivated) form of its downstream target, GSK-3β. Elevated levels of both phosphoproteins were detected in GK compared with control Wistar rats at both age groups measured (Fig. 9). Sections from both control Wistar and GK rats also revealed that increased levels of phospho-PKB/Akt and pGSK-3β were present in GK compared with control kidneys, with staining predominantly located in the kidney tubules (Fig. 10). These data suggest that PKB/Akt activity is involved not only in TGF-β1-induced EMT in kidney epithelial cells in vitro but is elevated in the diabetic kidney disease in GK rats.
Fig. 9.
PKB/Akt pathway signaling is elevated in GK rats. A: protein extracts from control Wistar rats and GK rat kidneys were probed with antisera raised against pSer473 of PKB/Akt or pSer9 of GSK-3β. Protein loading was controlled using a polyclonal antibody raised against total PKB/Akt and β-actin, respectively. Data are representative of 4 rats from both control and GK groups at ages 11–12 wk and 9–10 mo. B: band intensities were calculated using Scion Image software. Data are means (SD).
Fig. 10.
Elevated PKB/Akt and GSK-3β localize to the kidney tubules of diabetic rats. Paraffin-embedded kidney sections (5 μm) from control Wistar and diabetic GK rats ages 11–12 wk and 9–10 mo were incubated with a rabbit polyclonal antibody against pSer473 of PKB/Akt (A) or pSer9 of GSK-3β (B) as described in materials and methods. Immunoreactivity was visualized using diaminobenzidine staining at ×400 magnification. Arrows indicate staining in the proximal and distal tubules.
DISCUSSION
Tubulointerstitial fibrosis plays a key role in most forms of chronic kidney disease leading to renal failure. In diseases such as DN, chronic hyperglycemia increases profibrotic cytokines such as TGF-β1, which contribute to a cascade of signal transduction driving damage to both the glomerulus and kidney tubules. In the present study, we have demonstrated that activation of PKB/Akt is important for TGF-β1-induced EMT in rat kidney epithelial cells. In addition, elevated TGF-β1 signaling in the diabetic rat kidney coincides with increased PKB/Akt activation in renal tubules in vivo. Together, these data suggest that PKB/Akt may serve as an important downstream mediator of TGF-β1 actions during the pathogenesis of DN.
NRK52E cells treated with TGF-β1 underwent EMT-like changes (Fig. 1). TGF-β and peptide growth factors induced EMT in several cell lines, including mammary epithelial cells (3, 33) and primary lens epithelial cells (9). In both cell types, PI3K was shown to be a critical mediator of EMT. The role of TGF-β-induced PI3K activation in the context of EMT in kidney epithelial cells is not well understood. In our study, we demonstrated that TGF-β1 triggers PtdIns(3,4,5)P3 formation via PI3K in both MEF and NRK52E epithelial cells (Supplemental Fig. 1). These results were consistent with previous reports that PI3K was activated in response to TGF-β in other cell types and that the TGF-β1 receptors (Alk1/5) formed a complex with the p85 regulatory subunit of PI3K (3, 17, 24).
TGF-β1 activated PKB/Akt signaling in NRK52E rat kidney epithelial cells in a PI3K-dependent manner (Fig. 2A). Pharmaceutical inhibitors of PI3K, such as LY-294002, or PKB/Akt attenuated this activation. The PKB/Akt inhibitor used (Akt inhibitor II) blocks the binding of PtdIns(3,4,5)P3 to the pleckstrin homology (PH) domain of PKB/Akt. Since PKB/Akt requires PtdIns(3,4,5)P3 for full phosphorylation and activation, both these PKB/Akt processes are reduced in cells treated with this inhibitor (Fig. 2A, top). Expression of the lipid phosphatase PTEN also attenuated the activation of PKB/Akt (Fig. 2). A mutant form of PTEN with no lipid phosphatase activity (PTEN C124S) had no effect on PKB/Akt activation, emphasizing the requirement for PtdIns(3,4,5)P3 generation in this regard (Fig. 2). The PI3K→PKB/Akt pathway is typically activated by tyrosine kinase receptors whose phosphotyrosine residues engage the SH2 domains of the p85 regulatory subunit of PI3K and trigger activation of the p110 catalytic PI3K subunit, which converts PtdIns(4,5)P2 to PtdIns(3,4,5)P3 (13). TGF-β1 engages the Alk1/5 receptors to drive Smad2/3 phosphorylation, the formation of phospho-Smad2/3 dimers with Smad4, nuclear translocation of these complexes, and transcription regulation (28). In NRK52E cells, TGF-β1 triggers Smad3 phosphorylation independently of PI3K and PKB/Akt activity (Fig. 4), most likely via the above-described mechanism. Runyan et al. (37) demonstrated that TGF-β1-mediated Smad phosphorylation and activation is regulated by PI3K signaling in human mesangial cells. Thus cell type-specific signal transduction events downstream of TGF-β1 may exist in different kidney cells.
How is TGF-β1 activating PI3K→PKB/Akt signaling in NRK52E cells? Several groups have suggested that TGF-β directly activated PI3K→PKB/Akt via Alk1/5 receptor binding (3, 17, 24). In contrast, Horowitz et al. (18) observed that TGF-β-mediated PKB/Akt activation is p38 MAPK dependent in fetal lung fibroblasts. Liu colleagues (12, 25) demonstrated that TGF-β→Smad→ILK signaling results in activation of PKB/Akt and plays an important role in tubular EMT. Interestingly, two reports from 2004 detailed a direct cellular interaction between PKB/Akt and nonphosphorylated Smad3 that prevented Smad3 nuclear translocation (11, 35). This interaction was enhanced in the presence of insulin and inhibited in the presence of TGF-β1 (35). In our hands, overexpression of Smad3 or the p38 MAPK inhibitor SB 203580 had no effect on TGF-β-mediated activation of PKB/Akt (data not shown). We therefore conclude that TGF-β1-mediated PKB/Akt activation in NRK52E cells is PI3K dependent and is not influenced by Smad3 or p38 MAPK to any great extent. However, the activation of PKB/Akt by other signaling pathways such as ILK, ERK, and Notch cannot be excluded in this system.
Coincubation of NRK52E cells with PI3K or PKB/Akt inhibitors significantly reduced the ability of TGF-β1 to induce EMT (Fig. 5), suggesting that PI3K→PKB/Akt pathway was important for TGF-β1-induced EMT in these cells. The less dramatic effect seen on ZO-1 and vimentin expression in control cells in these experiments may be due to the shorter time of exposure to TGF-β1 compared with cells in Fig. 1 (3 vs. 6 days). This modification was necessary to avoid cell death due to the presence of the chemical inhibitors. Similar results were obtained by Bakin et al. (3), who showed that blockade of PI3K activity by synthetic inhibitor LY-294002 significantly inhibited EMT in mammary epithelial cells. The downstream target of PKB/Akt, GSK-3β, was recently implicated in EMT in both normal and transformed breast epithelial cells. Snail is a transcription factor that binds to the promoter region of E-cadherin and represses E-cadherin mRNA production, a key event in EMT (4, 8). Phosphorylation by GSK-3β inhibits Snail function via nuclear export and proteosome-mediated degradation (1, 50). Therefore, elevated PKB/Akt activation increases GSK-3β phosphorylation, leading to inhibition of this kinase, stabilization of Snail, and thus E-cadherin repression and EMT. Julien et al. (21) have shown that activation of NF-κB downstream of PKB/Akt increases Snail expression and induces EMT in squamous cell carcinoma cells. Thus multiple signaling events downstream of TGF-β1 may be necessary but not sufficient for EMT. Together with Smad signaling, p38 MAPK signaling, and ILK activation, PKB/Akt may contribute to TGF-β1→EMT in vitro and in vivo.
EMT-like changes were observed in GK rats exposed to chronic hyperglycemia (Figs. 6 and 8). Our results are consistent with previous studies demonstrating EMT in animal models such as the streptozotocin-induced type 1 diabetic mouse model and human kidney biopsies (12, 25, 32, 34, 41, 47). Interestingly, the observed EMT-like changes in GK rats occurred in parallel with moderate tubulointerstitial fibrosis (Fig. 6) and overt proteinuria (data not shown), hinting that EMT is not an early event in DN in this model. TGF-β1 is thought to be the primary cytokine driver of EMT in kidney, and levels of both TGF-β1 mRNA (data not shown) and Smad3 phosphorylation (Fig. 7) were elevated in GK rat kidneys at 9–10 mo. In addition, elevated PKB/Akt and GSK-3β phosphorylation was detected in GK rat kidneys compared with controls, with prominent tubular staining (Figs. 9 and 10). This increase in PKB/Akt activation preceded both increased TGF-β1 expression and EMT-like changes (Fig. 8), suggesting that other stimuli may be driving PKB/Akt activation at the 11- to 12-wk time point. Of note, two likely contributors to renal dysfunction in diabetes, high blood glucose and CTGF, are elevated at this time point in diabetic but not control rats (Kattla et al., unpublished observations).
There is increasing evidence in the literature regarding the function of PI3K→PKB/Akt signaling in the setting of diabetes. Feliers et al. (15) identified elevated PI3K and PKB/Akt activity in the renal cortex of db/db mice. Lloberas et al. (27) recently showed increased phosphorylated PKB/Akt in whole kidney at 4 wk of diabetes, and increased levels of total and phosphorylated PKB/Akt in mesangial cells in DN were observed by others (30). Gas6 growth factor-mediated glomerular hypertrophy in diabetic rats also involved PKB/Akt signaling (30), and Chuang et al. (10) demonstrated that PI3K signaling was required for p21WAF expression and consequent tubular cell hypertrophy in LLC-PK1 cells. Treatment of animals with rapamycin (sirolimus) attenuated diabetes-induced kidney disease in rats and also decreased elevated activated mTOR (mammalian target of rapamycin) and PKB/Akt in the glomerulus of these animals (27). Together with our data detailing the role of PKB/Akt in TGF-β1-induced EMT, these data suggest that future therapeutic strategies that target PKB/Akt signaling may be of potential benefit in treating diabetic nephropathy.
GRANTS
Work in the laboratory of D. P. Brazil is funded by Science Foundation Ireland and Health Research Board Ireland. C. Godson is funded by a Science Foundation Ireland Principal Investigator Award, the Wellcome Trust, and EU EICOSANOX Consortium Grant LSHM-CT-2004-005033. Funding from the Health Research Board Ireland is also gratefully acknowledged.
Supplementary Material
Acknowledgments
We thank Dr. Brian Hemmings (Friedrich Miescher Institute, Basel, Switzerland) for PKB/Akt reagents and helpful discussions, Prof. Nick Tonks (Cold Spring Harbor Laboratories) for PTEN constructs, and Prof. Takehito Sasaki (Akita University, Akita, Japan) for providing AKT-PH-MEF cells. Alfie Redmond [University College Dublin (UCD)] and Dr. Richie Talbot (UCD) are gratefully acknowledged for assistance with animal handling and MSD electrochemiluminescence, respectively. Confocal microscopy expertise was provided by Ann Cullen (UCD Conway Institute). Finally, we thank Prof. Finian Martin (UCD) for helpful discussions regarding this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol 168: 29–33, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 115: 3193–3206, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell 12: 27–36, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 7.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol 17: 2484–2494, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Cho HJ, Baek KE, Saika S, Jeong MJ, Yoo J. Snail is required for transforming growth factor-beta-induced epithelial-mesenchymal transition by activating PI3 kinase/Akt signal pathway. Biochem Biophys Res Commun 353: 337–343, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Chuang TD, Guh JY, Chiou SJ, Chen HC, Huang JS, Yang YL, Chuang LY. Phosphoinositide 3-kinase is required for high glucose-induced hypertrophy and p21WAF1 expression in LLC-PK1 cells. Kidney Int 71: 867–874, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Conery AR, Cao Y, Thompson EA, Townsend CM Jr, Ko TC, Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol 6: 366–372, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Dai C, Yang J, Liu Y. Transforming growth factor-beta 1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem 278: 12537–12545, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feliers D, Duraisamy S, Faulkner JL, Duch J, Lee AV, Abboud HE, Choudhury GG, Kasinath BS. Activation of renal signaling pathways in db/db mice with type 2 diabetes. Kidney Int 60: 495–504, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Goto Y, Suzuki K, Ono T, Sasaki M, Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat). Adv Exp Med Biol 246: 29–31, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Higaki M, Shimokado K. Phosphatidylinositol 3-kinase is required for growth factor-induced amino acid uptake by vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 2127–2132, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem 279: 1359–1367, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 156: 299–313, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen U, Phillips AO, Floege J. Rodent models of nephropathy associated with type II diabetes. J Nephrol 12: 159–172, 1999. [PubMed] [Google Scholar]
- 21.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 26: 7445–7456, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kattla JJ, Roxburgh S, Godson C, Brazil. DP. TGF β-mediated activation of protein kinase B (PKB/Akt) is important for tubulointerstitial fibrosis in diabetic nephropathy (Abstract). J Am Soc Nephrol 16: 610A, 2005. [Google Scholar]
- 24.Krymskaya VP, Hoffman R, Eszterhas A, Ciocca V, Panettieri RA Jr. TGF-β1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 273: L1220–L1227, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 112: 503–516, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17: 1395–1404, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Massague J How cells read TGF-β signals. Nat Rev Mol Cell Biol 1: 169–178, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA 95: 13513–13518, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai K, Matsubara T, Mima A, Sumi E, Kanamori H, Iehara N, Fukatsu A, Yanagita M, Nakano T, Ishimoto Y, Kita T, Doi T, Arai H. Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int 68: 552–561, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Nath KA Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest 108: 1853–1863, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-β type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 112: 4557–4568, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D'Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62: 137–146, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-β signalling through a direct interaction with Smad3. Nat Cell Biol 6: 358–365, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 341: 1127–1133, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem 279: 2632–2639, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Sheppard D Transforming growth factor β: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 3: 413–417, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slattery C, Campbell E, McMorrow T, Ryan MP. Cyclosporine A-induced renal fibrosis: a role for epithelial-mesenchymal transition. Am J Pathol 167: 395–407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 61: 1714–1728, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto H, Grahovac G, Zeisberg M, Kalluri R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56: 1825–1833, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Saika S, Ohnishi Y, Ooshima A, McAvoy JW, Liu CY, Azhar M, Doetschman T, Kao WW. Fibroblast growth factor 2: roles of regulation of lens cell proliferation and epithelial-mesenchymal transition in response to injury. Mol Vis 10: 462–467, 2004. [PubMed] [Google Scholar]
- 45.Ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29: 265–273, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, Sime PJ, Gauldie J, Collins SM. TGF-β1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol 289: G116–G128, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant 5: 1367–1374, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol 13: 96–107, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 159: 1313–1321, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6: 931–940, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.