Abstract
Glomerular sclerosis of diverse etiologies is characterized by mesangial matrix accumulation, with transforming growth factor-β (TGFβ) an important pathogenic factor. The GTPase RhoA mediates TGFβ-induced matrix accumulation in some settings. Here we study the role of the membrane microdomain caveolae in TGFβ-induced RhoA activation and fibronectin upregulation in mesangial cells (MC). In primary rat MC, TGFβ1 time dependently increased RhoA and downstream Rho kinase activation. Rho pathway inhibition blocked TGFβ1-induced upregulation of fibronectin transcript and protein. TGFβ1-induced RhoA activation was prevented by disrupting caveolae with cholesterol depletion and rescued by cholesterol repletion. Compared with wild types, RhoA/Rho kinase activation was absent in MC lacking caveolae. Reexpression of caveolin-1 (and caveolae) restored these responses. Phosphorylation of caveolin-1 on Y14, effected by Src kinases, has been implicated in signaling responses. Overexpression of nonphosphorylatable caveolin-1 Y14A prevented TGFβ1-induced RhoA activation. TGFβ1 also activated Src, and its inhibition blocked RhoA activation. Furthermore, TGFβ1 led to association of RhoA and caveolin-1. This was prevented by Src or TGFβ receptor I inhibition, and by caveolin-1 Y14A overexpression. Last, fibronectin upregulation by TGFβ1 was blocked by Src inhibition, not seen in caveolin-1 knockout MC, and restored by caveolin-1 reexpression in the latter. TGFβ1-induced collagen I accumulation also required caveolae. TGFβ1-mediated Smad2/3 activation, however, did not require caveolae. We conclude that RhoA/Rho kinase mediates TGFβ-induced fibronectin upregulation. This requires caveolae and caveolin-1 interaction with RhoA. Interference with caveolin/caveolae or RhoA signaling thus represents a potential target for the treatment of fibrotic renal disease.
Keywords: extracellular matrix, glomerular sclerosis, caveolin-1
renal fibrosis of diverse etiology is associated with progressive decline of renal function. Transforming growth factor-β1 (TGFβ1), through its role in matrix accumulation, is thought to play a central role in the pathogenesis of glomerular sclerosis (44). Mesangial cells (MC), the primary producers of glomerular extracellular matrix, increase the production and secretion of matrix proteins such as collagen and fibronectin in response to TGFβ1 (13, 54).
Signaling is initiated by TGFβ binding to its type II receptor, which then associates with, phosphorylates, and thereby activates the type I receptor. This receptor subsequently phosphorylates Smad2 and -3, which then associate with Smad4. The complex translocates to the nucleus where it regulates the expression of target genes containing Smad binding elements (2, 27). Smads generally require the cooperation of other transcription factors such as Sp1, ATF/CREB, and AP-1 to effect gene transcription (1, 42, 48, 59, 63). TGFβ1 has also been shown to activate non-Smad signaling pathways. The small GTPase RhoA and its downstream effector Rho kinase have been implicated in TGFβ matrix responses in some cells (13, 57). In breast cancer cells, RhoA activation by TGFβ was shown to be independent of Smads, whereas some studies conversely suggest a potential regulation of Smad signaling by RhoA/Rho kinase (13, 15). The mechanism of RhoA activation by TGFβ, however, has not been well defined.
Recently, membrane microdomains rich in glycosphingolipids and cholesterol, termed rafts, have been implicated in regulating TGFβ signaling. Caveolae are a type of raft and appear as noncoated 50- to 100-nm omega-shaped invaginations of the plasma membrane by electron microscopy (36). They are defined by the presence of caveolin, a 24-kDa integral membrane protein essential for their formation (30). Caveolae are found in most cells, including MC (30, 52). Three isoforms of caveolin exist; caveolin-1 (cav-1) has wide expression and is required for caveolar formation in nonmuscle cells (23, 35, 36). Numerous signaling molecules have been localized to rafts/caveolae, with these domains playing an important regulatory role in their function (24, 30). We have shown that RhoA partly localizes in rafts/caveolae in MC (32), and a proportion of cellular TGFβ receptors as well as Smad2 have also been found to reside in these microdomains (5, 38, 46).
Cav-1, through its association with signaling proteins, may impart a specific functional role to caveolae that is distinct from rafts. Caveolin is a transmembrane noncatalytic protein with both NH2 and COOH termini facing the cytoplasm (30). Interactions with caveolin are believed to sequester proteins within caveolae and modulate or suppress their catalytic activities (36, 37). The further modification of cav-1 through phosphorylation on tyrosine-14 has been demonstrated in response to various stimuli including osmotic and shear stress and growth factor receptor activation (17, 34, 39, 56). The full functional significance of cav-1 phosphorylation is not yet known, but it has been shown to modulate cav-1 association with other proteins (4, 17, 34). In MC, we have shown that mechanical stretch-induced RhoA activation depends on RhoA association with cav-1, and that this requires cav-1 to be phosphorylated at Y14 (32). Conversely, cav-1 phosphorylation in response to other stimuli such as angiotensin II has been shown to release proteins such as the epidermal growth factor receptor from cav-1 binding, with a resultant increase in its activation (55). Responses to cav-1 phosphorylation are thus dependent on the stimulus.
The potential role of caveolae and cav-1 in TGFβ-induced RhoA activation and their role in matrix upregulation have not yet been addressed. These studies demonstrate that TGFβ activates RhoA signaling in MC through the TGFβ receptors and implicate caveolae and cav-1 as necessary upstream regulators. They further show that Src activation and cav-1 phosphorylation on Y14 are important for the transduction of this signal. These studies outline a novel signaling pathway implicating caveolae as positive regulators of TGFβ non-Smad signaling.
MATERIALS AND METHODS
Cell culture.
Primary MC were obtained from glomeruli of Sprague-Dawley rats (Charles River) or mice (cav-1 knockout or their corresponding wild type, B6129SF1/J, both from Jackson Laboratory) by differential sieving and cultured in DMEM supplemented with 20% fetal calf serum (Invitrogen), streptomycin (100 μg/ml), and penicillin (100 units/ml) at 37°C in 95% air-5% CO2. Protocols were approved by an independent review committee (Animal Research Ethics Board, McMaster Univ., Hamilton, ON), and experiments were performed in accordance with Canadian Council on Animal Care guidelines. Experiments were carried out using cells between passages 6 and 15. 293T cells were cultured in DMEM supplemented with 10% serum. Confluent MC were made quiescent by serum deprivation for 24 h before treatment. TGFβ1 (R&D Systems) was used at 2 ng/ml. Pharmacological inhibitors were added at the indicated concentrations and times before TGFβ1: Y-27632 (Calbiochem), 10 μM for 30 min; HA-1077 (Calbiochem), 25 μM for 30 min; cyclodextrin (Calbiochem), 5 mM for 60 min; filipin III (Sigma), 2.5 μg/ml for 10 min; cholesterol (Sigma), 15 μg/ml for 60 min; SU6656 (Calbiochem), 10 μM for 30 min; and SB431542 (Tocris), 5 μM for 30 min.
Protein extraction and Western immunoblotting.
Cells were lysed, and protein was extracted as previously described by our laboratory (19). Briefly, cells were lysed in buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA, 1 mM sodium vanadate, 1 mM β-glycerophosphate, 1 mM sodium fluoride, 2 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were collected and centrifuged at 4°C at 14,000 rpm for 10 min to pellet cell debris. Supernatant (50 μg) was separated on SDS-PAGE, and Western blotting was performed as we have described previously (19). Antibodies used included monoclonal RhoA (1:500, Santa Cruz), polyclonal phospho-MYPT Thr696 (1 μg/ml, Upstate), polyclonal phospho-Smad2 Ser465/467 (1:2,000 Upstate), polyclonal phospho-Smad3 Ser433/435 (1:1,000, Cell Signaling), polyclonal Smad2/3 (1:1,000, Santa Cruz), polyclonal phospho-SrcY416 (1:500, Cell Signaling), polyclonal Src (1:1,000, Cell Signaling), monoclonal cav-1 (1:1,000, BD Biosciences), monoclonal β-actin (1:5,000, Sigma), and monoclonal collagen I (1:1,000, Sigma).
For assessment of secreted fibronectin, conditioned medium was collected and centrifuged (4,000 rpm, 5 min) to pellet debris, and 10 μg of protein were separated on a 7.5% gel. Membranes were probed with monoclonal anti-fibronectin (1:5,000, BD Biosciences).
RhoA pull-down assay.
This was performed as described previously (19). Briefly, cells were lysed in hypertonic buffer, and GTP-bound RhoA was immunoprecipitated from cleared lysate with 25 μg of glutathione-agarose bound GST-tagged rhotekin RhoA binding domain (purified from bacterial lysate). Beads were washed, and the immunoprecipitate was resolved on 15% SDS-PAGE. Membranes were probed with anti-RhoA antibody. Lysate (50 μg) was also probed for RhoA to ensure equality across conditions.
Northern blot analysis.
Total RNA (10 μg), extracted using Trizol (Invitrogen), was separated on a formaldehyde-agarose gel and transferred to a nylon membrane (Hybond, Amersham Biosciences). Hybridization was performed with random-primed digoxigenin-11-dUTP-labeled cDNA probes prepared from fibronectin, collagen IA1, or β-actin cDNA amplified by PCR. Hybridized probes were detected using alkaline phosphatase-labeled anti-digoxigenin antibodies and CDP-star as substrate. Kits and reagents were from Roche Applied Science.
Immunoprecipitation.
Cells were lysed with lysis buffer including 60 mM N-octyl-glucopyranoside. After clarification, equal amounts of lysate were incubated overnight with 2 μg of monoclonal RhoA antibody, rotating at 4°C, followed by 25 μl of protein G-agarose slurry for 1.5 h at 4°C. Immunoprecipitates were extensively washed, resuspended in 2× sample buffer, boiled, and analyzed by immunoblotting.
Infection of MC.
Rat cav-1 was amplified from MC cDNA and inserted into the retroviral vector pLHCX with an NH2-terminal FLAG. Using this as template, Y14 was mutated to alanine. Rat MC were infected with empty vector or FLAG-Cav-1Y14A, and cav-1 knockout MC were infected with cav-1 as described previously (19, 20, 62). In brief, competent virus capable of single infection was generated using the vesicular stomatitis virus system (Stratagene), and MC passages 5–12 were exposed to virus concentrated by centrifugation in the presence of polybrene. Seventy-two hours after infection, a two-week antibiotic selection period was begun. Experiments were performed using a population of pooled stably infected MC.
Statistical analyses.
Densitometry was obtained using Scion Image (NIH), and analysis was performed using one-way ANOVA with Tukey's honestly significant difference test for post hoc analysis (SPSS 14.0 for Windows). P value <0.05 (2-tailed) was considered significant. Data are represented as means ± SE. Experiments were repeated multiple times, with n as the number of repetitions.
RESULTS
TGFβ-mediated fibronectin upregulation requires RhoA/Rho kinase activation.
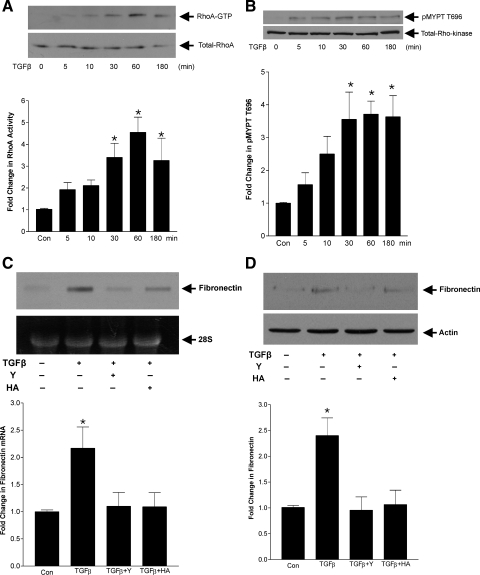
TGFβ has been shown to activate RhoA in several cell types (6, 25, 28, 50), although this has to date not been demonstrated in MC. Given the known importance of TGFβ in induction of matrix production by MC in disease states, we thus first assessed the ability of TGFβ1 (2 ng/ml) to activate RhoA signaling. MC were treated for 5 min to 3 h, and RhoA activity was assessed by pull-down assay of GTP-bound (active) RhoA. Figure 1A shows a time-dependent increase in RhoA activation, significant by 30 min and seen through 3 h. Similarly, downstream Rho kinase activation, as assessed by phosphorylation of the Rho kinase substrate MYPT on T696, was induced in response to TGFβ with kinetics similar to that of RhoA activation (Fig. 1B).
Fig. 1.
Transforming growth factor-β (TGFβ) activates RhoA/Rho kinase signaling to induce fibronectin upregulation. Mesangial cells (MC) were treated for the indicated times with TGFβ1 at 2 ng/ml with active (GTP bound) RhoA (24 kDa) immunoprecipitated as described in materials and methods [A; *P < 0.04 vs. control (con), n = 4] and Rho kinase activity assessed by phosphorylation of its substrate MYPT on T696 (MYPT, 130 kDa; Rho kinase, 160 kDa) (B; *P < 0.03 vs. con, n = 7). C: MC were treated with TGFβ1 for 48 h and pretreated with the Rho kinase inhibitors Y-27632 (Y; 10 μM, 30 min) and HA-1077 (HA; 25 μM, 30 min), and fibronectin upregulation was assessed by Northern analysis. Results were normalized to the intensity of the 28S band (*P < 0.05 vs. all other groups, n = 6). D: TGFβ-induced fibronectin protein upregulation was assessed by Western blot after treatment as in C, with actin used as loading control (fibronectin, 240 kDa; actin, 45 kDa; *P < 0.02 vs. all other groups, n = 5).
Rho/Rho kinase activation has been implicated in TGFβ-induced upregulation of the matrix protein collagen I (13) as well as in upregulation of connective tissue growth factor, an important mediator of TGFβ profibrotic effects (10, 31, 47). We have also shown it to be required for upregulation of fibronectin in response to mechanical stress (19). The involvement of this pathway in TGFβ-induced fibronectin upregulation, however, has not yet been assessed. We thus next examined this with the two distinct Rho kinase inhibitors Y-27632 (10 μM) and HA-1077 (25 μM). After exposure of MC to TGFβ for 48 h, fibronectin transcript upregulation and cellular protein levels were assessed by Northern analysis and Western immunoblotting, respectively. Figure 1C shows a significant increase in fibronectin transcript by TGFβ which was prevented by Rho kinase inhibition. Similar results were observed for fibronectin protein upregulation (Fig. 1D).
RhoA activation by TGFβ is dependent on TGFβ receptor activation.
The mechanism of RhoA activation by TGFβ has not been defined. In these studies, we first determined whether activity of the TGFβ receptors was required. Signaling through these receptors to the target proteins Smads is well established (48). More recently, a number of other proteins that may impact non-Smad pathways have also been shown to interact with TGFβ receptors (40). We used the type I receptor inhibitor SB431542 (5 μM) to determine its role in RhoA activation. Figure 2A shows that TGFβ-induced RhoA activity at 1 h was prevented by SB431542, and a dose-dependent effect was observed with 1 and 5 μM (not shown). Since both Smad and RhoA signaling are initiated through the TGFβ receptors, we next determined whether Rho kinase inhibition would prevent Smad2 and -3 activation as assessed by their COOH-terminal phosphorylation. Figure 2, B and C, shows that neither Rho kinase inhibitor Y-27632 nor HA-1077 affected TGFβ-induced phosphorylation of Smad2 or -3. An independence of Smad and RhoA activation has been observed in breast cancer cells (15), and here we show that Smad activation is independent of downstream Rho kinase.
Fig. 2.
TGFβ receptor I is required for RhoA activation, but RhoA activation is distinct from that of Smads. A: MC were treated for 1 h with TGFβ1 (2 ng/ml) and pretreated with the TGFβ receptor I inhibitor SB431542 (SB; 5 μM, 30 min), and RhoA activity was assessed (*P < 0.01 TGFβ vs. others, n = 4). B and C: dependence of Smad activation on RhoA signaling was assessed using the Rho kinase inhibitors Y-27632 (10 μM) and HA-1077 (25 μM), both for 30 min before the addition of TGFβ1. Phosphorylation at the COOH terminus of Smad2 or Smad3 was assessed by phosphospecific antibodies after 1 h of TGFβ (2 ng/ml) (Smad2, 60 kDa; Smad3, 52 kDa).
Caveolae are required for TGFβ-induced RhoA/Rho kinase activation.
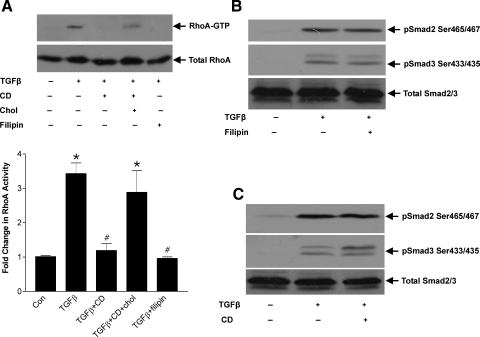
We have recently shown that mechanical stress-induced RhoA activation requires caveolae (32), and it is of interest that the TGFβ receptors have also been localized to these microdomains (38, 46). We thus next examined the requirement for caveolae in TGFβ-induced RhoA activation. We initially used the membrane-impermeable cholesterol binding agent cyclodextrin, which depletes cell surface cholesterol, and the membrane-permeable agent filipin III to perturb the formation of caveolae. Both have been shown to almost completely abolish the presence of caveolae by electron microscopy (45, 55). Figure 3A shows that both cyclodextrin (5 mM) and filipin (2.5 μg/ml) completely abrogated RhoA activation in response to TGFβ. Since caveolar disruption mediated by cyclodextrin resides in its ability to chelate extracellular cholesterol, hence making it unavailable for incorporation into caveolae (7), we tested whether the effect of cyclodextrin was reversible by co-incubation with excess cholesterol. As seen in Fig. 3A, cholesterol reversed the effects of cyclodextrin on RhoA activation, indicating that TGFβ-induced RhoA activity is dependent on the structural integrity of caveolae in MC.
Fig. 3.
Caveolar disruption prevents TGFβ-induced RhoA, but not Smad, activation. A: MC were treated with TGFβ (2 ng/ml, 1 h) in the presence or absence of pretreatment with the caveolar-disrupting agent cyclodextrin (CD; 5 mM, 60 min) or filipin (2.5 μg/ml, 10 min), and RhoA activity was assessed. Reversal of drug effects was sought with simultaneous cholesterol repletion (Chol; 15 μg/ml) given at the time of cyclodextrin administration (*P < 0.01 vs. control, #P < 0.001 vs. TGFβ, n = 6). B and C: TGFβ-induced COOH-terminal phosphorylation of Smad2 and Smad3 was assessed after 1 h of TGFβ with cyclodextrin or filipin as above.
We next assessed the effects of caveolar disruption on TGFβ-induced Smad2/3 phosphorylation. As seen in Fig. 3, B and C, neither filipin nor cyclodextrin prevented COOH-terminal phosphorylation of these Smads. This is consistent with the previously observed lack of efficacy of downstream Rho kinase inhibition on Smad2/3 activation. Hence, TGFβ-induced signaling may be divergent at the level of the TGFβ receptors, with subcellular localization determining the signaling pathway that is activated.
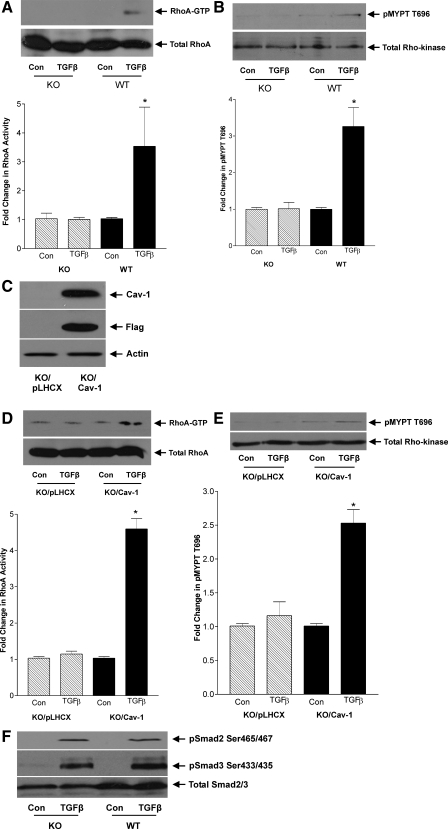
It is recognized that depletion of cholesterol does not specifically eliminate caveolae but also affects other cholesterol-enriched membrane microdomains such as rafts (18). To verify that caveolae mediate TGFβ-induced RhoA activation, we utilized MC derived from cav-1 knockout mice or their wild-type counterparts. These mice lack cav-1 and hence caveolae in all tissues (35). Absence of cav-1 expression in MC was confirmed by Western blotting (not shown). Figure 4, A and B, shows that TGFβ-induced RhoA and downstream Rho kinase activation were absent in knockout cells. To examine whether cav-1 reexpression could restore RhoA/Rho kinase activation, we generated knockout cells expressing FLAG-tagged cav-1. Stable expression of cav-1 after selection of a pooled population of cells is shown in Fig. 4C. Compared with cells infected with the empty vector pLHCX, both RhoA and Rho kinase activation in response to TGFβ were restored in knockout cells reexpressing cav-1 (Figs. 4, D and E). Finally, to confirm that caveolae do not function in TGFβ-induced Smad activation, Smad2 and -3 phosphorylation was assessed in knockout MC. Figure 4F shows no difference in response to TGFβ between knockout and wild-type MC. This is the first demonstration of a role for caveolae in enabling TGFβ-induced RhoA/Rho kinase activation, distinct from activation of the Smad signaling pathway.
Fig. 4.
TGFβ-induced RhoA/Rho kinase activation is absent in caveolin-1 knockout MC. MC from cav-1 knockout (KO) mice were compared with their wild-type (WT) counterparts in their response to TGFβ (2 ng/ml) for 1 h. A: RhoA activity was assessed by pull-down assay of GTP-bound RhoA (*P < 0.02 vs. WT con, n = 3). B: downstream Rho kinase activation was assessed by Western blot for phosphorylated substrate MYPT (*P < 0.05 vs. WT con, n = 3). C: KO MC reexpressing cav-1, tagged with FLAG, were generated and tested for overexpression. D and E: responses to 1 h of TGFβ were compared between KO MC expressing cav-1 and those expressing empty vector pLHCX for RhoA activity (D; *P < 0.001 for con KO/cav-1, n = 3) and Rho kinase activation (E; *P < 0.001 for con KO/cav-1, n = 6). F: KO and WT MC were treated with TGFβ for 1 h, and Smad2 and -3 COOH-terminal phosphorylation was assessed by Western blotting.
Src is required for TGFβ-induced RhoA signaling.
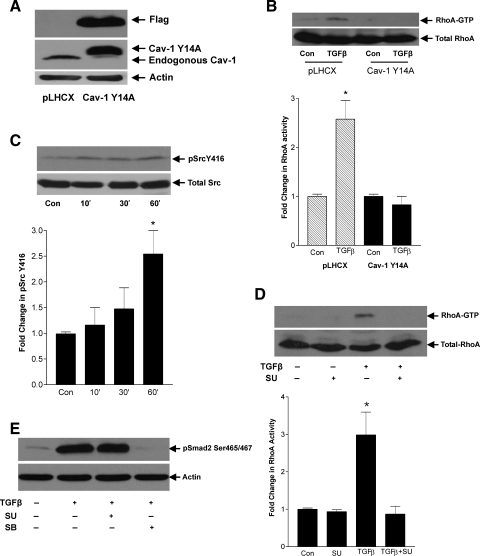
We have previously shown that phosphorylation of cav-1 on Y14 by Src kinases is an upstream event in mechanical stretch-induced RhoA activation in MC (32). Since TGFβ also depends on caveolae for RhoA activation, we examined whether Src kinases and cav-1 Y14 play a role in this setting. We first utilized MC overexpressing the nonphosphorylatable mutant cav-1 Y14A to determine whether Y14 phosphorylation was required. Overexpression of this construct in MC is shown in Fig. 5A. As seen in Fig. 5B, RhoA activation in response to TGFβ, observed in MC overexpressing the empty vector pLHCX, was clearly abrogated in MC with cav-1 Y14A.
Fig. 5.
Activation of Src and cav-1 Y14 phosphorylation are required for TGFβ-induced RhoA activation. A: MC overexpressing the nonphosphorylatable cav-1 mutant Y14A, tagged with FLAG, were generated and tested for overexpression. B: TGFβ-induced RhoA activation was assessed at 1 h in MC overexpressing cav-1 Y14A compared with MC with empty vector pLHCX (*P < 0.01 vs. con pLHCX, n = 4). C: since Y14 phosphorylation is known to be effected by Src kinases, Src activation in response to TGFβ (2 ng/ml) at the indicated times was assessed by Western blotting for phosphorylated Src Y416 (60 kDa). D: effect of pretreatment with the Src inhibitor SU6656 (SU; 10 μM, 30 min) on TGFβ-induced RhoA activity at 1 h was assessed (*P < 0.02 vs. others, n = 4). E: effect of pretreatment with the Src inhibitor SU6656 (10 μM, 30 min) or TGFβ receptor I inhibitor SB431542 (SB; 5 μM, 30 min) on Smad2 and -3 COOH-terminal phosphorylation in response to TGFβ at 1 h was assessed by Western blotting.
Since Src kinases are the only known cav-1 Y14 kinases (61), we next confirmed that TGFβ leads to the activation of Src. Figure 5C shows a time-dependent increase in the autophosphorylation of Y416 on Src, indicative of increased Src activity, in response to TGFβ. This is in agreement with Mishra et al. (29), who also recently observed TGFβ-induced Src activation in MC. Having implicated Src in RhoA activation by TGFβ, we next assessed the effects of the Src inhibitor SU6656 (10 μM). Figure 5D shows that SU6656 completely prevented TGFβ-induced RhoA activation. Consistent with our previous observation of divergence between RhoA and Smad2/3 signaling, we did not observe any effect of Src inhibition on TGFβ-induced Smad2 phosphorylation (Fig. 5E). The receptor-mediated activation of Smad2 was confirmed by abrogation of Smad2 phosphorylation with the TGFβ receptor I inhibitor SB431542 (5 μM).
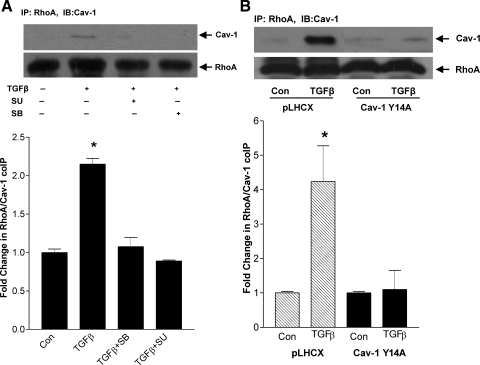
Since phosphorylation of cav-1 may alter its interaction with proteins (4), and we have shown cav-1 Y14 phosphorylation to be required for RhoA activation, we sought to determine whether phosphorylation enabled RhoA association with cav-1 in response to TGFβ. In Fig. 6A, cav-1 was observed in RhoA immunoprecipitates after TGFβ treatment. This association between RhoA and cav-1 was prevented by the Src inhibitor SU6656 (10 μM) and the TGFβ receptor I inhibitor SB431542 (5 μM). Furthermore, overexpression of the nonphosphorylatable cav-1 Y14A also prevented RhoA association with cav-1 in response to TGFβ (Fig. 6B). Thus, downstream of TGFβ receptors, activation of Src and consequent phosphorylation of cav-1 Y14 enable RhoA/cav-1 association and RhoA activation.
Fig. 6.
TGFβ leads to RhoA association with cav-1. A: MC were treated with TGFβ (2 ng/ml) for 1 h with or without the TGFβ receptor I inhibitor SB431542 (5 μM, 30 min) or Src inhibitor SU6656 (10 μM, 30 min) given before TGFβ1. RhoA was immunoprecipitated, and its association with cav-1 (24 kDa) was assessed by Western blotting. Membranes were reprobed for total RhoA to ensure equal immunoprecipitation across conditions (*P < 0.001 vs. others, n = 3). B: MC stably expressing the nonphosphorylatable mutant cav-1 Y14A or empty vector pLHCX were treated with TGFβ for 1 h, and RhoA association with cav-1 was assessed as in A (*P < 0.02, TGFβ vs. con in pLHCX, n = 4).
Caveolae and Src activation are necessary for TGFβ-induced fibronectin upregulation.
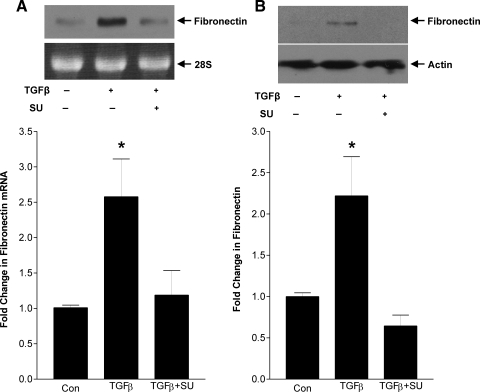
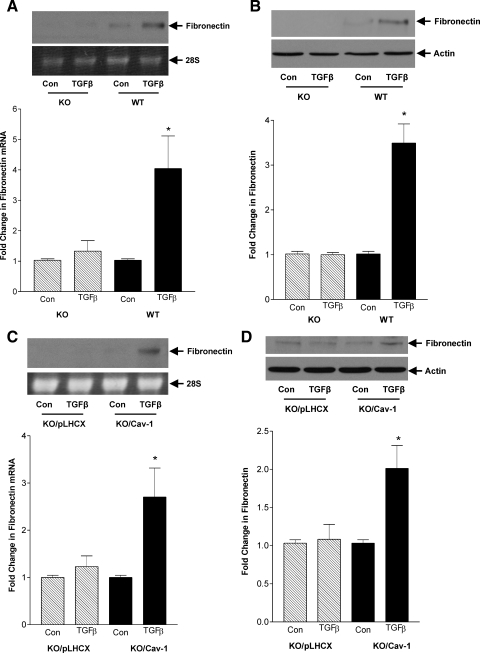
In Fig. 1, C and D, we have shown that TGFβ-induced fibronectin upregulation requires RhoA/Rho kinase signaling. Since Src activation and caveolae are required for induction of RhoA activity, their inhibition should also prevent fibronectin upregulation. Indeed, Fig. 7A shows that TGFβ-induced fibronectin transcript upregulation, as assessed by Northern analysis, was prevented by the Src inhibitor SU6656 (10 μM). Similarly, fibronectin protein upregulation was also prevented by SU6656, as shown in Fig. 7B. To determine the role of caveolae, we used cav-1 knockout cells. Figure 8, A and B, shows that TGFβ was unable to induce fibronectin upregulation in knockout compared with wild-type MC at both the transcriptional and protein levels. Both fibronectin transcript and protein upregulation were restored by reexpression of cav-1 in knockout cells (Figs. 8, C and D). Hence TGFβ-induced RhoA signaling, important for downstream upregulation of the matrix protein fibronectin, is dependent on Src activation and intact caveolae.
Fig. 7.
Src activity is required for fibronectin upregulation in response to TGFβ. MC were treated with TGFβ (2 ng/ml), with or without the Src inhibitor SU6656 (10 μM) added 30 min before TGFβ1, for 48 h. Fibronectin upregulation was assessed by Northern analysis (A; *P < 0.05 vs. others, n = 5) or Western blot (B; *P < 0.04 vs. others, n = 3). Loading controls were 28S for Northern analysis and actin for Western blot for 48 h.
Fig. 8.
Caveolae are required for TGFβ-induced fibronectin upregulation. Cav-1 KO and WT MC were treated with TGFβ (2 ng/ml) for 48 h, and fibronectin transcript (A) and protein (B) levels were assessed by Northern analysis (*P < 0.03, TGFβ vs. con for WT, n = 4) and Western blot (*P < 0.01, TGFβ vs. con for WT, n = 3), respectively. Loading controls were 28S for Northern analysis and actin for Western blot. Fibronectin expression in response to TGFβ was assessed in KO MC reexpressing cav-1 or empty vector pLHCX. Both Northern analysis (C; *P < 0.02, KO/cav-1 vs. con, n = 4) and Western blot (D; *P < 0.04, KO/cav-1 vs. con, n = 3) were used, with 28S and actin used, respectively, as loading controls.
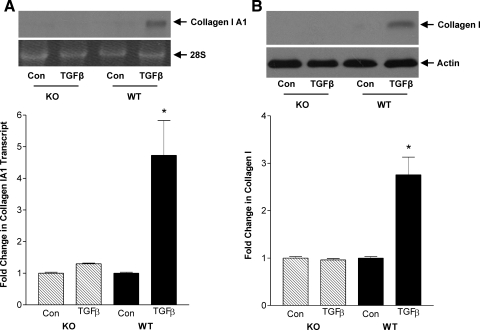
Finally, since Rho kinase was also shown to be important in TGFβ-induced collagen accumulation in MC (13), we assessed the role of caveolae in its upregulation. As seen in Fig. 9, A and B, TGFβ-induced collagen IA1 transcript and collagen I protein upregulation were also impaired in cav-1 knockout MC. This supports the importance of caveolae in mediating RhoA/Rho kinase signaling.
Fig. 9.
Caveolae are required for TGFβ-induced collagen I upregulation. Cav-1 KO and WT MC were treated with TGFβ (2 ng/ml) for 48 h, and collagen IA1 transcript (A) and collagen I protein (B; 95 kDa) levels were assessed by Northern (*P < 0.04 vs. all others, n = 2) and Western blotting (*P < 0.001 vs. all others, n = 6), respectively. Loading controls were 28S for Northern and actin for Western blotting.
DISCUSSION
TGFβ is an important pathogenic factor in chronic fibrotic renal disease of various etiologies, including hypertensive and diabetic nephropathy. The events involved in TGFβ signaling through Smad proteins have been extensively studied. However, activation of alternative signaling pathways that have an important role in mediating fibrotic responses are not well characterized. One such pathway is TGFβ-induced activation of RhoA. Here, we demonstrate that this occurs in MC and is mediated by TGFβ receptors, and we identify RhoA and downstream Rho kinase as necessary for TGFβ-induced fibronectin upregulation. In delineating the upstream signaling events, our studies identify a novel role for caveolae in TGFβ-induced RhoA activation, demonstrating divergence from receptor-induced Smad activation which is independent of these microdomains. We further show that TGFβ leads to the association of RhoA with cav-1, and that Src-mediated cav-1 phosphorylation at Y14 both enables this association and is required for downstream fibronectin upregulation. These studies shed light on non-Smad TGFβ signaling and highlight a novel pathway showing a requirement for caveolae in TGFβ matrix effects.
Caveolae have recently been implicated in TGFβ signaling to the Smad transcription factors. A proportion of both TGFβ receptor I and II has been found in rafts/caveolae in several cell lines (5, 38, 46, 64). This localization appears to be constitutive and is unaffected by TGFβ (5, 64). The inhibitory Smad7 and Smurf2 have also been found in these microdomains (5). Endocytosis of TGFβ receptors occurs through both clathrin and raft/caveolar pathways. Clathrin-dependent internalization facilitates TGFβ signaling, while receptors located in rafts/caveolae are not only potentially in greater association with inhibitory regulators but also undergo rapid degradation on endocytosis (5, 64). In 3T3 and pulmonary fibroblasts, cav-1 was shown to inhibit TGFβ-induced Smad2 phosphorylation and activity (38, 58). In our studies, however, we did not observe any effect on TGFβ-induced Smad2/3 phosphorylation with disruption or genetic absence of caveolae, suggesting that there may be cell type-specific requirements for caveolae in TGFβ signaling.
An assessment of the role of caveolae in other signaling pathways initiated by TGFβ is limited. In primary pulmonary fibroblasts, cav-1 overexpression inhibited TGFβ-induced Erk and JNK activation and production of the matrix proteins collagen I and fibronectin (58). Our studies, however, suggest a facilitative role for caveolae in matrix upregulation in MC. Using several methods including cav-1 knockout MC, which lack caveolae, coupled with reexpression of cav-1 in these cells, we show that caveolae are clearly required for TGFβ-induced activation of RhoA signaling and downstream fibronectin synthesis. Both Smad2/3 phosphorylation and RhoA activation, however, require the TGFβ I receptor, showing a divergence of signaling pathways downstream of the receptor. This suggests that in different cell types and tissues, alternate approaches to manipulation of cav-1 expression might be required for anti-fibrotic effects.
Cav-1 is known to interact with various signaling proteins. This interaction is most commonly inhibitory, with release from cav-1 enabling activation. A positive effect, however, has also been demonstrated, as is seen in insulin receptor signaling (60). In our studies, we have shown this interaction to be necessary for RhoA activation in response to TGFβ as well as in mechanical stress-induced signaling (32). Furthermore, the phosphorylation of cav-1 on Y14 is known to facilitate cav-1 interaction with other proteins (4). Using the nonphosphorylatable mutant cav-1 Y14A, we found that phosphorylation on this residue was important for TGFβ-induced association between cav-1 and RhoA and subsequent RhoA activation. While this interaction with phosphorylated cav-1 is often mediated through SH2 or phosphotyrosine domain interactions (9, 21), our studies and others have found that proteins without such domains, including RhoA and TRAF2, also preferentially associate with Y14-phosphorylated cav-1 (4). It is possible that other intermediary proteins containing these domains may assist in this association.
The Src family kinases are the only kinases known to phosphorylate cav-1 at Y14 (4, 22). Since the nonphosphorylatable mutant cav-1 Y14A prevented RhoA activation and cav-1/RhoA association, we examined the effects of TGFβ on Src in MC. Our data show Src activation in response to TGFβ, as assessed by its phosphorylation at Y416. This is in agreement with studies by others demonstrating TGFβ-induced Src activation (29, 41, 43). Since the Src inhibitor PP1, and to a lesser extent PP2, have been shown to inhibit TGFβ receptor activation (26), we used the more recently described SU6656 for Src inhibition studies. These confirmed a role for Src in TGFβ-induced cav-1/RhoA association, RhoA activation, and fibronectin synthesis.
The extent to which Smads are required for fibronectin gene upregulation by TGFβ in MC is uncertain. Isono et al. (14) showed that overexpression of the inhibitory Smad7 or dominant negative Smad3 prevented activation by TGFβ of a fibronectin promoter-luciferase construct, suggesting a role for Smad signaling in fibronectin upregulation. However, neither fibronectin transcript nor protein was assessed. Indeed, dominant negative Smad4 did not inhibit TGFβ-induced fibronectin upregulation as assessed by Northern analysis in MC (53). This is consistent with observations made in other cell types including Smad2-, -3-, and -4-deficient fibroblasts in which TGFβ-induced fibronectin upregulation was maintained (33, 49). Furthermore, although the peroxisome proliferator-activated receptor (PPAR)-γ ligand pioglitazone abrogated TGFβ-induced fibronectin transcript synthesis in MC, it had no effect on activation of Smad2 (8). These studies, in addition to our data showing that Smad COOH-terminal phosphorylation was unaffected by caveolar disruption or Rho kinase inhibition, suggest that Smad activity is not sufficient for the induction of fibronectin gene upregulation in MC. Of note, collagen I upregulation by TGFβ1 was also absent in cells without cav-1/caveolae in our studies. However, Smad signaling was shown to be required for TGFβ-induced collagen I upregulation in MC (53). It is likely that the requirement for cav-1/caveolae in collagen I synthesis reflects a deficiency in activation of a necessary co-regulatory pathway. Indeed, disruption of Rho kinase signaling in MC prevented TGFβ1-induced upregulation of collagen IA1 transcript (13). Together, these data suggest that RhoA/Rho kinase and Smad signaling cooperate in MC for specific downstream effects of TGFβ1.
TGFβ-induced fibronectin synthesis in MC was shown to require both p38 and Erk (54). Our studies now demonstrate a requirement for RhoA/Rho kinase signaling. It is possible that RhoA enables activation of p38 and Erk, as we have previously shown in mechanically stressed MC (unpublished data and Ref. 19) and as others have shown for Erk activation in response to serum in MC (16). Conversely, in other cells, p38 was found to be either upstream or independent of RhoA activation (15, 25). It is thus evident that responses to TGFβ are highly dependent on cell type. Indeed, in 3T3 cells, fibronectin upregulation was independent of Erk and p38 (11). When considering cell type dependence, it is thus important to focus on cells that play a central role in vivo in human disease. There is no doubt that the MC, with its response to TGFβ, is central to progressive glomerulosclerosis. The relationship between these signaling proteins in TGFβ-induced matrix upregulation in these cells, as well as the role of caveolae in p38/Erk activation by TGFβ, need further study.
While we have demonstrated a requirement for caveolae in fibronectin synthesis, these microdomains were also recently implicated in fibronectin turnover. Sottile et al. (51) showed that fibronectin degradation requires internalization through caveolae, with impairment in fibronectin turnover leading to loss of matrix fibrils. In fibronectin null mouse embryonic fibroblast cells, exogenous fibronectin co-localized with cav-1 by immunofluorescence and was found in low-density caveolar fractions. This seemed to be functionally important, since chemical disruption of caveolae prevented fibronectin-mediated cell contraction and growth (12). Additionally, RhoA was shown to promote fibronectin polymerization or assembly in Swiss 3T3 fibroblasts and epithelial cells (3, 65). Thus caveolae appear to have a multifaceted role in the production of fibronectin, important to its upregulation, polymerization, and turnover.
In conclusion, our data identify a novel role for caveolae in TGFβ signaling. The requirement for RhoA activation and fibronectin synthesis, independent of Smad activation, highlights a divergent set of signaling pathways downstream of the TGFβ receptors. We have also identified a new relevant target for TGFβ-induced Src activation, showing the importance of its phosphorylation of cav-1 in allowing cav-1/RhoA interaction with subsequent RhoA activation and fibronectin synthesis. These findings help to elucidate the initial events in RhoA activation by TGFβ and suggest potential targets for treatment of glomerular sclerosis marked by excessive TGFβ signaling.
GRANTS
This work was supported by funding to J. Krepinsky from the Canadian Diabetes Association and the Canadian Institutes of Health Research (CIHR). F. Peng is supported by a fellowship from the Father Sean O'Sullivan Research Center (Hamilton, ON, Canada). D. Wu is supported by a fellowship from the Krescent Program of the Kidney Foundation of Canada/CIHR.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol 12: 235–243, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science 296: 1646–1647, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Cali G, Mazzarella C, Chiacchio M, Negri R, Retta SF, Zannini M, Gentile F, Tarone G, Nitsch L, Garbi C. RhoA activity is required for fibronectin assembly and counteracts beta1B integrin inhibitory effect in FRT epithelial cells. J Cell Sci 112: 957–965, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem 277: 8771–8774, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell 13: 902–914, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fielding CJ, Fielding PE. Relationship between cholesterol trafficking and signaling in rafts and caveolae. Biochim Biophys Acta 1610: 219–228, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes 53: 200–208, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Han DC, Guan JL. Association of focal adhesion kinase with Grb7 and its role in cell migration. J Biol Chem 274: 24425–24430, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J Am Soc Nephrol 12: 1853–1861, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor beta (TGFbeta)-stimulated fibronectin synthesis through TGFbeta-activated kinase 1 and activation of the JNK pathway. J Biol Chem 280: 25920–25927, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hocking DC, Kowalski K. A cryptic fragment from fibronectin's III1 module localizes to lipid rafts and stimulates cell growth and contractility. J Cell Biol 158: 175–184, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubchak SC, Runyan CE, Kreisberg JI, Schnaper HW. Cytoskeletal rearrangement and signal transduction in TGF-beta1-stimulated mesangial cell collagen accumulation. J Am Soc Nephrol 14: 1969–1980, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Isono M, Chen S, Hong SW, Iglesias-De La Cruz MC, Ziyadeh FN. Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-beta-induced fibronectin in mesangial cells. Biochem Biophys Res Commun 296: 1356–1365, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem 280: 1024–1036, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A, Sharpe CC, Noor M, Hendry BM. The role of geranylgeranylated proteins in human mesangial cell proliferation. Kidney Int 70: 1296–1304, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kim YN, Wiepz GJ, Guadarrama AG, Bertics PJ. Epidermal growth factor-stimulated tyrosine phosphorylation of caveolin-1. Enhanced caveolin-1 tyrosine phosphorylation following aberrant epidermal growth factor receptor status. J Biol Chem 275: 7481–7491, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 1745: 273–286, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Krepinsky JC, Ingram AJ, Tang D, Wu D, Liu L, Scholey JW. Nitric oxide inhibits stretch-induced MAPK activation in mesangial cells through RhoA inactivation. J Am Soc Nephrol 14: 2790–2800, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Krepinsky JC, Li Y, Chang Y, Liu L, Peng F, Wu D, Tang D, Scholey J, Ingram AJ. Akt mediates mechanical strain-induced collagen production by mesangial cells. J Am Soc Nephrol 16: 1661–1672, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol 14: 1750–1775, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem 271: 3863–3868, 1996. [PubMed] [Google Scholar]
- 23.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J Cell Biol 140: 617–626, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem 277: 41295–41298, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol 101: 375–384, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Maeda M, Shintani Y, Wheelock MJ, Johnson KR. Src activation is not necessary for transforming growth factor (TGF)-beta-mediated epithelial to mesenchymal transitions (EMT) in mammary epithelial cells. PP1 directly inhibits TGF-beta receptors I and II. J Biol Chem 281: 59–68, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol 284: F911–F924, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mishra R, Zhu L, Eckert RL, Simonson MS. TGF-beta-regulated collagen type I accumulation: role of Src-based signals. Am J Physiol Cell Physiol 292: C1361–C1369, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273: 5419–5422, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M. Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem 278: 44305–44311, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Peng F, Wu D, Ingram AJ, Zhang B, Gao B, Krepinsky JC. RhoA activation in mesangial cells by mechanical strain depends on caveolae and caveolin-1 interaction. J Am Soc Nephrol 18: 189–198, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem 276: 19945–19953, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol 288: H936–H945, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Razani B, Lisanti MP. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res 271: 36–44, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 54: 431–467, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 276: 6727–6738, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285: H1720–H1729, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Runyan CE, Poncelet AC, Schnaper HW. TGF-beta receptor-binding proteins: complex interactions. Cell Signal 18: 2077–2088, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Samarakoon R, Higgins CE, Higgins SP, Kutz SM, Higgins PJ. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-beta1-stimulated smooth muscle cells is pp60(c-src)/MEK-dependent. J Cell Physiol 204: 236–246, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem 274: 8949–8957, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Sato M, Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Ohyama Y, Suga T, Maeno T, Aoki Y, Tamura J, Sakamoto H, Nagai R, Kurabayashi M. c-Src and hydrogen peroxide mediate transforming growth factor-beta1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arterioscler Thromb Vasc Biol 25: 341–347, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284: F243–F252, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol 127: 1217–1232, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J 390: 199–206, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharpe CC, Hendry BM. Signaling: focus on rho in renal disease. J Am Soc Nephrol 14: 261–264, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Sirard C, Kim S, Mirtsos C, Tadich P, Hoodless PA, Itie A, Maxson R, Wrana JL, Mak TW. Targeted disruption in murine cells reveals variable requirement for Smad4 in transforming growth factor beta-related signaling. J Biol Chem 275: 2063–2070, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Smith PC, Caceres M, Martinez J. Induction of the myofibroblastic phenotype in human gingival fibroblasts by transforming growth factor-beta1: role of RhoA-ROCK and c-Jun N-terminal kinase signaling pathways. J Periodontal Res 41: 418–425, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell 16: 757–768, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamai O, Oka N, Kikuchi T, Koda Y, Soejima M, Wada Y, Fujisawa M, Tamaki K, Kawachi H, Shimizu F, Kimura H, Imaizumi T, Okuda S. Caveolae in mesangial cells and caveolin expression in mesangial proliferative glomerulonephritis. Kidney Int 59: 471–480, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchida K, Zhu Y, Siva S, Dunn SR, Sharma K. Role of Smad4 on TGF-beta-induced extracellular matrix stimulation in mesangial cells. Kidney Int 63: 2000–2009, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Uchiyama-Tanaka Y, Matsubara H, Mori Y, Kosaki A, Kishimoto N, Amano K, Higashiyama S, Iwasaka T. Involvement of HB-EGF and EGF receptor transactivation in TGF-beta-mediated fibronectin expression in mesangial cells. Kidney Int 62: 799–808, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem 276: 48269–48275, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Volonte D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr(14)) via activation of p38 mitogen-activated protein kinase and c- Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem 276: 8094–8103, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of constitutively active cGMP-dependent protein kinase prevents glucose stimulation of thrombospondin 1 expression and TGF-beta activity. Diabetes 52: 2144–2150, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AM. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 203: 2895–2906, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong C, Rougier-Chapman EM, Frederick JP, Datto MB, Liberati NT, Li JM, Wang XF. Smad3-Smad4 and AP-1 complexes synergize in transcriptional activation of the c-Jun promoter by transforming growth factor beta. Mol Cell Biol 19: 1821–1830, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers MG Jr, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem 273: 26962–26968, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal 19: 1690–1700, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem 275: 39237–39245, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem 280: 12239–12245, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141: 539–551, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]