Abstract
Augmented intrarenal ANG II stimulates IL-6, which contributes to renal injury. The expression of intrarenal angiotensinogen (AGT) is enhanced by increased intrarenal ANG II in human renin/human AGT double transgenic mice. ANG II also augments AGT expression in hepatocytes and cardiac myocytes. However, the mechanisms underlying AGT augmentation by ANG II and the contribution of IL-6 to this system are poorly understood. This study was performed in human renal proximal tubular epithelial cells (HRPTECs) to test the hypothesis that IL-6 contributes to the upregulation of AGT expression by ANG II. Human kidney-2 (HK-2) cells, immortalized HRPTECs, were incubated with 10−7 M ANG II and/or 10 ng/ml IL-6 for up to 24 h. AGT mRNA and protein expressions were measured by real-time RT-PCR and ELISA, respectively. The activities of NF-κB and STAT3 were evaluated by Western blotting and EMSA. Stimulation with either ANG II or IL-6 did not significantly alter AGT mRNA or protein expression. In contrast, costimulation with ANG II and IL-6 significantly increased AGT mRNA and protein expressions (1.26 ± 0.10 and 1.16 ± 0.13 over control, respectively). Olmesartan, an ANG II type 1 receptor blocker, and an IL-6 receptor antibody individually inhibited this synergistic effect. NF-κB was also activated by costimulation with ANG II and IL-6. Phosphorylation and activity of STAT3 were increased by stimulation with IL-6 alone and by costimulation. The present study indicates that IL-6 plays an important role in ANG II-mediated augmentation of AGT expression in human renal proximal tubular cells.
Keywords: angiotensinogen, kidney, renin-angiotensin system, cytokines
the renin-angiotensin system (RAS) plays important roles in blood pressure control and the regulation of electrolytes and body fluid homeostasis. Previous studies have demonstrated the expression of renin and angiotensinogen (AGT), the precursor of ANG II, in multiple tissues (8, 37). These findings led to the concept that locally expressed RAS is involved in the regulation of individual organs in a tissue-specific manner (8, 28, 30). Nevertheless, since intrarenal ANG II is elevated in many forms of hypertension and blockade of RAS components constitutes potent therapies that control hypertension and attenuate renal damage, the renal RAS is acknowledged as a key target for clinical and biochemical studies (26). In the kidney, AGT is expressed mainly in the proximal tubule cells (13, 38), and the expressions of renal AGT mRNA and protein are enhanced in ANG II-infused rats (18, 34). We recently reported that intrarenal AGT mRNA and protein expression is enhanced by increased intrarenal ANG II production in human renin/human AGT double transgenic mice (19). Moreover, ANG II induces AGT expression in an immortalized rat renal proximal tubular cell line (12). These data provide a firm foundation for the hypothesis that ANG II-induced enhanced AGT production in renal proximal tubular cells contributes to further increases in intrarenal ANG II levels.
In rodent tissues and cells, some mechanisms underlying ANG II-induced AGT augmentation have been reported in hepatocytes (3, 16, 23). ANG II induces rat AGT expression via NF-κB activation (3, 16, 23). An acute-phase response element was identified in the rat AGT promoter region, which includes the NF-κB and CCAAT/enhancer-binding protein binding sites (2, 5). This finding revealed a molecular mechanism underlying the augmentation of rodent AGT by ANG II and some cytokines. On the other hand, little information is available regarding the cis- and trans-acting regulators of AGT expression in human cells (2). Although the regulatory mechanisms of human AGT have been clarified by investigations regarding human AGT polymorphism (9, 14), the existence of an NF-κB-binding site in the promoter region of human AGT has not been reported (10, 15). Therefore, it is not known whether NF-κB activity is involved in the expression of human AGT.
ANG II and cytokines contribute to various forms of renal dysfunction (35), and there is substantial evidence of interaction between these factors. In IL-6 knockout mice, the magnitude of ANG II-induced hypertension is attenuated (21). In the kidneys of ANG II-infused rats, IL-6 levels are elevated with the hypertension (31). Furthermore, the disruption of the IL-6 gene attenuated albuminuria, a marker for renal injury, in ANG II-induced hypertensive mice (24). In hepatocytes, IL-6 stimulated AGT secretion (27) and expression via STAT3 activation (15, 29). These data suggest that IL-6 plays a role in the ANG II-mediated AGT positive feedback system (4). However, direct interaction between AGT and IL-6 in the kidney has not been established. In particular, the mechanism underlying the augmentation of AGT by ANG II and the contribution of IL-6 to this system in human renal proximal tubular epithelial cells have not been demonstrated although it is clear that intrarenal AGT is primarily expressed in proximal tubule cells. This study was performed to test the hypothesis that IL-6 contributes to the upregulation of AGT expression by ANG II and that NF-κB and STAT3 participate in the regulation of AGT expression in human renal proximal tubular epithelial cells.
METHODS
Cell culture.
Human kidney-2 (HK-2) cells, which are immortalized human renal proximal tubular epithelial cells, were obtained from ATCC. The cells were cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen). They were plated at a density of 2 × 105 cells/well in six-well plates. Before stimulation, the cells were exposed to serum-free medium for 24 h. Thereafter, they were treated with 10−7 M ANG II (Phoenix Pharmaceuticals) and/or several concentrations of IL-6 (Peprotech) for up to 24 h (4, 12, or 24 h) in a medium containing 1% serum. To determine the contribution of ANG II type 1 receptor (AT1R) to the effect of ANG II and IL-6 on AGT expression, HK-2 cells were individually treated with ANG II and IL-6 and with a combination of ANG II and IL-6 in the presence of 1 μM olmesartan (Sankyo), an AT1R blocker, for 24 h. Moreover, the role of the IL-6 receptor (IL-6R) in the effects of ANG II and IL-6 was investigated using 2 μg/ml anti-IL-6R antibody (R&D Systems). To deduce the influence of NF-κB in human AGT expression, cells were treated with 10 μM parthenolide, an NF-κB inhibitor (32), for 24 h. To investigate the influence of STAT3 on human AGT expression, cells were treated with 0.2 μM JSI-124 (Calbiochem), an inhibitor of STAT3 activation (1). The treatment with JSI-124 was begun before 2 h of stimulation with ANG II and/or IL-6 because the inhibitory effect of JSI-124 is observed more slowly than STAT3 activation by IL-6 (39).
Quantitative real-time RT-PCR.
Quantitative real-time RT-PCR (qRT-PCR) was performed to evaluate human AGT mRNA expression using a TaqMan PCR system. For total RNA isolation, treated cells were washed with 3 ml of PBS. After PBS aspiration, total RNA was isolated from the cells using a BIO-ROBOT EZ 1 (Qiagen). Subsequently, qRT-PCR was performed as previously described (20). In brief, 20 ng total RNA from each sample was applied to an Mx3000P System (Stratagene) equipped with a Brilliant Single-Step QRT-PCR Master Mix II Kit (Stratagene). All samples were analyzed in triplicate, and the data were normalized based on the expression level of the human GAPDH mRNA. The information of sequences are as follows: human AGT mRNA: forward primer, 5′-GAG AGA GCC CAC AGA GTC TA-3′; reverse primer, 5′-GCT TTG ATC ATA CAC AGC AA-3′; and probe, 5′/6-FAM/CCA ACA GCT TAA CAA GCC TGA GGT/BHQ1/3′; and human GAPDH mRNA: forward primer, 5′-ATC ATC CCT GCC TCT ACT GG-3′; reverse primer, 5′-CTG CTT CAC CAC CTT CTT GA-3′; and probe, 5′/HEX/ACC TGA CCT GCC GTC TAG AAA AAC CTG/BHQ2/3′.
Human AGT ELISA.
To quantify human AGT protein in the culture medium and in the cell lysate, human AGT ELISA was performed. HK-2 cells were cultured in six-well plates with 1.5 ml of medium/well. After treatment with the various agents, the media were collected into tubes, and the supernatants were collected after centrifugation of the media for 5 min at 5,000 rpm. Cells were harvested with 100 μl lysis buffer (IBL), and the cell lysates were then incubated for 30 min at 4°C. Supernatants were collected after centrifugation for 10 min at 10,000 rpm. Human AGT ELISA was performed as previously described (17). Total protein concentrations in the media and cell lysates were quantified using Bio-Rad Protein Assay (Bio-Rad) and a Micro BCA Protein Assay Kit (Pierce), respectively. Data from human AGT ELISA were normalized based on protein concentration.
Western blot analysis.
To detect the expression levels of AT1R and IL-6R proteins, Western blotting was performed. After treatment for 24 h, cells were washed three times with 3 ml PBS. The cells were harvested with 80 μl lysis buffer containing 1% Triton X-100, 150 mmol/l NaCl, 1 mmol/l EDTA, 1% Nonidet P-40, 1 mmol/l Na3VO4, and 0.25% Protease Inhibitor Cocktail (Sigma). The lysates were sonicated three times for 10 s each and centrifuged at 13,000 rpm at 4°C for 30 min. Total protein concentration of the supernatant was quantified using a Micro BCA Protein Assay Kit (Pierce). Next, 5 μg (for AT1R) or 20 μg (for IL-6R) of total protein was applied to a precast NuPAGE 4–12% gel (Invitrogen). The separated proteins were transferred to a nitrocellulose membrane (Bio-Rad). A rabbit anti-AT1R antibody (1:350, Santa Cruz Biotechnology) and an IRDye-labeled anti-rabbit IgG antibody (1:15,000, Li-Cor) were used for AT1R detection. A goat anti-IL-6R antibody (1:150, R&D Systems) and an IRDye-labeled anti-goat IgG antibody (1:15,000, Li-Cor) were used for the detection of human IL-6R. Mouse anti-β-actin antibody (1:1,000, Abcam) and an IRDye-labeled anti-mouse IgG antibody (1:15,000, Li-Cor) were used for the detection of human β-actin. Detection was performed using an Odyssey System (Li-Cor). Data were normalized based on human β-actin protein expression levels. Phosphorylations of p65, a subunit of NF-κB, and STAT3 were detected using Western blot analysis to elucidate participations of these transcriptional factors in the effects mediated by ANG II and IL-6 on human AGT expression. Cells were treated with ANG II and/or IL-6 for up to 30 min. Mouse anti-phospho-p65 (Ser 536) antibody (1:1,000, Cell Signaling), mouse anti-phospho-STAT3 (Tyr 705) antibody (1:250, BD Transduction Laboratories), and an IRDye-labeled anti-mouse IgG antibody (1:15,000, Li-Cor) were used for the detection of phosphorylated p65 and STAT3. It is known that p65 protein is a subunit of NF-κB, and NF-κB activation involves p65 protein phosphorylation (33). Rabbit anti-p65 antibody (1:1,000, Cell Signaling), rabbit anti-STAT3 antibody (1:250, Thermo), and an IRDye-labeled anti-rabbit IgG antibody (Li-Cor) were used to detect total p65 and STAT3. The levels of phosphorylated p65 and STAT3 were normalized based on total p65 and STAT3, respectively.
EMSA.
Binding activities of NF-κB and STAT3 to DNA were tested using EMSA. After treatment for 15 min, the cells were washed with 3 ml cold PBS and then the cells were harvested. Nuclear protein extraction was performed using an NE-PER kit (Pierce). The protein concentration was determined using a Micro BCA Protein Assay Kit (Pierce). Three micrograms nuclear protein was incubated with NF-κB IRDye-700 (Li-Cor) or STAT3 IRDye-700 (Li-Cor) for 30 min. Reagents included in an EMSA Buffer Kit (Li-Cor) were used for the reaction. The reaction was applied to 6% Novex DNA Retardation Gel (Invitrogen). The detection and quantification of shift bands were performed using an Odyssey System. After detection of the shift bands, target bands were identified using the combination of anti-p65 with p50 antibodies (Cell Signaling) and anti-STAT3 antibody (Thermo).
Statistical analysis.
Data are expressed as means ± SD. The data were analyzed using one-way ANOVA followed by a Bonferroni/Dunn multiple comparison post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Effects of ANG II and IL-6 on human AGT mRNA expression.
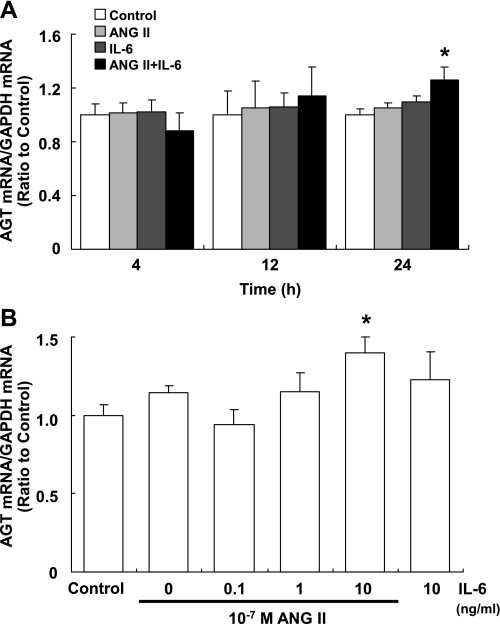
Augmentation of human AGT mRNA expression was not observed with any combination at either 4 or 12 h (Fig. 1A). The AGT mRNA expression level was not influenced by the addition of either 10−7 mol/l ANG II or 10 ng/ml IL-6 at each of these two time points (Fig. 1A). However, costimulation with 10−7 mol/l ANG II and 10 ng/ml IL-6 significantly increased the expression level of AGT mRNA (1.26 ± 0.10, ratio to control) at 24 h (Fig. 1A). AGT mRNA expression was not increased by any combination either at 48 h (data not shown). The increased expression of AGT mRNA induced by costimulation reached significance at 10 ng/ml IL-6 (Fig. 1B).
Fig. 1.
Effects of ANG II and IL-6 on the expression of angiotensinogen (AGT) mRNA. HK-2 cells were treated with 10−7 M ANG II and/or 10 ng/ml IL-6 for 4, 12, and 24 h (A; n = 4). Costimulation with ANG II and IL-6 significantly increased expression of AGT mRNA at 24 h (A). It augmented AGT mRNA expression in IL-6 concentration-dependent manner (B; n = 4). Expression levels of AGT mRNA were normalized based on GAPDH. Data are expressed as relative values compared with the control and represent means ± SD. *P < 0.05 vs. control.
Effects of ANG II and IL-6 on human AGT protein expression.
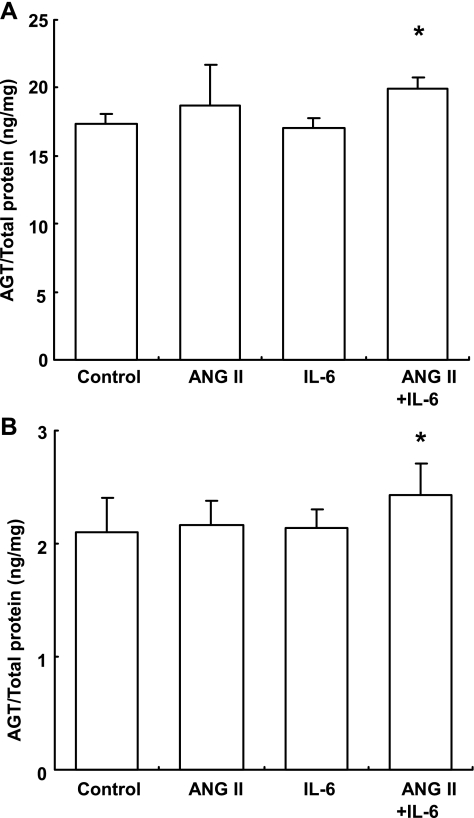
In addition to AGT mRNA expression, costimulation with ANG II and IL-6 increased the expression of human AGT protein in the media (Fig. 2A) and cell lysates (Fig. 2B) after 24 h. The increments in AGT protein expression induced by the costimulation were smaller than those increments in AGT mRNA expression (in medium: 1.15 ± 0.05, in cell lysate: 1.16 ± 0.13, ratio to control). However, the difference is significant. In both the medium and cell lysate, stimulation with either ANG II or IL-6 individually had no effect on the expression level of AGT protein when tested independently.
Fig. 2.
Effects of ANG II and IL-6 on the expression of AGT protein. HK-2 cells were treated with 10−7 M ANG II and/or 10 ng/ml IL-6 for 24 h. Costimulation with ANG II and IL-6 significantly increased the expression of AGT protein in the medium (A; n = 3) and cell lysate (B; n = 8). Expression levels of AGT protein were normalized based on the concentrations of total protein in the medium and cell lysate. Values are means ± SD. *P < 0.05 vs. control.
Effects of olmesartan and IL-6R antibody on AGT mRNA augmentation by costimulation with ANG II and IL-6.
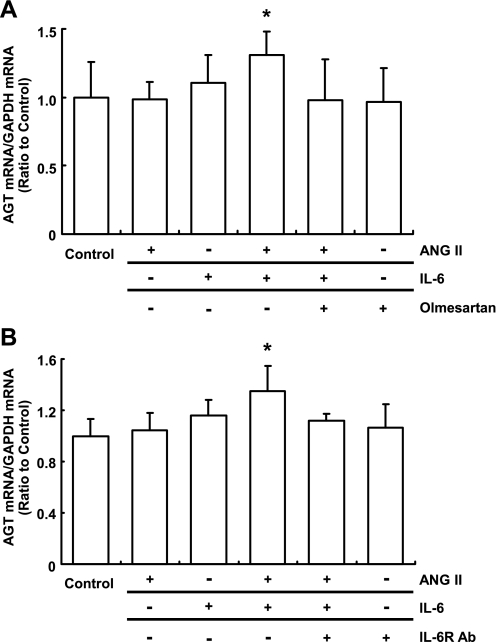
The augmentation of AGT mRNA induced by costimulation with ANG II and IL-6 was inhibited by 1 μmol/l olmesartan (Fig. 3A). In the absence of the costimulation, olmesartan did not influence the AGT mRNA level. We observed that 2 μg/ml IL-6R antibody neutralized the effect of costimulation with ANG II and IL-6 (Fig. 3B). Individually, neither olmesartan nor the IL-6R antibody had any effect on AGT expression.
Fig. 3.
Effects of olmesartan and anti-IL-6 receptor antibody (IL-6R Ab) on the augmentation of AGT mRNA by costimulation with ANG II and IL-6. HK-2 cells were treated with 10−7 M ANG II, 10 ng/ml IL-6, and/or 1 μM olmesartan for 24 h (A; n = 7). The cells were treated with 10−7 M ANG II, 10 ng/ml IL-6, and/or 2 μg/ml IL-6R Ab for 24 h (B; n = 4). Olmesartan and IL-6R Ab inhibited the synergistic effect of ANG II and IL-6 on the expression of AGT mRNA (A and B). Expression levels of AGT mRNA were normalized based on GAPDH. Data are expressed as relative values compared with the control and represent means ± SD. *P < 0.05 vs. control.
Effects of ANG II and IL-6 on AT1R and IL-6R protein expression.
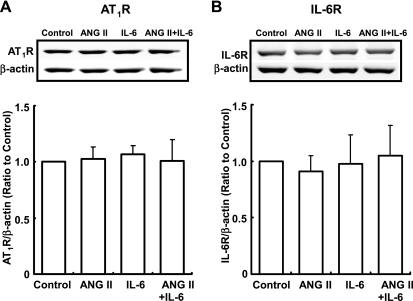
Addition of either ANG II or IL-6 individually did not induce AT1R expression at 24 h. Furthermore, costimulation with ANG II and IL-6 had no effect on AT1R expression either (Fig. 4A). Similarly, there was no change in the expression of IL-6R at 24 h (Fig. 4B).
Fig. 4.
Effects of ANG II and IL-6 on the expression of ANG II type 1 receptor (AT1R) and IL-6R proteins. HK-2 cells were treated with 10−7 M ANG II and/or 10 ng/ml IL-6 for 24 h. The expression of AT1R (A) and IL-6R (B) proteins was not influenced by either treatment. Results were normalized based on the expression levels of β-actin protein. Data are expressed as relative values compared with the control and represent means ± SD. *P < 0.05 vs. control.
Effects of ANG II and IL-6 on NF-κB activity.
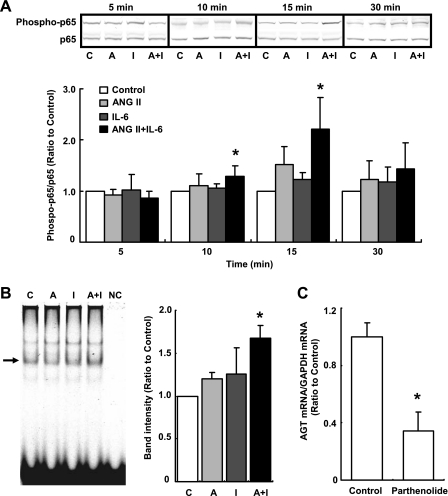
Phosphorylation of p65 protein was not affected by treatment with either ANG II or IL-6 at any time point. Although phosphorylation of p65 protein was not observed at 0 min (data not shown), it was detected even in the control after 5 min (Fig. 5A). Costimulation with ANG II and IL-6 facilitated p65 phosphorylation at 10 (1.28 ± 0.21, ratio to control) and 15 min (2.21 ± 0.62, ratio to control, Fig. 5A). The increment in p65 protein phosphorylation by the costimulation was not detected at 30 min (Fig. 5A).
Fig. 5.
Effects of ANG II and IL-6 on NF-κB activity. HK-2 cells were treated with 10−7 M ANG II and/or 10 ng/ml IL-6 for 5, 10, 15, and 30 min (n = 4). The level of phosphorylated p65 was increased by the costimulation with ANG II and IL-6 at 10 and 15 min. Phosphorylated p65 levels were normalized based on the expression levels of total p65 (A). After treatment of cells with ANG II and/or IL-6 for 15 min, the binding activity of NF-κB to DNA was determined and quantified using EMSA (n = 4). The arrow indicates a band identified by both anti-p65 and p50 antibodies. NF-κB activity was significantly increased by the costimulation (B). The inhibitory effect of parthenolide on the basal expression level of AGT mRNA was tested using quantitative real-time RT-PCR. Basal AGT expression was decreased by 10 μM of inhibitor at 24 h (C). C, control; A, ANG II; I, IL-6; A+I, ANG II+IL-6; NC, negative control (without nuclear protein). Data are expressed as relative values compared with the control and represent means ± SD. *P < 0.05 vs. control.
Binding activity of NF-κB to DNA was detected using EMSA. Costimulation with ANG II and IL-6 significantly enhanced the binding activity at 15 min (1.67 ± 0.15, ratio to control, Fig. 5B). Binding activity as well as p65 phosphorylation were detected even in the control (Fig. 5B).
Since the binding activity of NF-κB to DNA was detected even in the control, the contribution of NF-κB to human AGT expression was determined using parthenolide, an NF-κB inhibitor. The inhibitor attenuated the basal expression level of AGT (0.34 ± 0.13, ratio to control) at 24 h in the cell line used in this study (Fig. 5C).
Effects of ANG II and IL-6 on STAT3 activity.
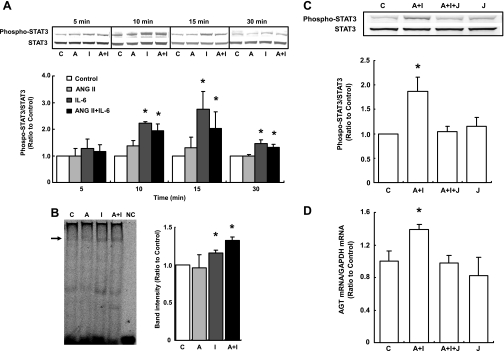
ANG II alone did not induce phosphorylation of STAT3 at any time point. STAT3 phosphorylation was increased by stimulation with IL-6 alone at 10, 15, and 30 min (2.22 ± 0.06, 2.75 ± 0.68, and 1.46 ± 0.14, respectively, ratio to control). Costimulation with ANG II and IL-6 also increased the phosphorylation at these time points (1.95 ± 0.26, 2.02 ± 0.63, and 1.32 ± 0.11, respectively, ratio to control, Fig. 6A).
Fig. 6.
Effects of ANG II and IL-6 on STAT3 activity. HK-2 cells were treated with 10−7 M ANG II and/or 10 ng/ml IL-6 for 5, 10, 15, and 30 min (n = 4). Phosphorylation of STAT3 was increased by stimulation with IL-6 alone and by costimulation with ANG II and IL-6. Phosphorylated STAT3 levels were normalized based on the levels of total STAT3 expression (A). After treatment of cells with ANG II and/or IL-6 for 15 min, the binding activity of STAT3 to DNA was determined and quantified using EMSA (n = 4). The arrow indicates a band identified using STAT3 antibody. STAT3 activity was significantly increased by stimulation with IL-6 alone and by costimulation (B). Inhibition of STAT3 activation was performed by JSI-124. The inhibitor suppressed STAT3 phosphorylation (C; n = 4) and increment in AGT expression (D; n = 4) by costimulation with ANG II and IL-6. C, control; A, ANG II; I, IL-6; A+I, ANG II+IL-6; NC, negative control (without nuclear protein); A+I+J, ANG II+IL-6+JSI-124; J, JSI-124. Data are expressed as relative values compared with the control and represent means ± SD. *P < 0.05 vs. control.
EMSA showed that the binding activity of STAT3 to DNA was significantly induced by IL-6 alone and by the costimulation at 15 min (1.16 ± 0.04 and 1.33 ± 0.04, respectively, ratio to control, Fig. 6B).
JSI-124 inhibited STAT3 phosphorylation (Fig. 6C) and increment in AGT mRNA expression (Fig. 6D) by costimulation with ANG II and IL-6.
DISCUSSION
The present results demonstrate the effects of combined ANG II and IL-6 on human AGT expression in human renal proximal tubular cells. Several in vivo studies in rodents have demonstrated ANG II-induced augmentation of renal AGT expression (18, 19, 34). Furthermore, in vitro studies have shown ANG II-induced augmentation of AGT expression in rat renal proximal tubular cells (12), hepatocytes (23), and cardiomyocytes (25). Previous studies have also shown that IL-6 stimulates AGT secretion (27) and expression (15, 29, 36) in hepatocytes. In the present study, stimulation with ANG II alone did not increase the expressions of human AGT mRNA or protein in HK-2 cells. Although stimulation of IL-6 alone also failed to increase human AGT expression, costimulation with ANG II and IL-6 increased the expression levels of both AGT mRNA and protein at 24 h. Since it is well known that ANG II increases intrarenal IL-6 levels (31), ANG II and the IL-6 induced by ANG II may collectively increase human AGT expression in vivo. Although AGT production in HK-2 cells is lower than that in HepG2 cells (7), renal proximal tubular cells are the main source of AGT in the kidney (13, 38). Therefore, ANG II and IL-6 can synergistically induce large changes in the amount of AGT in the human kidney even if its induction ratio is small, as shown in this study. Augmentation of AGT expression in the kidney by IL-6 has not been reported; thus this study provides novel information relevant to intrarenal AGT regulation. Moreover, the results indicate that ANG II-dependent induction of AGT expression requires IL-6 as a costimulatory factor in human renal proximal tubular cells.
It has been reported that oxalate and IL-1 stimulate IL-6 production in HK-2 cells, indicating that HK-2 cells can produce IL-6 by in response to various stimuli (6, 11). Therefore, it is possible that ANG II induces endogenous IL-6. The reason that single stimulation with ANG II alone did not induce AGT expression may be the low concentration of induced IL-6. In support of this, costimulation with ANG II and a low concentration of IL-6 did not influence AGT expression (Fig. 1B).
Olmesartan inhibited the effect of costimulation with ANG II and IL-6 on AGT expression, indicating that AT1R is critical for AGT enhancement by ANG II. Moreover, IL-6R antibody also neutralized the effect, suggesting that these agents exert a synergistic effect, even though they do not independently induce human AGT expression. These findings suggest that AT1R blockers may attenuate the secondary pathophysiological effects of ANG II even though ANG II alone does not exert any direct effects, and this may partially account for the powerful renoprotective effects of AT1R blocker beyond its depressor effect.
It has been reported that IL-6 increases AT1R expression in vascular smooth muscle cells (40). Thus we evaluated the possibility that ANG II increases IL-6R expression and/or IL-6 increases AT1R expression. However, treatment with either ANG II or IL-6 alone or with their combination did not increase the expression of AT1R or IL-6R (Fig. 4). Hence, these results indicate that the mechanism underlying human AGT upregulation by costimulation in this cell line is independent of the changes in the expression levels of their receptors.
Treatment of cells with the combination of ANG II and IL-6 facilitated the phosphorylation of p65 protein (Fig. 5A). Furthermore, NF-κB activation in response to costimulation with ANG II and IL-6 was revealed by EMSA (Fig. 5B). ANG II-induced NF-κB activation has been shown in many studies (3, 16, 23), and high basal activity of NF-κB in HK-2 cells has been demonstrated (6). In our study, high basal activity of NF-κB was observed (Fig. 5, A and B, in control), and basal AGT expression level was decreased by NF-κB inhibitor (Fig. 5C). Although an NF-κB-binding site in the human AGT promoter region has not been identified, these results indicate that NF-κB activity obviously relates to AGT expression in the human kidney as well as in the rodent kidney. High basal NF-κB activity may reflect the high production of AGT in proximal tubule cells in the kidney.
Interestingly, STAT3 phosphorylation was increased by IL-6 alone as well as by costimulation with ANG II and IL-6 (Fig. 6A). Furthermore, STAT3 activation by IL-6 alone and by costimulation with ANG II and IL-6 was revealed using EMSA (Fig. 6B). Moreover, JSI-124 inhibited STAT3 phosphorylation (Fig. 6C) and increment in AGT mRNA expression (Fig. 6D) by costimulation with ANG II and IL-6. IL-6 has been shown to stimulate NF-κB activity through the activation of STAT3 in renal proximal tubule cells (22). Therefore, NF-κB activation by costimulation with ANG II and IL-6 may be achieved through weak activation of NF-κB by ANG II, which is subsequently augmented by IL-6-mediated STAT3 activation. The human AGT promoter region contains STAT3-binding sites, and the activation of STAT3 contributes to the augmentation of AGT expression (15). However, independent stimulation with IL-6 had no effect on AGT expression in HK-2 cells. Although high basal activity of NF-κB might relate to this low sensitivity of human AGT expression to IL-6, further studies are required to confirm this possibility.
In summary, we have demonstrated that ANG II and IL-6 synergistically induce human AGT expression through the activation of NF-κB and STAT3 in HK-2 cells, indicating that IL-6 plays an important role in ANG II-mediated augmentation of AGT expression in human renal proximal tubular cells. Elucidation of the mechanisms underlying this augmentation may lead to new strategies to treat hypertension, cardiac diseases, and renal diseases.
GRANTS
This study was supported by National Institutes of Health Grants R01-DK-072408, P20-RR-017659, and R01-HL-026371; the Health Excellence Fund, Louisiana Board of Regents; Sankyo Co., Ltd. (Tokyo, Japan); and the Consortium for Southeastern Hypertension Control Warren Trust Fellowship Award program (R. A. Gonzalez-Villalobos).
Acknowledgments
The authors acknowledge the excellent technical assistance of Duy V. Tran and My-Linh Rauv (Tulane University).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63: 1270–1279, 2003. [PubMed] [Google Scholar]
- 2.Brasier AR, Han Y, Sherman CT. Transcriptional regulation of angiotensinogen gene expression. Vitam Horm 57: 217–247, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem 212: 155–169, 2000. [PubMed] [Google Scholar]
- 4.Brasier AR, Recinos A 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257–1266, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brasier AR, Ron D, Tate JE, Habener JF. A family of constitutive C/EBP-like DNA binding proteins attenuate the IL-1 alpha induced, NF kappa B mediated trans-activation of the angiotensinogen gene acute-phase response element. EMBO J 9: 3933–3944, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Haij S, Adcock IM, Bakker AC, Gobin SJ, Daha MR, van Kooten C. Steroid responsiveness of renal epithelial cells. Dissociation of transrepression and transactivation. J Biol Chem 278: 5091–5098, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Dickson ME, Zimmerman MB, Rahmouni K, Sigmund CD. The -20 and -217 promoter variants dominate differential angiotensinogen haplotype regulation in angiotensinogen-expressing cells. Hypertension 49: 631–639, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation 89: 493–498, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Fardella C, Zamorano P, Mosso L, Gomez L, Pinto M, Soto J, Oestreicher E, Cortes P, Claverie X, Montero J. A-6G variant of angiotensinogen gene and aldosterone levels in hypertensives. Hypertension 34: 779–781, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Fukamizu A, Takahashi S, Seo MS, Tada M, Tanimoto K, Uehara S, Murakami K. Structure and expression of the human angiotensinogen gene. Identification of a unique and highly active promoter. J Biol Chem 265: 7576–7582, 1990. [PubMed] [Google Scholar]
- 11.Huang MY, Chaturvedi LS, Koul S, Koul HK. Oxalate stimulates IL-6 production in HK-2 cells, a line of human renal proximal tubular epithelial cells. Kidney Int 68: 497–503, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol Renal Physiol 276: F218–F227, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest 85: 417–423, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest 99: 1786–1797, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S, Li Y, Patil S, Kumar A. HNF-1α plays an important role in IL-6-induced expression of the human angiotensinogen gene. Am J Physiol Cell Physiol 293: C401–C410, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Jamaluddin M, Meng T, Sun J, Boldogh I, Han Y, Brasier AR. Angiotensin II induces nuclear factor (NF)-kappaB1 isoforms to bind the angiotensinogen gene acute-phase response element: a stimulus-specific pathway for NF-kappaB activation. Mol Endocrinol 14: 99–113, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 293: F956–F960, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938–F945, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol 16: 2073–2080, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DL, Sturgis LC, Labazi H, Osborne JB Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lee YJ, Heo JS, Suh HN, Lee MY, Han HJ. Interleukin-6 stimulates α-MG uptake in renal proximal tubule cells: involvement of STAT3, PI3K/Akt, MAPKs, and NF-κB. Am J Physiol Renal Physiol 293: F1036–F1046, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the rel A (nuclear factor-kappaB p65) transcription factor: one mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol Endocrinol 10: 252–264, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Manhiani MM, Quigley JE, Socha MJ, Motamed K, Imig JD. IL6 suppression provides renal protection independent of blood pressure in a murine model of salt-sensitive hypertension. Kidney Blood Press Res 30: 195–202, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci USA 95: 5590–5594, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtani R, Yayama K, Takano M, Itoh N, Okamoto H. Stimulation of angiotensinogen production in primary cultures of rat hepatocytes by glucocorticoid, cyclic adenosine 3′,5′-monophosphate, and interleukin-6. Endocrinology 130: 1331–1338, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Ray S, Boldogh I, Brasier AR. STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology 129: 1616–1632, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Re RN Tissue renin angiotensin systems. Med Clin North Am 88: 19–38, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82: 12–22, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Saadane A, Masters S, DiDonato J, Li J, Berger M. Parthenolide inhibits IkappaB kinase, NF-kappaB activation, and inflammatory response in cystic fibrosis cells and mice. Am J Respir Cell Mol Biol 36: 728–736, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem 274: 30353–30356, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol Endocrinol Metab 263: E863–E869, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, Dechend R, Gratze P, Luft FC, Muller DN. Complement activation in angiotensin II-induced organ damage. Circ Res 97: 716–724, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Sherman CT, Brasier AR. Role of signal transducers and activators of transcription 1 and -3 in inducible regulation of the human angiotensinogen gene by interleukin-6. Mol Endocrinol 15: 441–457, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K, Tanimoto K, Takahashi S, Sagara M, Fukamizu A, Murakami K. Structure and expression of the mouse angiotensinogen gene. Jpn Heart J 33: 113–124, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int 43: 1251–1259, 1993. [DOI] [PubMed] [Google Scholar]
- 39.van Kester MS, Out-Luiting JJ, von dem Borne PA, Willemze R, Tensen CP, Vermeer MH. Cucurbitacin I inhibits Stat3 and induces apoptosis in sezary cells. J Invest Dermatol. In press. [DOI] [PubMed]
- 40.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 94: 534–541, 2004. [DOI] [PubMed] [Google Scholar]