Abstract
The mechanism of action of cisplatin as a chemotherapeutic agent has been attributed to DNA binding, while its mechanism of action as a nephrotoxin is unresolved. Only ∼1% of intracellular cisplatin interacts with DNA, primarily forming intrastrand cross-linked adducts, and many studies have implicated both nuclear and cytoplasmic causes of cisplatin-induced death in cultured cells. We have demonstrated that cisplatin cytotoxicity depends on cdk2 activity, which is at least partly through the cdk2-E2F1 pathway. The mechanism of the dependency on cdk2, and whether cdk2 activation of E2F1 represents the only cell death pathway involved, is still unclear. Our previous work showed that deletion of the nuclear localization signal from p21WAF1/CIP1, a cdk2 inhibitor, did not alter its protective action against cisplatin cytotoxicity. Active cdk2-cyclin complexes are localized in both the nucleus and cytoplasm, and it was reported that cdk2 translocated to the cytoplasm after an apoptotic stimulus. Herein, we show that cisplatin caused cell death in enucleated mouse kidney proximal tubule cells (TKPTS), which was prevented by cdk2 inhibition. Also, we localized cytoplasmic cdk2 to both the endoplasmic reticulum (ER) and Golgi compartments, and ER stress was blocked by specific cdk2 inhibition. We conclude that cisplatin can induce nuclear independent apoptosis, cisplatin cytotoxicity can be initiated by cytoplasmic events, and cytoplasmic cdk2 plays an important role in apoptosis signaling.
Keywords: cdk2, apoptosis, endoplasmic reticulum stress
cisplatin [or cis-diamminedichloroplatinum (II)] is a widely used chemotherapeutic agent against several types of solid tumors (30). However, its clinical use is limited by its potent nephrotoxicity, which leads to acute renal failure (7, 46). It is believed that DNA damage and subsequent induction of apoptosis is the primary cytotoxic mechanism of cisplatin (13). However, cisplatin-induced cell death does not always correlate with DNA damage, since cell death occurs at concentrations that do not inhibit synthesis of DNA (49, 50). Cisplatin is a reactive drug that interacts not only with DNA but also with proteins, and it is possible that damage to cytoplasmic proteins is an early process that initiates cisplatin-induced apoptosis (14, 17, 39). In addition, recent studies showed that cisplatin is involved in the endoplasmic reticulum (ER) stress response in both cancer cells (35) and normal kidney proximal tubule cells (34), also indicating that cisplatin may initiate apoptosis from the cytoplasm.
Cdk2-cyclin complexes, the major cell cycle regulators, easily commute between the nuclear and cytoplasmic compartments (5, 8, 16, 25, 27, 42, 53). Although primarily localized in the nucleus in proliferating cells, cdk2 translocated to the cytoplasm upon apoptotic stimulus (24). The p21WAF1/CIP1 protein, usually associated with nuclear Cdk-cyclin complexes, translocated to the cytoplasm in association with its antiapoptotic function (3, 48, 59). Similarly, we found that deletion of the nuclear localization signal from p21 did not alter its protective action against cisplatin cytotoxicity (56). Our previous work demonstrated that the mechanism of p21 protection from cisplatin was by direct inhibition of cdk2 (56), and cisplatin cytotoxicity was cdk2 dependent in vitro and in vivo (41). This evidence indicates that the cytoplasmic localization of cdk2-cyclin complexes may be important to trigger apoptotic signaling, in contrast to the role of nuclear cdk2 in controlling cell proliferation.
Our present study was designed to examine whether cisplatin cytotoxicity in mouse kidney proximal tubule (TKPTS) cells has a mechanism other than DNA damage and whether cytoplasmic cdk2 plays a role in initiating apoptosis signaling. We report that cisplatin caused cell death in enucleated TKPTS cells, which was prevented by cdk2 inhibition. Etoposide, which initiates cell death only by nuclear mechanisms, did not cause apoptosis in enucleated cells. In addition, we show that cytoplasmic cdk2 was colocalized with ER/Golgi compartments and cdk2 inhibition prevented ER stress-induced apoptosis. Thus our present work demonstrates that cisplatin cytotoxicity, while possibly being amplified by nuclear interactions, can be initiated by cytoplasmic events.
MATERIALS AND METHODS
Cell culture and treatment.
TKPTS cells (11) were grown at 37°C with 5% CO2 in DMEM+Ham's F-12 medium supplemented with 50 μU/ml insulin and 7% FBS. Culture conditions followed the same schedule. Cultured TKPTS cells were maintained for 30 h after splitting before adenovirus was added; purvalanol A (Tocris Cookson,) was added at 44 h; actinomycin D (Sigma-Aldrich) was added at 47 h; and cisplatin, etoposide (Sigma-Aldrich), or ER stressors (tunicamycin or brefeldin A) (Sigma-Aldrich) were added at 48 h, as indicated. Cytoplasts were plated, incubated as TKPTS cells, and maintained for 4 h before being washed with fresh medium; purvalanol A was added at 4 h; cisplatin or etoposide was added at 5 h; and cells were harvested at 21 h.
Cisplatin, etoposide, tunicamycin, or brefeldin A was added to cultures to final concentrations of 25 μM, 5 μM, 1 μg/ml, or 2 μg/ml, respectively, when cells were ∼75% confluent, and the cells were grown for an additional 24 or 16 h as indicated. DN-cdk2 expression adenoviruses were added to a final multiplicity of infection of 100 for 18 h before ER stressor treatment. Purvalanol was dissolved in DMSO and added to 9 μM 4 h before cisplatin or ER stressor treatment. In enucleation experiments, purvalanol was added to 9 μM or roscovitine was added to 45 μM 1 h before cisplatin. Actinomycin D was added to 10 nM 1 h before cisplatin treatment.
Cytoplast preparation.
Enucleation was performed by a modified procedure of Wigler et al. (38, 54). Briefly, 40% (wt/vol) Ficoll-70 (Sigma-Aldrich) was prepared in 25 mM Tris·HCl (pH 7.5) and diluted as indicated with PBS (pH 7.5). Cells (0.5–1 × 108) were harvested by trypsinization and resuspended in 3 ml of 12.5% Ficoll-70 supplemented with cytochalasin B (100 μg/ml). The cell suspension was layered onto a density gradient prepared in ultracentrifuge tubes with layers of 20, 16, 15, 14, and 13% Ficoll. The gradient was supplemented with 100 μg/ml of cytochalasin B and preequilibrated at 37°C in 5% CO2 overnight. The cells were centrifuged in a prewarmed (32°C) Beckman SW40 swinging bucket rotor for 1 h at 25,000 rpm. Enucleated cytoplasts were collected at the interface between the 13 and 14% Ficoll layers and were washed with PBS. Cytoplasts were plated into culture dishes to recover for 4 h with complete medium, washed with complete medium, and only viable attached cytoplasts were used for drug treatment.
The purity of the cytoplast preparation was determined by fluorescence-activated cell sorter analysis (FACS) analysis (see FACS analysis). Propidium iodide (PI) fluorescence of cytoplasts and intact cells were analyzed by way of electronic gating. The purity of attached cytoplasts was confirmed by double staining with MitoTracker Red and 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI) (see Light microscopy and immunofluorescence microscopy).
Adenoviruses.
Human DN-cdk2 cDNA plasmid was obtained from Dr. Sander van den Heuvel (Massachusetts General Hospital, Boston, MA) (52). The DN-cdk2 adenoviruses were constructed by insertion of a BamHI fragment that contained the cDNA for the protein into the BglII site of the pAdTrack-CMV plasmid according to protocols from Dr. Bert Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MD) (22). After selection for clones with the proper orientation of cDNA insertion by restriction analysis, adenoviruses were constructed as described previously (41, 56).
Cdk2-green fluorescent protein fusion protein construction and expression.
Human wild-type cdk2 cDNA plasmid was obtained from Dr. Sander van den Heuvel (Massachusetts General Hospital) (52). The cdk2 fragment was generated by PCR amplification (primers: 5′-GAA CTA GTG GAT CCA TGG AGA-3′ and 5′-CGT GAA TTC CCG GGA AGC ATA ATC TGG CAC ATC-3′) and ligated into pEGFP-N1 vector (BD Clontech). The plasmid expressing the cdk2-green fluorescent protein (GFP) fusion protein was transiently transfected into TKPTS cells using Lipofectamine 2000 (Invitrogen).
FACS analysis.
TKPTS cells were harvested by trypsinization. Cell preparation, including PI staining and FACS analysis, were performed as described previously (9, 40). Samples were analyzed using FACSCalibur (Becton Dickinson). At least 10,000 cells were analyzed for each culture condition. The percentage of cells in sub-G1/G0, G1/G0, S, and G2/M phases was determined using a cell-cycle analysis program (WinMDI 2.8, Scripps Institute). Cells in the subdiploid (sub-G1/G0) region of the histogram are classified as apoptotic (9, 40). These analyses were performed on at least four separate cultures.
Light microscopy and immunofluorescence microscopy.
TKPTS cells were photographed with an inverted microscope (Nikon Eclipse TE200) using Hoffman optics before harvesting (40).
For immunostaining, TKPTS cells grown on cover slides were transfected with plasmid encoding the cdk2-GFP fusion protein. For MitoTracker Red (Molecular Probes) staining, the dye was added at a final concentration of 100 nM for 30 min in culture condition. Cells were fixed in 4% neutral buffered formaldehyde for 10 min followed by incubation with blocking serum for 10 min. After that, cells were subjected to probing with antibodies at 37°C for 1 h. The primary antibodies included a Golgin-160 polyclonal antibody (supplied by Dr. C. E. Machamer, Johns Hopkins University School of Medicine), GM-130 monoclonal antibody (BD Transduction Laboratories), 1,4,5-trisphosphate receptor polyclonal antibody (Affinity BioReagents), PCNA monoclonal antibody, and cytochrome c polyclonal antibody (Santa Cruz Biotechnology). Alexa Fluor 546 goat anti-mouse antibody and Alexa Fluor 546 goat anti-rabbit antibody (Molecular Probes) were used for detection of immunofluorescent staining. DAPI (Vector) staining was performed at the final step for 10 min to visualize nuclei. Fluorescent images were taken using a fluorescence microscope (Nikon Eclipse E800) or with a deconvolution microscope (Zeiss Axioplan 2e) with DAPI, rhodamine, and FITC filters. The fluorescence images were analyzed by Zeiss Axiovision 4.0 and Adobe-Photoshop 7.0 software.
Western blot analysis.
Proteins were extracted from TKPTS using a lysis buffer that contained 50 mM Tris·HCl (pH 7.4), 50 mM NaCl, 0.5% NP-40, and 1% Triton X-100 with phosphatase inhibitor I and II, and a proteinase inhibitor (Sigma-Aldrich). Western blot analyses were as described previously (40, 56). Protein concentration was determined using a Bio-Rad protein assay. Samples (100 μg protein/lane from intact cells, 300 μg protein/lane from cytoplasts, or 40 μl/lane from fractions) were boiled, electrophoresed using a 15% SDS-polyacrylamide gel (29), and transferred to polyvinylidene difluoride membranes. After blocking with 5% nonfat dry milk in TBST, the membrane was incubated at 4°C overnight with primary antibody. After washing, horseradish peroxidase-conjugated secondary antibody (anti-mouse or anti-rabbit) was applied and visualized using ECL (Amersham Biosciences). The primary antibodies included histone H1 (AE4) monoclonal antibody (Santa Cruz Biotechnology), cdk2 (M2) polyclonal antibody (Santa Cruz Biotechnology), cdk2 monoclonal antibody (Upstate Biotechnology), caspase-3 polyclonal antibody (Cell Signaling), and β-actin monoclonal antibody (Sigma-Aldrich).
For the cdk2-GFP fusion protein, protein extracts (200 μg) from transfected TKPTS cells were immunoprecipitated by GFP polyclonal antibody (BD Clontech), or cdk2 (M2) polyclonal antibody (Santa Cruz Biotechnology) for 4 h at 4°C with constant rocking followed by Western blotting.
Kinase assay for cdk2.
TKPTS cells were washed twice with PBS and lysed in cold lysis buffer (as in Western blot analysis). Samples were kept on ice for 30 min and centrifuged for 30 min at 10,000 g. Kinase activity of cdk2 was assayed by a modified histone H1 kinase assay (19, 28, 56). Briefly, protein extracts (200 μg) were immunoprecipitated by agarose-immobilized cdk2 antibody (Santa Cruz Biotechnology) for 4 h at 4°C with constant rocking, and washed three times with lysis buffer and once with kinase buffer that contained 20 mM Tris·HCl (pH 7.4), 10 mM MgCl2, and 1 mM dithiothreitol. Agarose beads were resuspended in 20 μl of kinase buffer that contained 2 μg of histone H1 (Upstate Biotechnology), 20 μM ATP, and 10 μCi of [γ-32P]ATP (Amersham Biosciences) and incubated for 30 min at 30°C. The reaction was stopped by Laemmli buffer (29). Samples were boiled and electrophoresed by SDS-PAGE as described above and autoradiographed.
Statistical analyses.
Statistical analysis was performed with ANOVA and a t-test. Results are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
Cdk2 inhibition protects TKPTS from cisplatin-induced nucleus-independent apoptosis.
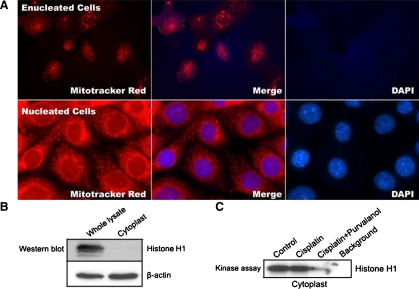
To investigate whether cisplatin can induce nucleus-independent apoptosis and whether cdk2 inhibition can prevent it, TKPTS cells were enucleated as described (see materials and methods). The purity of the cytoplast preparation was first determined by FACS analysis. The result showed that 99.5% of the sample was PI-stained negative compared with intact cells, of which >95% were PI-stained positive. The cytoplasts were then plated in culture dishes to recover for 4 h. Only reattached cytoplasts were used for experiments. The function and purity of attached cytoplasts were determined by double-staining with MitoTracker Red and DAPI. MitoTracker Red only stains mitochondria in living cells, and its accumulation is dependent on mitochondrial membrane potential. DAPI is a fluorescent dye that binds tightly to DNA, staining nuclei blue under fluorescent light. As shown in Fig. 1A, cytoplasts (“Enucleated cells”) were unstained by DAPI compared with positive-stained nucleated cells (Fig. 1A). The cytoplasts and nucleated cells were both stained with MitoTracker Red, indicating that the cytoplasts were viable and functional. Lysates isolated from cytoplasts were subjected to Western blotting with histone H1 antibody to confirm the purity of cytoplasts. Histone H1, a nuclear-specific protein, was only present in lysates from whole cells, whereas β-actin, a cytoplasmic protein, was present in both lysates (Fig. 1B).
Fig. 1.
Fluorescence microscopy of cytoplasts (enucleated) or intact mouse kidney proximal tubule cells (TKPTS; nucleated) cells. A: cytoplasts or intact cells were stained with MitoTracker Red and 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI). Fluorescence images were taken using a fluorescence microscope with DAPI and Rhodamine filters with a ×100 objective. B: purity of cytoplasts compared with intact cells (whole lysate) was determined by Western blotting of protein extracts with histone H1 antibody. Protein extracts were probed with β-actin monoclonal antibody as a loading control. C: cdk2 activity in untreated or cisplatin-treated cytoplast extracts. Cytoplasts were either untreated or treated with 25 μM cisplatin for 16 h. Purvalanol A (9 μM) was added 1 h before cisplatin. Cytoplast extracts were immunoprecipitated by cdk2 antibody followed by kinase assay using histone H1 as a substrate. Samples were electrophoresed by SDS-PAGE followed by autoradiography.
Cdk2-cyclin A/E complexes were localized to both nucleus and cytoplasm, although they were primarily in the nucleus (5, 24, 25, 27, 42, 53). We tested whether cdk2 activity can be detected in cytoplasts in the presence or absence of cisplatin and whether the kinase can be inhibited by purvalanol A (41). The same level of cdk2 kinase activity was detected in lysates from cytoplasts treated or untreated with cisplatin and was inhibited by purvalanol A (Fig. 1C), demonstrating that cytoplasmic cdk2 was activated and its activity could be inhibited by specific chemical inhibitors. Although we previously showed that cdk2 activity could be blocked by dominant-negative cdk2-expressing adenovirus, this is not possible in cytoplasts, which are deficient in transcription.
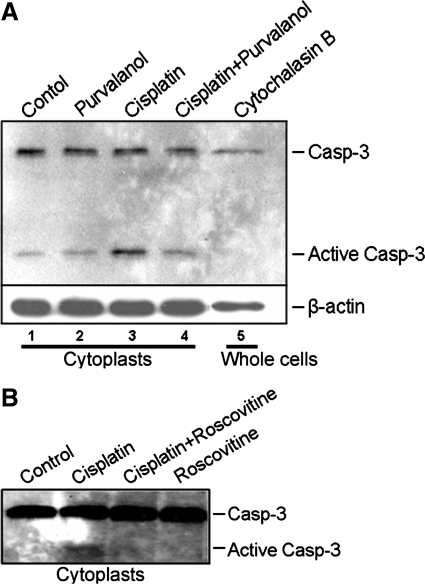
To analyze whether cisplatin can induce nuclear-independent apoptosis, cytoplasts grown in culture dishes were treated with 25 μM cisplatin for 16 h. Purvalanol A was added as indicated 1 h before cisplatin treatment to a final concentration of 9 μM. Similarly, another cdk2-inhibitory drug, roscovitine, was added as indicated 1 h before cisplatin treatment to a final concentration of 45 μM. Apoptosis was assessed by caspase-3 activation using an antibody that recognizes active (cleaved) caspase-3. As shown in Fig. 2A, cytochalasin B treatment did not induce caspase-3 activation in whole lysates from intact cells (lane 5) although the ultracentrifugation procedure for generation of enucleated cells sometimes induced a low level of active caspase-3 (lane 1). This basal level of active caspase-3 had little noticeable effect on the viability and function of cytoplast mitochondria (Fig. 1A). After cisplatin treatment, caspase-3 activation was markedly increased compared with untreated or purvalanol A-treated cytoplasts (Fig. 2A, lanes 3, 1, 2, respectively). Both purvalanol A and roscovitine, cdk2-inhibitory drugs, lowered cisplatin-induced caspase-3 activation to basal levels (Fig. 2A, lane 4 and 2B, lane 3). These results demonstrated that cisplatin induced nucleus-independent apoptotic signaling that was dependent on cytoplasmic cdk2 activity.
Fig. 2.
Cdk2 inhibition protects from cisplatin-induced nucleus-independent caspase-3 (Casp) activation. Cytoplasts were either untreated (Control) or treated with 25 μM cisplatin for 16 h. A: purvalanol A (9 μM) was added 1 h before cisplatin. In addition, whole cells were treated with cytochalasin B and were not subjected to ultracentrifugation (Cytochalasin B). Protein extracts were Western blotted with antibody to caspase-3 and active caspase-3. β-Actin was used as a loading control. B: similar to A, but roscovitine (45 μM) was added 1 h before cisplatin.
Etoposide-induced apoptosis in TKPTS cells requires nuclear events.
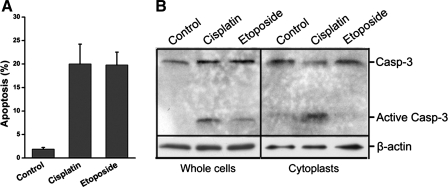
To demonstrate the specificity of cisplatin in initiating nucleus-independent apoptosis, both intact cells and cytoplasts were treated with etoposide. Etoposide is a topoisomerase II-inhibitor that induces apoptosis exclusively by DNA damage. TKPTS cells were treated with 5 μM etoposide or 25 μM cisplatin for 24 h. As shown in Fig. 3A by FACS analysis, etoposide induced apoptosis to the same extent as cisplatin. Untreated TKPTS cells had a background of 1.9 ± 0.3% apoptotic cells. Treatment with cisplatin or etoposide resulted in 20.0 ± 4.2 and 19.8 ± 2.7% of cells being apoptotic, respectively (Fig. 3A). Similarly, untreated cells had no activated caspase-3, whereas active caspase-3 was observed in both cisplatin- and etoposide-treated TKPTS cells (Fig. 3B, left). However, in cytoplasts, only cisplatin treatment caused significant caspase-3 activation; no activated caspase-3 was induced by etoposide, which was similar to untreated cytoplasts (Fig. 3B, right). The evidence that etoposide failed to induce caspase-3 activation in enucleated cells indicated that the cytotoxic effect of etoposide was dependent on DNA damage and provided further proof of the nuclear-free purity of the cytoplast preparation. In contrast, cisplatin was able to induce nucleus-independent apoptosis, which indicated that DNA damage (or any other nuclear event, such as mRNA transcription, endonuclease cleavage, etc.) is not required for cisplatin-induced cell death.
Fig. 3.
Etoposide-induced apoptosis requires nuclear events. A: fluorescence-activated cell sorter (FACS) analysis of cisplatin- and etoposide-induced apoptosis. Cells were either untreated or treated with 25 μM cisplatin or 5 μM etoposide for 24 h. The samples were analyzed using FACSCalibur. The percentage of cells in sub-G1/G0 (apoptotic fraction) was determined using a cell-cycle analysis program (WinMDI 2.8). B: etoposide does not induce apoptosis in enucleated cells. Whole cells or cytoplasts grown in culture dishes were either untreated or treated with 25 μM cisplatin or 5 μM etoposide for 16 h. Lysates were separated by 15% SDS-PAGE followed by Western blotting with an antibody to caspase-3 and active caspase-3. β-Actin was used as a loading control.
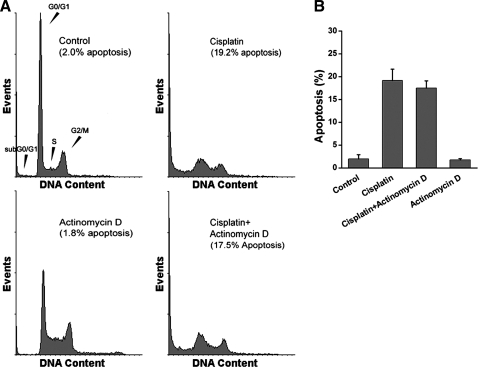
To confirm that nuclear transcription was not required for cisplatin-induced apoptosis, TKPTS cells were treated with actinomycin D, an inhibitor of DNA-primed RNA synthesis. Actinomycin D treatment had no effect on cell viability by itself (1.80 ± 0.3% vs. control, 2.0 ± 0.9% apoptosis) or together with cisplatin (17.5 ± 2.7% vs. cisplatin, 19.2 ± 4.3% apoptosis) (Fig. 4).
Fig. 4.
Inhibition of transcription has no effect on cisplatin-induced apoptosis. A: TKPTS cells were untreated or treated with 25 μM cisplatin. Actinomycin D (10 nM) was added 1 h before cisplatin. Cells were fixed and stained with propidium iodide. The samples were analyzed using FACSCalibur. B: bar graph of FACS analyses for apoptosis (percentage of cells in sub-G1/G0 fraction). Values are means ± SE from 4 separate cultures which were treated as described in A.
Cdk2 colocalized with ER/Golgi compartments in the cytoplasm.
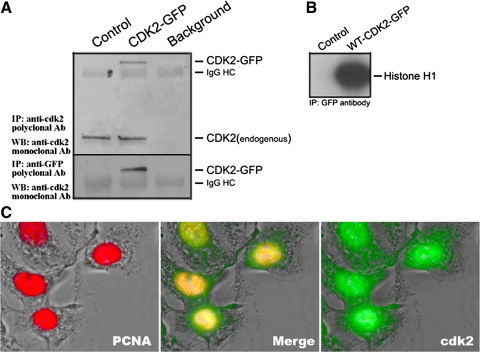
We showed the dependence of cisplatin-induced cell death in vitro and in vivo on cdk2 (41), and here we show that apoptosis can be initiated from the cytoplasm. Thus subcellular localization of cdk2 may provide a clue to the mechanism by which cisplatin initiates nucleus-independent apoptosis. To colocalize cdk2 with cellular organelles, TKPTS cells were transiently transfected with a plasmid encoding the cdk2-GFP fusion protein. The expression of the cdk2-GFP fusion protein was detected by immunoprecipitation with a cdk2 antibody (Fig. 5A, top) or GFP antibody (bottom) followed by Western blotting with an antibody against cdk2. To ensure that the cdk2-GFP fusion protein was functional, a histone H1 kinase assay was performed. Lysates from TKPTS cells transfected with plasmids encoding GFP protein (control) or the cdk2-GFP fusion protein were immunoprecipitated with a GFP antibody followed by kinase assay using histone H1 as a substrate. The cdk2-GFP fusion protein had normal cdk2 kinase activity (Fig. 5B). After transfection, TKPTS cells were fixed and immunostained with an antibody to proliferating cell nuclear antigen (PCNA). The fluorescence images showed that although the cdk2-GFP fusion protein was primarily localized to the nucleus, significant cdk2-GFP also had cytoplasmic localization (Fig. 5C). This was similar to the localization of endogenous cdk2 (data not shown), and in contrast to the purely nuclear localization of other “nuclear-centric” proteins, such as PCNA (Fig. 5C, left) or histones (data not shown).
Fig. 5.
Expression of cdk2-green fluorescent protein (GFP) fusion protein. A: cdk2-GFP fusion protein was immunoprecipitated either with cdk2 antibody or GFP antibody followed by Western blot analysis using cdk2 antibody. B: cdk2-GFP fusion protein has kinase function. Proteins were immunoprecipitated with GFP antibody, and kinase activity was determined using histone H1 as a substrate. C: subcellular localization of cdk2-GFP fusion protein. Colocalization of cdk2-GFP (green) and nuclear PCNA (red) appears as yellow.
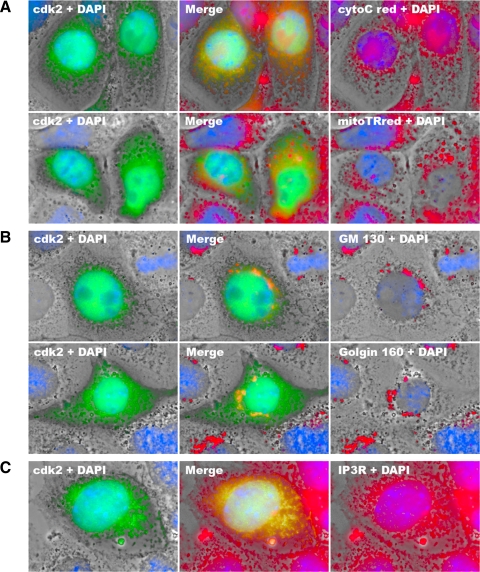
The colocalization of the cdk2-GFP fusion protein with cellular organelles was determined by immunostaining with organelle marker proteins (Fig. 6). Cdk2 did not colocalize with mitochondria as indicated by MitoTracker Red and marker protein-cytochrome c (Fig. 6A). In addition, we could not detect colocalization of cdk2 with lysosomes (data not shown). However, we detected the colocalization of cdk2 with ER and Golgi compartments. As shown in Fig. 6B, cdk2 colocalized with the Golgi apparatus, which was indicated by Golgi markers GM-130 and golgin-160 (23). The colocalization of cytoplasmic cdk2 with the ER marker protein inositol 1,4,5-trisphosphate receptor (IP3R) located in the ER membrane was also found (Fig. 6C). These observations suggested that substrates of cdk2 involved in the initiation of cisplatin cytotoxicity may also localize to ER/Golgi compartments.
Fig. 6.
Colocalization of cdk2 with endoplasmic reticulum (ER)/Golgi compartments. TKPTS cells transfected with plasmid encoding cdk2-GFP fusion protein and grown on cover slides were immunostained with cytochrome c antibody (A, top) or stained with MitoTracker Red (A, bottom) as mitochondria markers, GM-130 (B, top) or golgin-160 (B, bottom) as Golgi markers, or 1,4,5-trisphosphate receptor (IP3R; C) as an ER marker. DAPI staining was performed at the final step of staining. Fluorescence images were taken using a deconvolution microscope with a ×100 oil objective. Colocalization of cdk2-GFP (green) and red color appears as yellow.
Cdk2 inhibition protects TKPTS from ER stress-induced apoptosis.
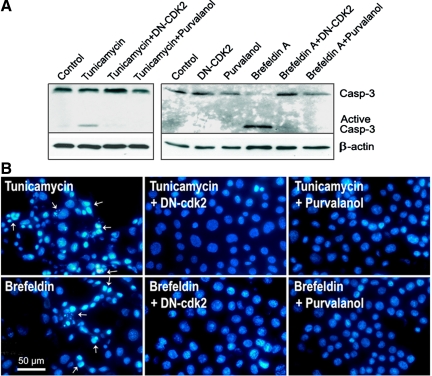
Recent studies showed that cisplatin may be involved in the ER stress response in kidney proximal tubular cells (34). To investigate whether cdk2 activity is required for apoptosis signaling initiated from ER/Golgi compartments, we tested whether cdk2 inhibition had any effect on ER stress-induced apoptosis. Apoptosis was detected by Western blot analysis for caspase-3 activation (Fig. 7A) and by nuclear morphology (Fig. 7B). Apoptosis induced by either tunicamycin or brefeldin A was completely blocked by pretreatment with either a dominant-negative cdk2-expression adenovirus or the cdk2 inhibitor purvalanol A. Apoptosis induced by thapsigargin, another ER stressor, was also inhibited (data not shown). Thus the results demonstrated that cdk2 activity is required for ER stress-induced apoptosis.
Fig. 7.
Cdk2 inhibition prevents ER-stress induced apoptosis. A: cdk2 inhibition blocks ER stress-induced caspase-3 activation. TKPTS cells were either untreated or treated with 1 μg/ml of tunicamycin or 2 μg/ml of brefeldin for 24 h. Adenoviruses expressing DN-cdk2 were added to a final multiplicity of infection of 100 for 18 h before ER stressors or for a total of 42 h. Purvalanol A (9 μM) was added 4 h before ER stressors or for a total of 28 h. Protein extracts were separated by 15% SDS-PAGE followed by Western blotting with an antibody to caspase-3 and active caspase-3. β-Actin was used as a loading control. B: cdk2 inhibition protects nuclear morphological changes caused by ER stress. The treatments were the same as described in A. Fluorescence images of DAPI-stained nuclei were taken using a fluorescence microscope with a DAPI filter with a ×100 objective.
DISCUSSION
The mechanisms and pathways leading to cisplatin cytotoxicity in quiescent renal proximal tubular epithelial cells vs. actively dividing tumor cells have not been elucidated. Approximately 1% of intracellular cisplatin reacts with nuclear DNA to cause intra-and interstrand DNA cross links and DNA-protein cross links (10, 26, 39). Besides DNA, cisplatin also reacts with other biomolecules, including cellular proteins, membrane phospholipids, and RNA (2, 26). Moreover, previous studies have indicated that inhibition of DNA synthesis may not be the critical step in cisplatin cytotoxicity in cancer cells (35, 49). Since many of the unwanted side effects of cisplatin chemotherapy involve nephrotoxicity, we examined whether cisplatin can induce apoptosis independently of genomic DNA interaction in kidney proximal tubule (TKPTS) cells.
We first established a method to generate enucleated TKPTS cells. It was reported that cytoplasts derived from several types of cells are viable and functional for up to 36 h with cellular functions (44, 54, 55). We found that enucleated TKPTS cells were able to survive more than 30 h (data not shown) with preserved mitochondrial and enzyme functions (Fig. 1, A and C). Enucleation followed by ultracentrifugation can cause significant damage to cells. To minimize unwanted cell death from the enucleation procedure, only attached viable cytoplasts were used for further experiments. Even with this selection, a small amount of caspase-3 activation was still found in viable untreated cytoplasts (Fig. 2). Results from the enucleation experiments (Figs. 2 and 3) demonstrated that cisplatin induced nucleus-independent apoptosis signaling. This contrasted with the DNA damage-dependent apoptosis induced by etoposide, which activated caspase-3 in whole cells but not in enucleated cytoplasts.
We next investigated the involvement of cdk2 in cisplatin-induced cytoplasmic apoptosis. The cdk2 activities detected in untreated and cisplatin-treated cytoplasts were at similar levels (Fig. 1C). In nucleated TKPTS cells, we reported that cisplatin caused a marked increase in cdk2 activity (41, 56). This increased activity is likely to be dependent on nuclear events, such as mRNA transcription, which is not possible in enucleated cells. Similar to nucleated cells, inhibition of cdk2 blocked cisplatin-induced apoptosis in cytoplasts (Fig. 2). Thus the data from enucleation experiments confirm our previous finding that cisplatin cytotoxicity depends on cdk2 (41, 56). The data using enucleated cells also suggest that although cisplatin-induced apoptosis requires cdk2 activity, in nucleated cells increased cdk2 activity may not be necessary for the initiation of apoptosis (Figs. 1C and 2).
We previously showed that cisplatin-induced cell death depended on cdk2 both in vitro and in vivo (41, 56) and that cdk2 activity and cisplatin-dependent cell death were inhibited by expression of p21WAF1/CIP1 protein (40, 41). Removal of the nuclear localization signal from p21 had no effect on its ability to protect (56), and cdk2 was found to be translocated to the cytoplasm after an apoptotic stimulus (24). We hypothesize that cytoplasmic subcellular localization of cdk2 may indicate the location of cdk2 substrates that are involved in the initiation of cisplatin cytotoxicity.
Consistent with other reports that cdk2 has both nuclear and cytoplasmic localization (5, 8, 16, 25, 27, 42, 53), we first showed that active cdk2-cyclin complexes existed in the cytoplasm (Fig. 1C). Studies from normal cells and cancer cells indicated that functions of several organelles were altered after cisplatin treatment, including mitochondrial (18, 43), lysosomal (6), and ER/Golgi compartments (32–35, 45). To determine putative organelles where cisplatin may initiate apoptosis, we studied the colocalization of cdk2 with organelle markers. As shown in Fig. 6, B and C, in the cytoplasm cdk2 is likely to colocalize with the ER and Golgi but not with mitochondria or lysosomes (Fig. 6A and data not shown). This result indicated that cdk2 substrates localized in these compartments may be phosphorylated in response to cisplatin and subsequently initiate apoptosis signaling. It also suggested that cdk2 activity may be critical for cell death signaling initiated from the ER and/or Golgi. Since ER stress-induced apoptosis is the only pathway known to be initiated from the ER/Golgi compartments, we tested whether ER stressor-initiated cell death can be blocked by cdk2 inhibition. ER stressors used to induce apoptosis include tunicamycin, which inhibits N-glycosylation, brefeldin A, which causes disassembly of the Golgi complex and accumulation of secretory proteins in the ER, and thapsigargin, which inhibits the ER Ca2+-ATPase. We found that cdk2 inhibition either by DN-cdk2 or purvalanol A protected TKPTS cells from ER stress-induced apoptosis (Fig. 7 and data not shown).
The present work demonstrated that cisplatin cytotoxicity, while possibly being augmented by nuclear events, can be initiated from the cytoplasm. Several lines of evidence indicate that cdk2 activity is important for promoting apoptosis and manipulations that decrease this activity prevent death (1, 12, 15, 20, 21, 24, 31, 36, 37, 47, 58). It is still not clear how cdk2 activity, which normally promotes cell cycle progression, contributes to apoptosis. Cdk-cyclin complexes have a broad range of substrates (51) and can commute between the nuclear and cytoplasmic compartments (8, 16, 24, 25). We recently reported that E2F1 is an important downstream effector of cdk2 in cisplatin nephrotoxicity (57). In its role as a positive and negative regulator of nuclear transcription, E2F1 involvement may implicate nuclear events in cisplatin cytotoxicity, although nuclear-independent apoptotic functions of E2F1 have been described (4). E2F1 gene deletion provided significant protection of kidney function and morphological damage from cisplatin-induced toxic injury in vivo, but this was less than that afforded by cdk2 inhibition (41). This indicated that other cdk2-dependent cell death pathways may be involved in cisplatin cytotoxicity. We and others (24) provided the evidence that cytoplasmic cdk2 is important in initiating cell death signaling. It is possible that subcellular localization of cdk2 determines its substrate specificity and is a mechanism by which cdk2 controls cell fate. Further investigations are required to identify substrates phosphorylated by cdk2 during cisplatin-induced apoptosis.
GRANTS
This work was supported in part by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-54471) and a VA Merit Review and with resources and the use of facilities at the John L. McClellan Memorial Veterans' Hospital (Little Rock, AR).
Acknowledgments
We thank Drs. Sander van den Heuvel and Ed Harlow (Massachusetts General Hospital) for providing clones of human wild-type and DN-cdk2 cDNA, Dr. Elsa Bello-Reuss (University of Texas Medical Branch) for providing the TKPTS, Dr. C. E. Machamer (Johns Hopkins University School of Medicine) for providing golgin-160 polyclonal antibody, and Dr. Bert Vogelstein (Johns Hopkins University School of Medicine) for providing adenoviral construction materials and protocols.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adachi S, Ito H, Tamamori-Adachi M, Ono Y, Nozato T, Abe S, Ikeda M, Marumo F, Hiroe M. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res 88: 408–414, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Andrews PA, Howell SB. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells 2: 35–43, 1990. [PubMed] [Google Scholar]
- 3.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J 18: 1223–1234, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell LA, O'Prey J, Ryan KM. DNA-binding independent cell death from a minimal proapoptotic region of E2F-1. Oncogene 25: 5656–5663, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Castro A, Jaumot M, Verges M, Agell N, Bachs O. Microsomal localization of cyclin A and cdk2 in proliferating rat liver cells. Biochem Biophys Res Commun 201: 1072–1078, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan SS, Liang XJ, Su AW, Pai-Panandiker A, Shen DW, Hanover JA, Gottesman MM. Reduced endocytosis and altered lysosome function in cisplatin-resistant cell lines. Br J Cancer 88: 1327–1334, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol 2: 80–91, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Coqueret O New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13: 65–70, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Darzynkiewicz Z, Li X, Gong J. Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol 41: 15–38, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Eastman A Mechanisms of resistance to cisplatin. Cancer Treat Res 57: 233–249, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Finkielstein CV, Chen LG, Maller JL. A role for G1/S cyclin-dependent protein kinases in the apoptotic response to ionizing radiation. J Biol Chem 277: 38476–38485, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Fisher DE Apoptosis in cancer therapy: crossing the threshold. Cell 78: 539–542, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Fuertesa MA, Castillab J, Alonsoa C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 10: 257–266, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gil-Gomez G, Berns A, Brady HJ. A link between cell cycle and cell death: Bax and Bcl-2 modulate cdk2 activation during thymocyte apoptosis. EMBO J 17: 7209–7218, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golsteyn RM Cdk1 and cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett 217: 129–138, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol 59: 657–663, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gordon JA, Gattone VH. Mitochondrial alterations in cisplatin-induced acute renal failure. Am J Physiol Renal Fluid Electrolyte Physiol 250: F991–F998, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281: 533–538, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hakem A, Sasaki T, Kozieradzki I, Penninger JM. The cyclin-dependent kinase cdk2 regulates thymocyte apoptosis. J Exp Med 189: 957–968, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey KJ, Lukovic D, Ucker DS. Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J Cell Biol 148: 59–72, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks SW, Machamer CE. The NH2-terminal domain of Golgin-160 contains both Golgi and nuclear targeting information. J Biol Chem 277: 35833–35839, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hiromura K, Pippin JW, Blonski MJ, Roberts JM, Shankland SJ. The subcellular localization of cyclin dependent kinase 2 determines the fate of mesangial cells: role in apoptosis and proliferation. Oncogene 21: 1750–1758, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Jackman M, Kubota Y, den Elzen N, Hagting A, Pines J. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol Biol Cell 13: 1030–1045, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 99: 2467–2498, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Keenan SM, Bellone C, Baldassare JJ. Cyclin-dependent kinase 2 nucleocytoplasmic translocation is regulated by extracellular regulated kinase. J Biol Chem 276: 22404–22409, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Kranenburg O, de Groot RP, van der Eb AJ, Zantema A. Differentiation of P19 EC cells leads to differential modulation of cyclin-dependent kinase activities and to changes in the cell cycle profile. Oncogene 10: 87–95, 1995. [PubMed] [Google Scholar]
- 29.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 30.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 34: 1522–1534, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM, Ross R. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of cdk2: role of a caspase cascade. Mol Cell 1: 553–563, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Liang XJ, Shen DW, Garfield S, Gottesman MM. Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines. Cancer Res 63: 5909–5916, 2003. [PubMed] [Google Scholar]
- 33.Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int 63: 1687–1696, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16: 1985–1992, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem 278: 9100–9106, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Meikrantz W, Gisselbrecht S, Tam SW, Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci USA 91: 3754–3758, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meikrantz W, Schlegel R. Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J Biol Chem 271: 10205–10209, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Nanda A, Brumell JH, Nordstrom T, Kjeldsen L, Sengelov H, Borregaard N, Rotstein OD, Grinstein S. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+-ATPases. J Biol Chem 271: 15963–15970, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Perez RP Cellular and molecular determinants of cisplatin resistance. Eur J Cancer 34: 1535–1542, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol 286: F378–F384, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, Megyesi J. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol 17: 2434–2442, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pujol MJ, Jaime M, Serratosa J, Jaumot M, Agell N, Bachs O. Differential association of p21Cip1 and p27Kip1 with cyclin E-CDK2 during rat liver regeneration. J Hepatol 33: 266–274, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Qian W, Nishikawa M, Haque AM, Hirose M, Mashimo M, Sato E, Inoue M. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol 289: C1466–C1475, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Roos D, Voetman AA, Meerhof LJ. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol 97: 368–377, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safaei R, Katano K, Larson BJ, Samimi G, Holzer AK, Naerdemann W, Tomioka M, Goodman M, Howell SB. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin Cancer Res 11: 756–767, 2005. [PubMed] [Google Scholar]
- 46.Safirstein R, Winston J, Goldstein M, Moel D, Dikman S, Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis 8: 356–367, 1986. [DOI] [PubMed] [Google Scholar]
- 47.Shi L, Chen G, He D, Bosc DG, Litchfield DW, Greenberg AH. Granzyme B induces apoptosis and cyclin A-associated cyclin-dependent kinase activity in all stages of the cell cycle. J Immunol 157: 2381–2385, 1996. [PubMed] [Google Scholar]
- 48.Shim J, Lee H, Park J, Kim H, Choi EJ. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature 381: 804–806, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Sorenson CM, Eastman A. Influence of cis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excision repair proficient and deficient Chinese hamster ovary cells. Cancer Res 48: 6703–6707, 1988. [PubMed] [Google Scholar]
- 50.Sorenson CM, Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res 48: 4484–4488, 1988. [PubMed] [Google Scholar]
- 51.Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO. Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864, 2003. [DOI] [PubMed] [Google Scholar]
- 52.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262: 2050–2054, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Verges M, Castro A, Jaumot M, Bachs O, Enrich C. Cyclin A is present in the endocytic compartment of rat liver cells and increases during liver regeneration. Biochem Biophys Res Commun 230: 49–53, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Wigler MH, Weinstein IB. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun 63: 669–674, 1975. [DOI] [PubMed] [Google Scholar]
- 55.Wright WE, Hayflick L. Formation of anucleate and multinucleate cells in normal and SV 40 transformed WI-38 by cytochalasin B. Exp Cell Res 74: 187–194, 1972. [DOI] [PubMed] [Google Scholar]
- 56.Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289: F514–F520, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Yu F, Megyesi J, Safirstein RL, Price PM. Involvement of the CDK2–E2F1 pathway in cisplatin cytotoxicity in vitro and in vivo. Am J Physiol Renal Physiol 293: F52–F59, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Zhou BB, Li H, Yuan J, Kirschner MW. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc Natl Acad Sci USA 95: 6785–6790, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3: 245–252, 2001. [DOI] [PubMed] [Google Scholar]