Abstract
Our recent studies showed that Src family kinases (SFKs) are important mediators of proliferation in renal proximal tubular cells (RPTC). In this study, we elucidate the signaling mechanisms that mediate SFK regulation of cell proliferation and cycle protein expression, and identify the SFK member responsible for these responses in a mouse RPTC line. Akt, a target of phosphoinositide-3-kinase (PI3K), and ERK1/2 were constitutively phosphorylated in RPTC cultured in the presence of serum. While treatment of cells with PP1, a specific SFK inhibitor, completely blocked phosphorylation of ERK1/2 and Akt, only inhibition of PI3K/Akt resulted in decreased RPTC proliferation. Incubation of cells with PP1 decreased cyclin D1 expression, decreased p27 and p57 phosphorylation, and increased p27 and p57 expression, two cyclin-dependent kinase inhibitors. Inhibition of the PI3K pathway decreased expression of cyclin D1 without altering expression of p27 and p57. In contrast, PP1 and PI3K inhibition had no effect on cyclin E and p21. Although RPTC expressed Src, Fyn, and Lyn, only siRNA-mediated knockdown of Src decreased RPTC proliferation, decreased cyclin D1 expression, and increased p27 and p57 expression. These data reveal that Src is a crucial mediator of RPTC proliferation and Src-mediated proliferation is associated with PI3K-dependent upregulation of cyclin D1 and PI3K-independent downregulation of p27 and p57.
Keywords: signal transduction processes, tissue regeneration
the src family kinases (SFKs) are a subclass of membrane-associated nonreceptor tyrosine kinases (RTKs) involved in a variety of signal transduction processes, including proliferation, migration, adhesion, and differentiation (15, 36). Of the nine known members of the Src family, six (Lyn, Lck, Hck, Blk, Fgr, and Yrk) are expressed primarily in hematopoietic cells (36), whereas three (Src, Yes, and Fyn) are expressed ubiquitously (19). All SFKs have a common structure and mode of regulation. Tyr416 is an autophosphorylation site that is highly conserved and phosphorylation of this residue leads to Src activation (3, 18). SFKs are involved in the signaling of many receptor tyrosine kinses including epidermal growth factor (EGF) receptor, fibroblast growth factor (FGF) receptor, insulin-like growth factor-1(IGF-1) receptor, and hepatocyte growth factor (HGF) receptor (3). These receptors are expressed in renal epithelial cells and mediate cell proliferation (27).
SFKs activate multiple intracellular signaling pathways including phosphoinositide-3-kinase (PI3K) and extracellular signaling-regulated kinase1/2 (ERK1/2). Both pathways are activated in response to stimulation by a variety of growth factor receptors. Akt/protein kinase B has been identified as a downstream target of PI3K. Activated Akt and ERK1/2 translocate to the nuclei and transactivate transcription factors, changing gene expression to promote cell proliferation in different models. Our previous studies showed that PI3K, but not ERK1/2, mediates cellular proliferation in the primary culture of rabbit renal proximal epithelial cells (43).
Proliferation signals are required for cell progression through the various steps of the cell cycle. The cell cycle is tightly controlled by both positive and negative regulators. Positive regulators include the cyclins and their catalytic partners cyclin-dependent kinases (CDK), which are essential for cells to progress through each phase of the cell cycle (10, 37). Negative regulators include the cyclin kinase inhibitors (CKI) which inhibit the cell cycle at multiple checkpoints through inactivation of cyclin-CDK complexes (10). Cyclin D1 and cyclin E are two of the major cyclins involved in cell cycle progression and p21, p27, and p57 are three CKI, which bind to the cyclin-Cdk2 complex to inhibit the kinase activity and prevent transition to the S phase (10, 37). Cyclins and CKI are regulated by transcriptional and posttranscriptional-dependent mechanisms that are linked to a variety of signaling pathways including PI3K/Akt and ERK1/2 (9, 22, 23, 31). Recently, it was reported that Src and Fyn can directly phosphorylate p27 at tyrosine residues, and promote its degradation by the SCF-SKP2 ubiquitin-proteasome pathway in breast cancer cells (4, 11).
Several lines of evidence suggest the importance of the SFKs in tissue regeneration. Yamada et al. (40) showed that activated Src is localized to the edge of scrape wounds made in keratinocyte monolayers. Takikita-Suzuki et al. (35) demonstrated that active Src is expressed in renal tubular cells after renal ischemia-reperfusion injury. Recently, we found that activation of Src regulates proliferation of renal proximal tubular cells (RPTC) following oxidant injury (44). However, the specific SFKs that are involved in and the signaling pathway that transduces SFK activation to cell proliferation remain to be studied in renal epithelial cells.
The purpose of this study was to identify the SFK(s) responsible for growth and proliferation, and to elucidate the signaling mechanisms that mediate SFK regulation of cell cycle protein expression in a mouse RPTC line.
MATERIALS AND METHODS
Chemicals and reagents.
PP1 and PP3 were purchased from Biomol Research Laboratories (Plymouth Meeting, PA). U0126 and LY294002 were obtained from Calbiochem (San Diego, CA). Antibodies to phospho-Akt (Tyr473), Akt, phospho-ERK1/2 (Thr202/Tyr204), phospho-Src (Tyr416), phospho-p27 (Thr187), Src, Fyn, Lyn, and Yes were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to cyclin D1, cyclin E, p27, p57, and p21, siRNA for Fyn and Lyn, as well as scrambled siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). siRNA for Src was purchased from Dharmacon (Lafayette, CO). All other chemicals were from Sigma (St. Louis, MO).
Cell culture.
Immortalized mouse RPTC were kindly provided by Dr. E. Bello-Reuss and were cultured in DMEM/F12 with 5% FBS at 37°C in 5% CO2. This cell line has been well characterized and expresses P-glycoprotein, has a brush border, and demonstrates a conserved epithelial morphology (6). In experiments using pharmacological inhibitors, control cells were treated with an equivalent amount of vehicle. For some experiments, trypan blue and 4′-6-diamidino-2-phenylindole (DAPI) staining were conducted to determine necrotic or apoptotic cells, respectively.
Transfection of siRNA into cells.
A pool of three to four complementary siRNA oligonucleotide duplexes targeted specifically to mouse Src, Fyn, or Lyn was used in this experiment. c-Src, Fyn, or Lyn siRNA (2 μM) was transfected into RPTC using the Nucleofector Kit V and the Amaxa Nucleofector device according to the manufacturer's instructions (Gaithersburg, MD). In parallel, scrambled siRNA (2 μM) was used to control for off-target changes in RPTC. After transfection, cells were cultured for 48 h in the DMEM with 0.5% serum and then switched to the same culture medium with 5% FBS for an additional 48 h before cell proliferation was measured.
MTT assay.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was added (final concentration, 0.5 mg/ml) to individual cultures for 1 h. Tetrazolium was released by dimethyl sulfoxide, and the optical density was determined with a Spectramax M5 plate reader.
Cell proliferation assay.
The assay was performed using a CyQUANT cell proliferation assay kit (Molecular Probes, Eugene, OR). At the end of various treatments, the cells were harvested, stored for overnight at −70°C, thawed at room temperature, and the CyQUANT GR dye/cell lysis buffer was added. After incubating for 5 min, the fluorescence was measured using a Spectramax M5 plate reader.
BrdU incorporation assay.
The assay was performed using a colorimetric BrdU cell proliferation enzyme-linked immunosorbent assay kit (Roche Applied Science, Penzberg, Germany). Briefly, BrdU labeling solution was added to cells and then incubated for 4 h. At the end of incubation, the labeling medium was removed, the cells were fixed, and the DNA was denatured. After addition of the anti-BrdU-peroxidase conjugate, the immune complexes were detected by subsequent reaction with tetramethylbenzidine as substrate for 20 min. The reaction product was quantified using a Spectramax M5 plate reader.
Protein determination.
Protein concentrations were determined using the bicinchoninic acid assay method as described by Sigma.
Immunoprecipitation.
Cells were harvested in RIPA buffer and 1 mg total protein was incubated overnight with an antibody against phosphotyrosine proteins (PY20) at a concentration of 4 μg/ml. The immune complexes were collected and the pellets were analyzed by SDS-PAGE.
Immunoblot analysis.
Equal amounts of cellular protein lysate were separated on 10% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. After treatment with 5% skim milk at 4°C overnight, membranes were incubated with various antibodies for 1 h and then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Bound antibodies were visualized following chemiluminescence detection on autoradiographic film.
Statistical analysis.
Each experiment was conducted three times. Data are presented as means ± SD and were subjected to one-way ANOVA. Multiple means were compared using Tukey's test. The differences between two groups were determined by Student's t-test. P < 0.05 was considered a statistically significant difference between mean values.
RESULTS
Mouse RPTC express multiple SFKs.
SFKs are composed of nine members in mammalin cells, of which Src, Fyn, Yes are expressed widely (5) and Lyn has been shown to be expressed in bronchial epithelial cells (42). We first examined the expression of these SFKs in RPTC whole cell lysates by immunoblot analysis using specific antibodies to Src, Fyn, Yes, or Lyn. As shown in Fig. 1, Src, Fyn, and Lyn were highly expressed in RPTC, and Yes was expressed at low levels. Actin was used as a loading control. Therefore, Src, Fyn, and Lyn are the three major SFKs expressed in RPTC.
Fig. 1.
Expression of Src family kinases in renal proximal tubular cells (RPTC). RPTC were cultured for 24 h and then cell lysates were prepared and subjected to immunoblot analysis using antibodies to Src, Fyn, Lyn, Yes, or actin.
SFK activity is required for RPTC proliferation.
To investigate the overall function of SFKs in renal epithelial cell proliferation in vitro, we cultured RPTC in the DMEM with 5% FBS for 24 h and then added PP1, a selective SFK inhibitor (14), or PP3, an analog of PP1 without the ability to inhibit SFKs. After incubation for an additional 48 h, RPTC monolayers reached confluence in cells treated with vehicle or 10 μM PP3. In contrast, RPTC growth was inhibited in the presence of 10 μM PP1 (Fig. 2A).
Fig. 2.
Effect of PP1 on RPTC proliferation. RPTC were cultured for 24 h and then incubated for an additional 48 h in the presence and absence of 10 μM PP1 or 10 μM PP3. A: phase contrast photographs (×40) were taken. RPTC were cultured for 24 h and then incubated for an additional 24 h with varying concentrations of PP1 or PP3 (B), or 10 μM PP1 and 10 μM PP3 (C, D). Cell proliferation was determined using the MTT assay (B), BrdU incorporation assay (C), or CyQUANT assay (D). Data are expressed as means ± SD (n = 3). *P < 0.05, compared with controls.
PP1 decreased RPTC proliferation in a concentration-dependent manner. Using the MTT assay, a significant reduction in cell number was observed when RPTC were treated with 1 μM PP1 and further decreased with increasing concentrations of PP1. At 20 μM PP1, RPTC proliferation was inhibited by ∼50%. In contrast, RPTC proliferation was not affected in cells treated with the same concentration of PP3 (Fig. 2B). To confirm these observations, we measured monolayer DNA content and BrdU incorporation. As shown in Fig. 2, C and D, PP1 treatment for 24 h decreased RPTC proliferation. Decreases in cell growth were not caused by cell death under these conditions because treatment of cells with PP1 did not increase trypan blue or DAPI-positive staining cells (data not shown). These data reveal that SFKs are critically involved in proliferation of RPTC in response to serum growth factors.
SFK mediates phosphorylation of Akt and ERK1/2.
The PI3K/Akt and ERK pathways mediate cell proliferation in different cell types and these pathways can be activated by multiple stimuli (16, 20). To assess whether SFK activate these two pathways in RPTC, we determined the effect of PP1 treatment on PI3K/Akt and ERK1/2 activation in the presence of serum. PI3K and ERK1/2 activation was measured using immunoblot analysis and antibodies that recognize phosphorylated Akt (p-Akt; a target of PI3K) and ERK1/2 (p-ERK1/2), respectively. Total Akt and ERK1/2 content was measured using immunoblot analysis and antibodies that recognize the Akt and ERK1/2 independent of their phosphorylation state. Constitutive phosphorylation of Akt and ERK1/2 was observed in RPTC undergoing proliferation and was blocked by PP1 in a concentration-dependent fashion (Fig. 3). At 20 μM, PP1 completely blocked phosphorylation of Akt and ERK1/2. In addition, we monitored SFK activation using the phosphospecific antibody to pY416 in the presence and absence of PP1. This antibody recognizes the conserved phosphotyrosine residue in the activation loop of SFKs (33, 39). SFKs were constitutively phosphorylated in proliferating RPTC and PP1 reduced SFKs phosphorylation in a concentration-dependent manner (1∼20 μM; Fig. 3). These data illustrate that the PI3K and ERK1/2 pathways are activated and regulated by SFKs during RPTC proliferation.
Fig. 3.
Effect of PP1 on phosphorylation of Src, Akt, and ERK1/2 in RPTC. RPTC were cultured for 48 h and then treated with various concentrations of PP1 or 20 μM PP3 for 1 h. A: cell lysates were prepared and subjected to immunoblot analysis using antibodies to phospho-Akt, Akt, phospho-ERK1/2, or ERK1/2 or using the pY 416 phosphospecific antibody to Src family kinases (SFKs). Representative immunoblots from 3 or more experiments are shown. B: immunoblotted phospho-SFKs were quantified by densitometry, and data are expressed as percentage of expression level relative to control.
PI3K but not ERK mediates RPTC proliferation.
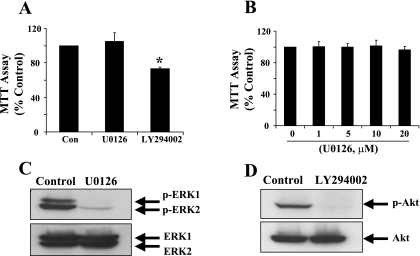
To determine whether PI3K/Akt and/or ERK1/2 activation mediates RPTC proliferation, we assessed proliferation in the presence and absence of the PI3K inhibitor LY294002 (20 μM) (38) and the MEK1/2 inhibitor U0126 (10 μM) (7). Inhibition of PI3K decreased proliferation (Fig. 4). In contrast, inhibition of MEK1/2, the direct upstream activator of ERK1/2, with increasing concentrations of U0126, had no effect on proliferation. LY294002 (20 μM) blocked Akt phosphorylation and 10 μM U0126 blocked ERK1/2 phosphorylation of RPTC, ensuring their efficacy in this model (Fig. 4). It should be noted that the LY294002 inhibited cell proliferation to a smaller degree (29%) compared with PP1-treated cells (58%; Figs. 2B, 4A). LY294002 treatment did not induce RPTC necrosis and/or apoptosis under these conditions as determined by trypan blue and DAPI staining (data not shown). These data illustrate that the PI3K/Akt, but not ERK, pathway mediates proliferation in this model and that Src-mediated proliferation in RPTC may be through PI3K-dependent and -independent pathways.
Fig. 4.
Effect of ERK1/2 and PI3K pathway inhibitors on RPTC proliferation. RPTC were cultured in the presence of serum for 24 h and then incubated for an additional 48 h with 10 μM U0126 or 20 μM LY294002 (A), or various concentrations of U0126 as indicated (B). Cell proliferation was determined using the MTT assay. Data are expressed as means ± SD of the percentage of MTT activity compared with controls grown with diluent (n = 3). *P < 0.05, compared with control group. B: RPTC were cultured for 48 h and then treated with 10 μM U0126 or 20 μM LY294002 for 30 min. Cell lysates were prepared and subjected to immunoblot analysis using antibodies to phospho-ERK1/2, phospho-Akt, total ERK1/2, or total Akt. Representative immunoblots from 3 or more experiments are shown.
Role of SFK and PI3K in cell cycle protein expression.
Cell proliferation is regulated positively by cyclins and negatively by CKI (37). Two cyclins (cyclin D1 and cyclin E) and three CKI (p21, p27, p57) are major cell cycle regulators (37). To investigate the role of SFK in regulating the expression of cyclin D1, cyclin E, p21, p27, and p57, we examined the expression of these proteins in the presence or absence of PP1 at different time points (2–24 h). As shown in Fig. 5, PP1 treatment resulted in a decrease in the expression of cyclin D1, which was observed at 4 h and further decreased over time. At 24 h, cyclin D1 was not detectable. In contrast, expression of cyclin E was not affected by PP1 at any time point. Similarly, p21, p27, and p57 expression patterns differed in response to SFK inhibition. Expression of p57 and p27 increased at 4 and 8 h, respectively, after PP1 treatment and was sustained for 24 h. However, p21 expression was not altered over the time course. Therefore, expression of cyclin D1, p27, and p57, but not cyclin E and p21, is under the control of SFKs in RPTC grown in the presence of serum.
Fig. 5.
Effect of PP1 and LY294002 on expression of cell cycle proteins and phosphorylation of p27 and p57. RPTC were cultured in the presence of serum for 24 h and then incubated for the indicated times with 10 μM PP1 (A), or 24 h with 20 μM LY294002 (B) or 10 μM PP1 (C). Cell lysates were prepared and subjected to immunoblot analysis using antibodies to cyclin D1, cyclin E, p27, phospho-p27 (T187), p57, p21, or actin (A, B). Tryosine-phosphorylated proteins were immunoprecipitated from cell lysates using PY20. Precipitates were analyzed using antibodies to p27 or p57 (C). Representative immunoblots from 3 or more experiments are shown.
Because Fig. 3 revealed that activation of the PI3K pathway is regulated by SFKs and involved in RPTC proliferation, we examined the effect of PI3K inhibition on the expression of these cell cycle proteins. Similar to PP1, LY294002 treatment decreased expression of cyclin D1 and did not alter cyclin E and p21 expression after 24 h. Interestingly, LY294002 did not stimulate or inhibit the expression of p27 and p57 (Fig. 5B). Therefore, we suggest that SFKs regulate cell cycle protein expression through two mechanisms: 1) Src-mediated cyclin D1 expression through activation of the PI3K/Akt pathway and 2) Src-regulated expression of p27 and p57 through a PI3K-independent mechanism.
Inhibition of SFKs leads to dephosphorylation of p27 and p57.
Recently, it was reported that Src and Lyn can directly phosphorylate tyrosine on p27 and reduce its inhibitory action on cyclin E-CDK2, facilitating subsequent p27 proteolysis through SKP2 ubiquitin ligase-dependent mechanism in breast cancer cells (4, 11). To determine whether SFK activity is required for phosphorylation of p27 and p57 in proliferating RPTC, we examined the effect of PP1 on tyrosine phosphorylation of p27 and p57. Because specific antibodies to tyrosine-phosphorylated p27 or p57 are not commercially available, we immunoprecipitated tyrosine phosphoproteins using PY20 and then analyzed p27 and p57 using their specific antibodies. As shown in Fig. 5, tyrosine-phosphorylated p27 and p57 were constitutively expressed in RPTC grown in serum containing medium and their phosphorylation was inhibited at 24 h in the presence of PP1. Therefore, SFK activity is also required for the regulation of p27 and p57 tyrosine phosphorylation.
Phosphorylation of p27 at threonine187 has been reported to promote the interaction of p27 with Skp2 ligase, which results in its degradation (4). Accordingly, we also examined the effect of PP1 on p27 phosphorylation at T187 by immunoblot analysis using an antibody against phosphorylated p27 at threonine 187. p27 Phosphorylated at this residue was constitutively expressed in RPTC grown in serum containing medium and its phosphorylation was inhibited by PP1 (Fig. 5A). This inhibition was observed at 8 h and was persistent at 24 h. Interestingly, p27 phosphorylation status and its expression levels were reciprocally changed: p27 dephosphorylation at T187 was accompanied by increased p27 expression. These data reveal that p27 degradation is associated with Src-mediated p27 threonine phosphorylation in proliferating RPTC.
Src, but not Fyn and Lyn, mediates RPTC proliferation.
Because Src, Fyn, and Lyn are highly expressed in RPTC (Fig. 1), we determined their individual roles in proliferation. RPTC were transfected with siRNA targeting Fyn, Lyn, or Src. As a control, RPTC were transfected with scrambled siRNA. While the gene-specific siRNA decreased expression of Fyn, Lyn, and Src ∼60% compared with cells treated with scrambled siRNA at 48 h after transfection (Fig. 6), RPTC proliferation, using MTT and Brdu assays, only decreased in cells transfected with Src siRNA (Fig. 7). In addition, transfection of Src siRNA in combination with siRNA specific to either Fyn or Lyn did not further inhibit RPTC proliferation than Src siRNA alone (data not shown). These results provide evidence that RPTC proliferation is specifically regulated by Src. The partial decrease in Src by siRNA is consistent with the partial decrease in RPTC proliferation.
Fig. 6.
Effect of specific siRNA on Fyn, Lyn, or Src expression. RPTC were transfected with scrambled siRNA or siRNA specific to Fyn, Lyn, or Src and then cultured for 48 h in DMEM with 0.5% FBS. Cells were harvested and immunoblot analysis was carried out using antibodies against Fyn, Lyn, Src, or actin. Representative immunoblots from 3 experiments are shown (A, C, E). Expression levels of Fyn, Lyn, or Src were quantified by densitometry, and expressed as the percentage of individual protein levels in RPTC treated with the scrambled siRNA (B, D, F).
Fig. 7.
Effect of siRNA specific to Fyn, Lyn, or Src on RPTC proliferation. RPTC were transfected with scrambled siRNA or siRNA specific to Fyn, Lyn, or Src and then cultured for 48 h in DMEM with 0.5% FBS and then incubated in 5% FBS for an additional 48 h. Cell numbers were evaluated using the MTT and Brdu incorporation assay. Data are expressed as means ± SD of the percentage of MTT activity or Brdu incorporation relative to that in cells transfected with scrambled siRNA (n = 3). *P < 0.05, compared with control groups treated with the scrambled siRNA.
Src siRNA decreases Akt phosphorylation and cyclin D1 expression, and increases p27 and p57 expression.
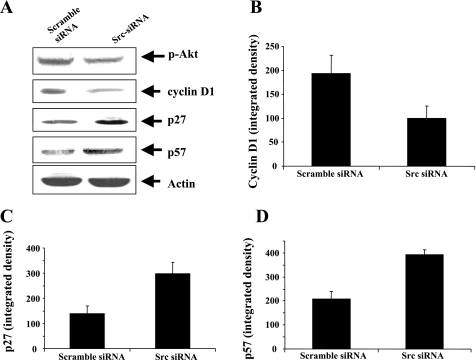
We further examined the effect of decreased Src on Akt activation and expression of cell cycle proteins that are regulated by SFKs in RPTC. As shown in Fig. 8, siRNA downregulation of Src decreased Akt phosphorylation and expression of cyclin D1, and increased p27 and p57 expression. These data are consistent with the results obtained using PP1, suggesting that Src is the major member of the SFK that mediates RPTC proliferation.
Fig. 8.
Effect of Src siRNA on phosphorylation of Akt, or ERK1/2, and expression of cyclin D1, p27, or p57. RPTC were transfected with scrambled siRNA or Src siRNA and cultured for 48 h in DMEM with 0.5% FBS. Then, cells were incubated in the DMEM with 5% FBS for an additional 48 h, and then cell lysates were prepared and subjected to immunoblot analysis using antibodies to Akt, phospho-Akt, cyclin D1, p27, p57, or actin. A: representative immunoblots from 3 or more experiments. Expression levels of cyclin D1 (B), p27 (C), or p57 (D) were quantified by densitometry and expressed as means ± SD (n = 3).
DISCUSSION
The kidney has the ability to restore the structural and functional integrity of the proximal tubule after acute injury. During the recovery process, the surviving epithelial cells dedifferentiate, proliferate, and migrate to denuded areas, and then redifferentiate to restore the integrity of the epithelium (2, 12, 28, 45). Tubular cell multiplication occurs locally in the internal milieu of the kidney and is subject to regulation by multiple growth factors following injury (2, 27, 28). Although the growth factors exert their actions through distinct receptors, they may share a common signaling pathway(s) to regulate cell proliferation. In this study, we examined the role of SFKs in proliferation of RPTC using the pharmacological inhibitor PP1 and demonstrated that SFKs are critically involved in regulation of renal epithelial cell proliferation and cell cycle protein expression. Using RNA interference technology, we further showed that Src is the member of SFKs that mediate these responses in RPTC.
Src-mediated RPTC proliferation is through PI3K-dependent and -independent mechanisms. Previously, we demonstrated that the PI3K/Akt, but not ERK, pathway mediates proliferation of rabbit proximal tubular cell in primary cultures (43). Here, we confirm this observation in RPTC and further demonstrate that Src mediates activation of the PI3K/Akt pathway and activation of Src/PI3/Akt pathway is required for upregulation of cyclin D1. As upregulation of cyclin D1 is an important mechanism that promotes cell progression, we suggest that PI3K/Akt pathway plays a role in transducing Src activation to cell cycle progression and renal epithelial cell proliferation. However, blocking the PI3K/Akt pathway only exhibited a partial inhibitory effect on cell proliferation, suggesting that other signaling pathways or mechanisms are also involved in these processes. In this regard, it has been reported that Src can activate Stat-3 and Stat-5b (1, 24), both of which mediate proliferation in other epithelial cell types (13, 25). Further studies are needed to clarify the role of these two kinases in Src regulation of mitogenic signaling in RPTC.
Src also appears to regulate cell proliferation through modulating cell cycle inhibitor proteins. Recently, it has been reported that p27 stability can be regulated by Src via phosphorylation of tyrosines 87 and 88 (4). Phosphorylation of p27 at these sites impairs the Cdk2 inhibitory action of p27 and reduces its steady-state binding to Cdk2. As a result, Cdk2 is able to phosphorylate p27 at threonine 187. Phosphorylation of p27 at threonine 187 promotes the interaction of p27 with Skp2 ligase, which results in its degradation (4). In agreement with this model, we demonstrate that blocking Src suppresses tyrosine phosphorylation of p27, phosphorylation of p27 at threonine 187, and increases expression of p27 in RPTC. In addition, we show that Src inhibition increases p57 expression and decreases its tyrosine phosphorylation. Compared with p27, the functional role of p57 is less characterized and the mechanism underlying Src regulation of p57 has not been elucidated. However, since Y88 is conserved in p57, it is possible that Src may also promote p57 proteolysis through phosphorylation and subsequent ubiquitination, similar to p27. Because expression of both p27 and p57 is associated with inhibition of cell cycle progression by regulating the progression from the quiescent state into the G1 phase and from the G1 phase into S-phase (26), we suggest that Src also regulates cell cycle progression and proliferation through p27 and p57 in RPTC.
Although p21 is expressed in renal epithelial cells, inactivation of SFKs by PP1 does not affect p21 expression. We suggest that p21 is not the target of SFKs. Interestingly, Hughes et al. (17) recently reported that a targeted disruption of the p27 gene, but not p21, decreased proliferation of renal epithelial cells following unilateral ureteric obstruction. In contrast, p21 expression has been reported to be associated with proliferation of glomerular epithelial cells and fibroblasts in the kidney (17). Therefore, the role of p21 in regulating proliferation may be dependent on the cellular context.
It has been reported that proximal tubular cells expressed high levels of active Src after ischemia-reperfusion injury (35). The remaining epithelial cells after injury may receive multiple signals to initiate proliferation. The oxidant exposure from the ischemia-reperfusion may directly activate Src or the EGF receptor (29, 34, 41). In addition, a signal may result from autocrine/paracrine mechanisms in which a growth factor is released locally and binds to a growth factor receptor. For example, it has recently been shown that RPTC proliferation can be mediated by heparin-binding EGF produced through an autocrine/paracrine mechanism (44). Finally, the bathing serum contains various growth factors that may initiate or propagate epithelial proliferation. Src is activated in response to a variety of growth factors including EGF (8), HGF (30), IGF-1 (21), and FGF (32) and the receptors for these growth factors are expressed in proximal tubular cells (27). In all of these cases, Src activation may serve as an essential and common signaling mechanism regulating renal epithelial cell proliferation.
In summary, our results demonstrated that Src-mediated proliferation occurs by PI3K-dependent and -independent pathways in RPTC. Src-dependent activation of the PI3K/Akt pathway is required for upregulation of cyclin D1, whereas PI3K/Akt-independent pathway is associated with downregulation of p27 and p57 (Fig. 9). Therefore, the potential role of Src in regulating renal regeneration warrants investigation in an animal model of ischemia-reperfusion-induced acute kidney injury.
Fig. 9.
Schematic illustration of Src-mediated signaling pathway leading to RPTC proliferation. Src is activated in response of the bath serum containing a variety of growth factors (GF). Src-dependent activation of the PI3K/Akt pathway is required for upregulation of cyclin D1, whereas PI3K/Akt-independent pathway is associated with downregulation of p27 and p57.
GRANTS
This work was supported by National Institutes of Health Grant DK-071997.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amorino GP, Deeble PD, Parsons SJ. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene 26: 745–756, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14, Suppl 1: S55–S61, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23: 7957–7968, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, Sun P, Tan CK, Hengst L, Slingerland J. p27 Phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128: 281–294, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtneidge SA, Fumagalli S, Koegl M, Superti-Furga G, Twamley-Stein GM. The Src family of protein tyrosine kinases: regulation and functions. Dev Suppl 57–64, 1993. [PubMed]
- 6.Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513–44521, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Z, Kumar A, Marques M, Cortes I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J 25: 655–661, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golias CH, Charalabopoulos A, Charalabopoulos K. Cell proliferation and cell cycle control: a mini review. Int J Clin Pract 58: 1134–1141, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jakel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128: 269–280, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hallman MA, Zhuang S, Schnellmann RG. Regulation of de-differentiation and re-differentiation in renal proximal tubular cells by the EGF receptor. J Pharmacol Exp Ther In press. [DOI] [PubMed]
- 13.Hambek M, Baghi M, Strebhardt K, May A, Adunka O, Gstottner W, Knecht R. STAT 3 activation in head and neck squamous cell carcinomas is controlled by the EGFR. Anticancer Res 24: 3881–3886, 2004. [PubMed] [Google Scholar]
- 14.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271: 695–701, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Haskell MD, Slack JK, Parsons JT, Parsons SJ. c-Src tyrosine phosphorylation of epidermal growth factor receptor, P190 RhoGAP, and focal adhesion kinase regulates diverse cellular processes. Chem Rev 101: 2425–2440, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol 194: 243–256, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hughes J, Brown P, Shankland SJ. Cyclin kinase inhibitor p21CIP1/WAF1 limits interstitial cell proliferation following ureteric obstruction. Am J Physiol Renal Physiol 277: F948–F956, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell 6: 209–214, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kefalas P, Brown TR, Brickell PM. Signalling by the p60c-src family of protein-tyrosine kinases. Int J Biochem Cell Biol 27: 551–563, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Kyosseva SV Mitogen-activated protein kinase signaling. Int Rev Neurobiol 59: 201–220, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR. The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J Biol Chem 281: 25041–25053, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Gac L, Alvarez B, Garcia Z, Marques M, Arrizabalaga M, Carrera AC. Phosphoinositide 3-kinase and Forkhead, a switch for cell division. Biochem Soc Trans 32: 360–361, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26: 3227–3239, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Mirmohammadsadegh A, Hassan M, Bardenheuer W, Marini A, Gustrau A, Nambiar S, Tannapfel A, Bojar H, Ruzicka T, Hengge UR. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 kinases. J Invest Dermatol 126: 2272–2280, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol 155: 531–542, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickeleit I, Zender S, Kossatz U, Malek NP. p27kip1: a target for tumor therapies? Cell Div 2: 13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigam S, Lieberthal W. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol 279: F3–F11, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Nony PA, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther 304: 905–912, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Otani H Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal 6: 449–469, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Rahimi N, Hung W, Tremblay E, Saulnier R, Elliott B. c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J Biol Chem 273: 33714–33721, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol 22: 7226–7241, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodier JM, Valles AM, Denoyelle M, Thiery JP, Boyer B. pp60c-src is a positive regulator of growth factor-induced cell scattering in a rat bladder carcinoma cell line. J Cell Biol 131: 761–773, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem 277: 45680–45687, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases. Antioxid Redox Signal 5: 789–794, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol 163: 277–286, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609, 1997. [DOI] [PubMed] [Google Scholar]
- 37.van den Heuvel S Cell-cycle regulation. WormBook September 21: 1–16, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248, 1994. [PubMed] [Google Scholar]
- 39.Wilson MB, Schreiner SJ, Choi HJ, Kamens J, Smithgall TE. Selective pyrrolo-pyrimidine inhibitors reveal a necessary role for Src family kinases in Bcr-Abl signal transduction and oncogenesis. Oncogene 21: 8075–8088, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Yamada T, Aoyama Y, Owada MK, Kawakatsu H, Kitajima Y. Scraped-wounding causes activation and association of C-Src tyrosine kinase with microtubules in cultured keratinocytes. Cell Struct Funct 25: 351–359, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Yano T, Yazima S, Hagiwara K, Ozasa H, Ishizuka S, Horikawa S. Activation of epidermal growth factor receptor in the early phase after renal ischemia-reperfusion in rat. Nephron 81: 230–233, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem 281: 19501–19511, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang S, Dang Y, Schnellmann RG. Requirement of the epidermal growth factor receptor in renal epithelial cell proliferation and migration. Am J Physiol Renal Physiol 287: F365–F372, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang S, Kinsey GR, Rasbach K, Schnellmann RG. Heparin binding epidermal growth factor and Src family kinases in proliferation of renal epithelial cells. Am J Physiol Renal Physiol In press. [DOI] [PubMed]
- 45.Zhuang S, Yan Y, Han J, Schnellmann RG. p38 kinase-mediated transactivation of the epidermal growth factor receptor is required for dedifferentiation of renal epithelial cells after oxidant injury. J Biol Chem 280: 21036–21042, 2005. [DOI] [PubMed] [Google Scholar]