Abstract
The kidney is capable of regeneration following injury, particularly following acute insults. Although the mechanisms underlying cellular regeneration are incompletely understood, emerging evidence suggests a role for cells of renal origin in the repair and replacement of damaged renal tubule cells. The overall hypothesis of this study is that native kidney cells that reside in a niche in the kidney provide robust contribution to the repair of kidney tubules following injury. To test this hypothesis, we utilized a model of renal ischemia-reperfusion injury that results in extensive morphological changes, particularly in the outer medulla. Renal tissue obtained from mice constitutively expressing Escherichia coli β-galactosidase (ROSA26) was dissected from the cortex, outer medulla, or papilla and implanted under the renal capsule of the injured mice. Mice were allowed to recover for 7 days. Sections through the injured kidney demonstrated the presence of implant-derived cells in renal tubules in the outer medulla. The implanted renal region that exhibited the most robust response was the papilla, whereas tissue pieces from the cortex and outer medulla showed less contribution to recipient renal tubules. These results provide proof-of-principle evidence that renal-derived reparative cells reside in all regions of the kidney, perhaps more predominantly in the renal papilla. A greater understanding of the cell biology of renal repair by native kidney cells will provide further insight into the design of novel therapies in acute kidney injury, and the subcapsular implant technique described in this study may offer unique advantages to evaluate renal repair mechanisms.
Keywords: epithelial repair, ischemia-reperfusion injury, progenitor cells
therapy for acute kidney injury (AKI) remains a formidable challenge. Morbidity and mortality levels in AKI have not been altered significantly even with numerous investigations into the pathology of and therapeutic intervention in the disease. Despite the known regenerative capacity of the kidney to acute insults, the mechanisms of cellular repair remain elusive.
AKI results in cell death within the renal epithelium, and reparative cells must cover the denuded basement membrane and reestablish the normal cellular architecture of the tubule. Investigation of the process of this cellular reconstitution of the nephron following AKI has led to a variety of hypotheses as to the source of these reparative cells. Early work in the identification of cellular repair of the kidney focused on the role of bone marrow-derived mesenchymal stem cells, which may provide protective humoral or paracrine effects in the kidney after AKI (11, 12). In rodent studies, evidence exists to suggest cellular repair by surviving adult epithelial cells (4, 6). Furthermore, isolated renal cells grown in culture, which demonstrate some characteristics consistent with a renal stem cell derivation, have shown a capacity to incorporate into the nephron following AKI (1, 2, 5, 8). It is possible that more than one mechanism of tubule repair is employed following AKI, with the predominant mechanism dependent on the degree or type of injury. To date, consensus regarding the source and identity of reparative cells in AKI has not been reached.
The subcapsular implant model described in this study provides a simple approach with potential clinical application. This technique allows for introduction of cells or tissues as a source of reparative cells for scientific investigation as well as potential clinical therapy. Because an intact tissue is implanted, no bias for or against any renal cell population is introduced, and the cells remain in juxtaposition with different neighboring cells in an in vivo configuration. The study presented describes the implant method and its use in identifying renal regions that potentially contain reparative cells that are responsive to AKI.
MATERIALS AND METHODS
All protocols used in these studies were approved by the University of Alabama at Birmingham's Institutional Animal Care and Use Committee.
Preparation of implant tissue.
Kidneys were removed from healthy untreated mice (12-wk-old female ROSA26) that constitutively express Escherichia coli β-galactosidase (β-gal), providing a “marked” cellular source. The renal capsule was removed, and slices (∼1–2 mm) were prepared. Renal tissue pieces were then dissected from the cortex, outer medulla, and papilla under a surgical microscope.
Ischemia-reperfusion renal injury.
Male C57BL/6J mice (12 wk old) were subjected to right nephrectomy followed by left renal ischemia for 30 min (10). At the end of ischemia, reperfusion was confirmed visually. This model of ischemia-reperfusion renal injury (IRI) was previously confirmed by harvesting kidneys and serum at 24 and 48 h and evaluating biochemical and histological parameters (10). Animals undergoing sham surgery were used as controls.
Subcapsular implantation of marked tissue.
Immediately following reperfusion, IRI mice (n = 9) were implanted with marked tissue pieces. Under an operating microscope, a small breach was made in the renal capsule on the lateral surface of the lower pole of the injured kidney. Renal tissue pieces (n = 2–3) from the cortex, outer medulla, or papilla were inserted beneath the renal capsule (Fig. 1) immediately after return of blood flow to the kidney. The breach in the capsule was cauterized, the kidney was repositioned, and the incision was closed in layers. Sham mice (n = 3) were similarly implanted. A separate group of mice underwent IRI (n = 1) or sham injury (n = 1) without implantation of renal tissue pieces.
Fig. 1.
Diagram of the subcapsular implant technique. Ischemia-reperfusion injury (IRI) was induced in C57BL/6 mice by clamping of the renal pedicle for 30 min (A). Fragments of renal tissue from the cortex, outer medulla, or papilla were dissected from kidneys of ROSA26 mice, which constitutively express Escherichia coli β-galactosidase (β-gal) and provide a marked tissue source (B). Immediately following reperfusion, marked renal tissue pieces from one of the regions were implanted under the kidney capsule of the IRI mouse (C). Seven days later, the IRI kidney was removed and sectioned. Tubules in cross sections exhibited implant-derived cells (D).
Evaluation of β-gal enzyme activity and immunofluorescence to identify nephron regions.
At 7 days post-IRI, kidneys were removed, placed in fixative (2% paraformaldehyde in PBS for 2 h), and processed for frozen embedding in preparation for the detection of E. coli β-gal enzyme activity to identify implant-derived cells and immunofluorescence to identify specific regions of the nephron. To evaluate the β-gal activity, Xgal solution [60 mM Na2HPO4, 34 mM NaH2PO4, 2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 1 mg/ml Xgal, pH 7.4] was incubated at room temperature for 16–18 h on frozen sections (5 μm), conditions determined to be optimal for detection of E. coli β-gal reactivity exclusive of mammalian β-gal reactivity. Positive and negative controls for the Xgal reaction were run in parallel to establish positive reactivity (ROSA26 kidney section) and negative reactivity (C57BL/6 kidney section). As an additional negative control, kidneys from C57BL/6 mice that underwent IRI or sham injury but did not receive implanted tissues were similarly incubated with the Xgal solution to identify any false-positive reaction product. After washing, sections were subsequently processed for immunofluorescence. Immunostaining for prominin-1 (CD133/AC133) was performed on sections that were pretreated with 0.1% Triton X-100 in PBS for 10 min. Immunostaining for stem cell antigen 1 (Sca-1) required no pretreatment. Sections stained for aquaporin-2 (AQP2) were pretreated with 1% SDS in PBS for 5 min. In all cases, nonspecific antibody binding was blocked by subsequent incubation of the sections with 1.5% normal horse serum in PBS for 1 h, and the primary antibodies to prominin-1 (unlabeled rat isotype IgG1; eBiosciences, San Diego, CA; 2 μg/ml), Sca-1 (fluorescein-labeled rat isotype IgG2a; BD Biosciences, San Jose, CA; 10 μg/ml), and AQP2 (unlabeled goat IgG; Santa Cruz Biotechnology, Santa Cruz, CA; 4 μg/ml) were incubated overnight at 4°C. For prominin and AQP2 immunostaining, secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), fluorescein-labeled donkey anti-rat IgG (prominin-1), or fluorescein-labeled donkey anti-goat IgG (AQP2) were subsequently incubated on the sections. Sections were mounted with VectaShield (Vector Laboratories, Burlingame, CA) and examined using standard microscopy (Leica DM IRB). To confirm the specificity of the primary antibody binding, tissue sections were incubated in parallel with nonspecific isotype IgG or without primary antibody. These control sections demonstrated no fluorescent label (data not shown).
RESULTS AND DISCUSSION
The present study demonstrates a novel technique (Fig. 1) to examine the contribution to cellular repair following AKI by subcapsular implantation of a labeled tissue source immediately following IRI. After 7 days, cells derived from the E. coli β-gal+ (marked) implanted tissue source were localized within the native kidney. Surprisingly, reparative cells from all gross regions of the kidney were capable of migrating and incorporating into the regenerating tubule.
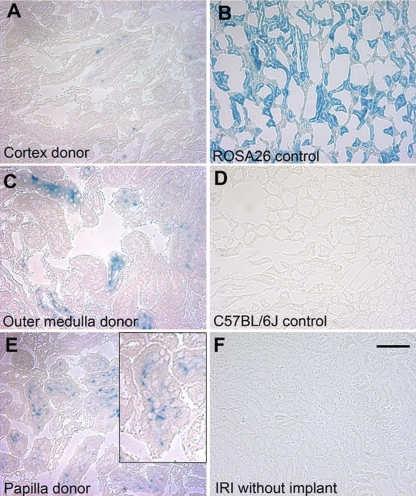
E. coli β-gal reactivity demonstrated that the kidney implants retained Xgal reactivity at 7 days post-IRI, suggesting continuing viability of the implanted tissue (Fig. 2). Implant-derived cells were detected in the outer medullary region of the IRI kidneys that received implants of cortex (Fig. 3A), outer medulla (Fig. 3C), or papilla (Fig. 3E). A more robust response was seen in mice that received an implant of papilla than of cortex or outer medulla tissue. Infrequently, labeled implant-derived cells were identified in sham-operated animals that received implanted tissue pieces after undergoing nephrectomy without contralateral IRI (data not shown). An injury response has been detected previously in sham mice (10). The positive reaction was evident in kidney sections of a ROSA26 mouse, the mouse strain that served as the marked tissue source (Fig. 3B), whereas no positive signal was observed in the C57BL/6 mouse, which served as the IRI mouse (Fig. 3D), or in a C57BL/6 mouse that underwent IRI but did not receive an implant (Fig. 3F). These results indicate that the blue reaction product from E. coli β-gal reactivity in the outer medulla of the experimental mice was specific to the implant-derived cells.
Fig. 2.
Representative image of a subcapsular implant at 7 days post-IRI. Reaction product (blue) resulting from incubation of a kidney section with Xgal solution demonstrates the presence of β-gal activity remaining in the implanted tissue. Implanted renal tissue is indicated by an asterisk; the demarcation between the implant and the native kidney is indicated by the arrows. The image was obtained at ×100 magnification; the scale bar represents 100 μm.
Fig. 3.
Implant-derived cellular contribution to the epithelium of the outer medullary region of IRI kidney. Product (blue) from E. coli β-gal enzyme activity demonstrates implant-derived cells in the outer medullary region of kidney sections from IRI mice (C57BL/6J) that received a subcapsular implant of cortex (A), outer medulla (C), or papilla (E) tissue from a ROSA26 mouse. Inset in E is a higher magnification of the positive signal. A control section through the kidney of a ROSA26 mouse (B) demonstrates the positive signal from the Xgal reaction. A negative control section through the kidney of a C57BL6 mouse (D) demonstrates no label from the Xgal reaction. Similarly, a section through the kidney of a mouse that underwent IRI but did not receive an implant (F) shows no reactivity to Xgal. All images were obtained at ×400 magnification; the scale bar in F represents 50 μm.
Cellular repair was seen predominantly in the proximal tubule, a major site of cellular injury in AKI. After incubation of tissue sections with Xgal solution to elucidate β-gal enzyme activity (Fig. 4, A, C, and E), labeling of the same sections for prominin-1, which is expressed in the proximal tubule, demonstrated a colocalization of implant-derived cells in the proximal tubule (Fig. 4B). Similar labeling of Sca-1, which localizes to the distal tubule (Fig. 4D), or AQP2, which is found in the collecting duct (Fig. 4F), demonstrated few, if any, implant-derived cells in the distal nephron or collecting duct.
Fig. 4.
Histochemical detection of β-gal+ implant cells by enzyme activity and immunolabeling of markers of specific nephron regions within the same section of tissue. Prominin-1 (B), a marker of proximal tubule, stem cell antigen 1 (Sca-1; D), a marker of distal tubule, and aquaporin-2 (AQP2; F), a marker of collecting duct, were identified using specific antibodies in sections that previously were reacted with Xgal (A, C, and E) to demonstrate implant-derived cells (blue reaction product). Side-by-side images are the same field taken under phase (left) and standard fluorescence microscopy (right). Arrows indicate tubules that are positive for Xgal reaction product; arrowheads outline tubules in both phase and fluorescent images that are positive for the marker but do not exhibit reaction product from the Xgal reaction. All images were obtained at ×400 magnification; the scale bar in E represents 50 μm.
These results provided proof-of-principle evidence that cells from all gross regions of the kidney have the capacity to contribute to cellular reconstitution of the nephron following AKI, particularly in the proximal tubule. Findings of contribution to cellular repair by all gross regions of the kidney are consistent with results of prior studies using bromodeoxyuridine (BrdU) retention in slow-cycling cells. These BrdU label-retaining cells were identified predominantly in the outer medulla (7) or in the papilla (8) in two contrasting studies; both populations appear to undergo proliferation after IRI.
Differences seen with the regional sources of implanted tissue may be due to variable potential for contribution to repair. The ability of these cells to respond to injury signals by migration and/or proliferation once incorporated may vary. In addition, variability in the response may be due to differences in the inherent tissue structure within these regions. For example, the amount of interstitial space, as well as the concomitant interstitial matrix, differs between the cortex and medullary regions (3) and may contribute to differences in the capacity of cells in these regions to migrate from the implant into the recipient tissue. Because of the inherently lower oxygen tension of the papilla, it also is possible that the papillary tissue is better able to withstand the ischemia associated with the implantation process. Alternatively, the greater response by papillary tissue may reflect a survival response by cells that are accustomed to a less oxygen-rich environment.
AKI results in significant tubular cell death during the injury phase and subsequent cell migration and proliferation as part of the resolution of the injury and the repair phase. Several signaling molecules have been identified as potential mediators of AKI or subsequent cellular repair processes. Injured tissues release a variety of factors, including growth factors, cytokines, and chemokines, some of which may signal for an immune response during the injury phase and some of which may be important to initiate the migration, epithelial incorporation, and differentiation by reparative cells. Importantly, this technique allows for the separation of the injury signal, which derives from the recipient IRI kidney, from the microenvironment of the reparative cell, which lies in the marked tissue implant. The ability to isolate the two aspects of injury and repair may allow for specific investigation of injury signals and repair responses and for the interaction between these. For example, the use of implanted tissue from a transgenic mouse null in a candidate mediator may allow for examination of the necessity and sufficiency of this mediator in initiating cellular repair. The subcapsular implantation technique also allows examination of reparative cells without selection or bias toward one population of renal cells while maintaining cell-cell and cell-matrix interactions that may be important for eliciting the reparative response. Simultaneous examination of individual cell types separately or in competition can be accomplished by implanting differently marked tissues from two donors into the same unmarked IRI recipient. Finally, because the subcapsular space is accessible in human kidneys, this method holds the promise of clinical application, particularly in the setting of kidney transplantation.
In summary, these studies demonstrate a unique and potentially clinically relevant technique to study cellular repair following AKI. Our results indicate the potential for contribution to renal epithelial repair by endogenous renal cells that reside in all three gross regions of the kidney. These reparative cells may be renal epithelial or interstitial cells or may be progeny that derive from a renal stem cell. The renal papilla may contain a significant niche of renal reparative cells, since it provided a robust response in migration to and epithelial incorporation into the outer medulla; however, all three regions were capable of contributing to cellular reconstitution of the nephron following IRI. Although these studies do not exclusively support the existence of a renal stem cell population, it is noteworthy that the renal papilla has a lower oxygen tension, which is a characteristic of the bone marrow stem cell niche (9). Regardless of whether or not the reparative cells of the kidney derive from a renal stem cell, the existence of a lower oxygen tension in the papilla may provide an environment for reparative cells of the kidney that is more protected from IRI. These findings may indicate multiple renal reparative cell populations residing in different locations in the kidney or a single renal reparative cell population that is present throughout the kidney. Future studies to identify the specific cells capable of renal epithelial repair within each gross region of the kidney may provide the foundation necessary for cell therapy in AKI.
GRANTS
This work was supported by a University of Alabama at Birmingham Department of Medicine Multi-investigator Planning Grant through the Nephrology Research Training Center, as well as National Institutes of Health (NIH) Grants R01-DK-46199 (P.W. Sanders) and R01-DK-59600 (A. Agarwal). L. M. Curtis was supported by NIH Training Grants T32 AR07450-23 in Rheumatic Diseases Research and T32 DK07545-17 in Cellular Physiology.
Acknowledgments
We acknowledge David Doo for technical assistance in acquiring these data and David Fisher (Medical Education and Design Services, University of Alabama at Birmingham) for contributions to Fig. 1.
Present address of S. Chen: Fudan University, Shanghai, China.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166: 545–555, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol 17: 3028–3040, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Howie AJ, Lote CJ. Interactions between renal tubules and interstitium. J Clin Pathol 49: 783–786, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J 19: 1789–1797, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Lin F, Patel V, Li L, Shao X, Igarashi P. Direct evidence of renal regeneration from mature tubular epithelial cells in mice expressing tamoxifen-inducible epithelial specific cre recombinase (creERT2Ksp) (Abstract). J Am Soc Nephrol 17: 662A, 2006. [Google Scholar]
- 7.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14: 3138–3146, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 104: 5431–5436, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts J, Chen B, Curtis LM, Agarwal A, Sanders PW, Zinn KR. Detection of early changes in renal function using 99mTc-MAG3 imaging in a murine model of ischemia-reperfusion injury. Am J Physiol Renal Physiol 293: F1408–F1412, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007. [DOI] [PubMed] [Google Scholar]