Abstract
Aldosterone is the principal regulator of Na homeostasis, and thereby blood pressure. One of the main targets of aldosterone is the epithelial Na channel (ENaC) located in the apical membrane of target cells. Previous studies identified several genes involved in the regulation of ENaC such as SGK1; however, SGK1 knockout mice have only a mild salt-losing phenotype, indicating that further genes must be involved in the action of aldosterone. In our search for further aldosterone-regulated genes, we discovered that aldosterone, at physiological concentrations, induces the expression of the promyelocytic leukemia zinc finger protein (PLZF) in renal cortical collecting duct (CCD) cell lines that stably express mineralocorticoid receptors (MRs). This effect is rapid and does not require de novo protein synthesis, suggesting a direct action. Surprisingly, stable overexpression of human or mouse PLZF isoforms significantly decreased transepithelial Na transport in CCD cells while having no effect on the integrity of the monolayers. In parallel with the decline in Na transport, PLZF suppressed the mRNA levels of β- and γ-ENaC subunits. These observations suggest that PLZF is a negative regulator of ENaC in renal epithelial cells and might be part of a negative feedback loop that limits aldosterone's stimulatory effects on sodium reabsorption.
Keywords: aldosterone, kidney, epithelia, gene induction
aldosterone, the main mineralocorticoid hormone, is a key regulator of Na homeostasis and consequently plays a central role in the regulation of blood pressure. High blood pressure affects ∼25% of the adult population in industrialized countries, but the etiology of most cases of hypertension remains unknown. Importantly, mutations in monogenic forms of hypertension converge on the pathway of aldosterone-regulated renal Na reabsorption. Our understanding of the molecular events that occur between aldosterone binding to the mineralocorticoid receptors (MR) and the resulting stimulation of epithelial Na channel (ENaC) activity is still incomplete. A better understanding of these steps is important, since activating mutations in proteins involved in the cascade of events initiated by aldosterone could lead to the development of high blood pressure.
Most, if not all, of the actions of aldosterone on Na transport are mediated through transcriptional events (29). An increase in ENaC activity can be observed as early as 1.5 h following aldosterone, a time point at which increased transcription or translation of ENaC subunits was not yet observed (28). Consequently, it is generally assumed that the initial response to aldosterone is mediated through “early-response” genes encoding ENaC-regulatory proteins (25). Recent work demonstrated that the serum- and glucocorticoid-induced kinase 1 (SGK1) is rapidly induced by aldosterone (5, 19, 24) and, at least in cultured cells, SGK1 is critical for steroid-induced epithelial Na transport (1, 13). Although SGK1 knockout mice exhibit impaired renal Na retention (30), the Na-wasting phenotype is milder than that of MR knockout mice, which die shortly after birth (3). These results suggest that SGK1 is an important mediator of, but does not fully account for, mineralocorticoid regulation of Na homeostasis. Indeed, work by others and by our laboratory identified several aldosterone-induced gene products that have been shown to affect Na transport in epithelial cells, among them the kidney-specific (KS) WNK1 (21), GILZ (26), or the recently described ubiquitin-specific protease Usp2-45 (8).
To search for further genes that might mediate the early effects of aldosterone, we used a novel cortical collecting duct (CCD) cell line that stably expresses MR (21). Microarray analysis revealed several genes that were rapidly upregulated by aldosterone, among them the promyelocytic leukemia zinc finger protein (PLZF; also called ZBTB16).
PLZF is a transcriptional repressor that belongs to the family of Krüppel-like zinc finger proteins. It has nine C2H2-type zinc fingers and an NH2-terminal BTB/POZ domain (12, 16) that mediates homo- and heterodimerization, nuclear localization, and binding of corepressors such as NCoR or SMRT (10, 11). PLZF is involved in cell cycle control, cell differentiation, and limb development (2). Relatively little is known about the regulation of PLZF expression, except that it is induced by activated steroid hormone receptors that bind to similar response elements as the MR, i.e., by progesterone and glucocorticoids in the uterus (7) and by androgens in the prostate (15). However, the role of aldosterone in the regulation of PLZF expression and the function of PLZF in epithelial cells remain to be established. The goal of the present work was to verify regulation of PLZF by aldosterone in renal collecting duct cells and to examine the effects of PLZF on sodium transport in these cells. We report here that PLZF is an aldosterone-regulated gene and that it inhibits the activity of ENaC and this action involves transcriptional suppression of two ENaC subunits.
MATERIALS AND METHODS
M1 cell lines stably expressing MR.
M1 cell lines that constitutively express the rat MR have been described previously (21). These cell lines will be referred to as M1-MR+ cells. M1 cells expressing a tetracycline (Tet)-inducible MR have been generated recently in our laboratory (17). These cells will be referred to as M1/TetON-MR cells.
Generation of ml cell lines stably expressing a Tet-inducible PLZF.
The human PLZF pIRES2-EGFP construct was generously provided by Drs. Z. Wang and A. J. Saporita (Dept. of Urology, Northwestern University, Chicago, IL), while the mouse PLZF2 (RIKEN Mouse Fantom PS Clone ID A430077H20; GenBank AK040211) in the FPLC1 vector was obtained from DNAForm (Tokyo, Japan). The human PLZF was subcloned into the pREV-SG-TRE retroviral construct by ligating an EcoRI-BamHI fragment of hPLZF in pIRES2-EGFP into MluI-BamHI-cut pRev-SG-TRE. Mouse PLZF2 was also subcloned into the retroviral pREV-SG-TRE vector. Details of construct generations are available on request. Retroviri were generated in amphotropic Phoenix cells, and M1-pBTE cells were cultured and infected with retroviri as described above. Clones stably expressing the Tet-inducible PLZF were selected with 200 μg/ml hygromycin and expanded. The cell lines expressing the inducible human PLZF or mouse PLZF-2 will be referred to as M1/TetON-hPLZF and M1/TetON-mPLZF2 cell lines, respectively.
Cell culture, RNA preparation, and electrophysiology.
Parent M1 cells, M1/TetON-hPLZF, and M1/TetON-mPLZF2 clones were seeded onto Millicell permeable membranes (Millipore, Bedford, MA) at a density of 4 × 105 cells/filter. After seeding, cells were bathed in complete PC1 medium (BioWhittaker, Walkersville, MD) with 5% FBS until they formed confluent monolayers (∼3–4 days). After reaching confluence, cells were maintained in steroid-free medium (composed of DMEM/F12, 5% FBS stripped three times with charcoal to remove steroids, 15 mM HEPES, 2 mM glutamine, and antibiotics) for 2 days, and then treated (or not) with 500 ng/ml doxycycline (Dox) for different periods to induce the expression of PLZF.
To determine the effect of aldosterone on PLZF mRNA expression levels, M1/MR+ cells (21) or M1/TetOn-MR cells (17) were grown to confluence in PC1 medium and then maintained in a steroid-free medium for 2 days. For M1/MR+ cells that express MR constitutively, the medium was then changed to one containing 0–10 nM aldosterone for different times. In M1/TetOn-MR cells, expression of MR was induced first by maintaining the cells in steroid-free medium with 500 ng/ml Dox for 24–48 h; then, cells were treated with 0–10 nM aldosterone. The effect of protein synthesis inhibitors on the induction of PLZF by aldosterone was tested by preincubating cells for 30 min with vehicle, 5 μg/ml of cycloheximide (CHX), or 10 μM anisomycin; then, 1 nM aldosterone or vehicle was added, and cells were incubated for an additional 2 h before RNA was prepared. Cells were lysed in Tri-Reagent, total RNA was prepared, and cDNA was synthesized as described elsewhere (9).
To determine whether the suppression of ENaC mRNA by PLZF requires histone deacetylation, M1-PLZF cells were grown in complete medium until reaching confluence. Cells were then steroid-starved for 48 h before treatment with either of the following agents or combinations: 1) 500 ng/ml Dox; 2) 100 ng/ml trichostatin A (TSA); 3) 100 μM curcumin; 4) Dox+TSA; 5) Dox+curcumin; or 6) steroid-free medium alone. After 24 h, RNA was extracted from the cells using Tri-Reagent.
Transepithelial voltage (VTE) and resistance (RTE) values were determined with an epithelial voltohmeter (World Precision Instruments, Sarasota, FL), and the equivalent short-circuit current was (ISC) calculated. We previously demonstrated that in M1 cells ISC mainly represents transepithelial Na current (20). This was verified by treating cells with 1 μM amiloride from the apical side, which resulted in an almost total elimination of the lumen negative VTE and associated Isc.
Real-time PCR.
To determine the levels of the full-length human PLZF (FL-hPLZF), full-length mouse PLZF (FL-mPLZF), the COOH terminally truncated splice variant mPLZF2, and ENaC subunit mRNAs, we used real-time quantitative RT-PCR. For FL-hPLZF expression, a 194-bp product was amplified by the primers: sense (5′-TGC ACA GTT TTC GAA GGA GGA TG -3′) and antisense (5′-GGC TGG GGA AGG TGC GGT TG -3′). To determine the relative expression of mouse PLZF isoforms, we designed two primer pairs that specifically amplify either the FL-mPLZF (GenBank AK142947) or mPLZF2 (GenBank AK040211). Primers sense 5′ TCA GGC ACA AAG CGC ACT C-3′ and antisense 5′-GCA CTC AAA GGG CTT CTC A-3′ amplify a 315-bp product only from the FL-mPLZF, whereas primers sense 5′-TCA GGC ACA AAG CGC ACT C-3′ and antisense 5′-TCT CTC CGG TTG AGC CTT GAA C-3′ amplify a 399-bp amplicon only from mPLZF2.
For α-ENaC expression, a 159-bp product was amplified with the primers sense 5′-GGC AGC CCA CCG AGG AGG A-3′ and antisense 5′-GCC ACA GCA CCG CCC AGA A-3′. An 84-bp product of β-ENac was amplified with the primers sense 5′-CAG TTG CCA TAA TCA GGG TAG AAG A-3′ and antisense 5′-CAT AAT CCT AGC TTG CCT GTT TGG A-3′. A 146-bp product of γ-ENaC was amplified with the primers sense 5′-TCA GAA GCC TCA GAT GGC CAC T-3′ and antisense 5′-CCC AAC TGG ATG TAT TGC TAC T-3′. The expression of β-actin was determined using primers sense 5′-CAA TAG TGA TGA CCT GGC CGT-3′ and antisense 5′-AGA GGG AAA TCG TCG GTG AC-3′ that amplified a 138-bp product. β-Actin mRNA levels were similar in both Dox-treated and control groups.
Real-time PCR was performed in triplicate using iTaq SYBR Green Supermix with Rox (Bio-Rad, Hercules, CA) according to the manufacturers' protocol with 200 nM of each sense and antisense primer and 30–40 ng of cDNA. The thermal cycling parameters were denaturation at 95°C for 3 min, followed by 40 cycles at 95°C for 15 s, 59°C (for hPLZF), or 57°C (for mPLZF isoforms and for ENaC) for 30 s. The PCR products and threshold cycle were determined and analyzed using AB 7300 (Applied Biosystems, Foster City, CA). Changes between the ENaC mRNA levels were based on the number of cycles at which the amplified product reached threshold (Ct). Relative expression (RE) of the mRNA was quantified using the equation RE = 2 − (Ct gene of interest − Ct β-actin). To apply the equation RE = 2 − (Ct gene of interest − Ct β-actin), we calculated the PCR efficiency of each amplicon from standard curves based on a dilution series. The results were graphed as the log of ng cDNA vs. Ct: efficiency = 10(−1/slope). The calculated PCR efficiency for each ENaC amplicon and β-actin amplicon was 2.0. To initially verify the results from the real-time PCR, traditional PCRs were performed with the cDNA. Reactions were performed under standard conditions with AmpliTaq DNA polymerase (Roche, Indianapolis, IN). Furthermore, to verify that the real-time signal did not contain signal from nonspecific amplification, a dissociation stage was performed: 95°C for 15 s, 59°C (for hPLZF) and 57°C (for mPLZF and ENaC) for 30 s, and 95°C for 15 s.
PLZF immunoblotting.
Fifteen micrograms of total protein from M1/Tet-ON/hPLZF or M1/Tet-ON/mPLZF2 cells was separated on 10% SDS-PAGE gel and transferred to an Immobilon-P membrane (Millipore). The membrane was incubated for 1 h at room temperature in blocking buffer (5% dry milk, 150 mM NaCl, 0.05% Tween, 124 μM thimerosal, and 10 mM Tris, pH 7.5) and then for 1 h at room temperature with a 1:1,000 dilution of the mouse monoclonal anti-PLZF antibody (Calbiochem) that recognizes human, mouse, and rat PLZF. After sufficient washing, the membrane was incubated at 1:5,000 dilution of horseradish peroxidase-linked rabbit anti-mouse antibody (Cell Signaling, Beverly, MA) for 45 min at room temperature. Signals were visualized using SuperSignal West Dura substrate (Pierce, Rockford, IL) and scanned using the FluoroChem 8900 imaging system (Alpha Innotech, San Leandro, CA) in conjunction with the ChemiImager 5500 software (Alpha Innotech).
RESULTS
Aldosterone rapidly induces PLZF mRNA expression in renal CCD cells.
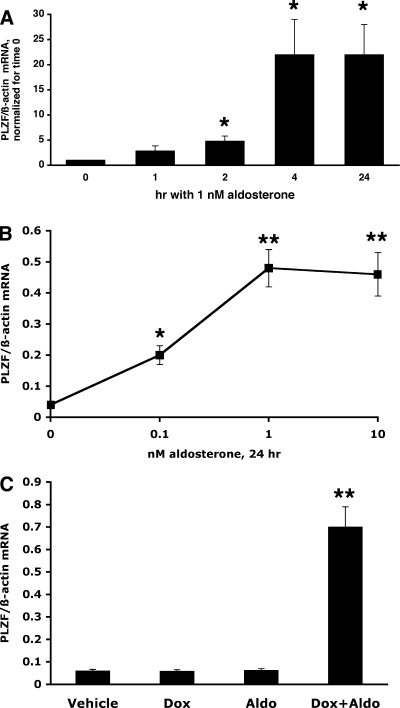
We used two M1 CCD cell lines that stably express MRs to test the effect of aldosterone on the expression of PLZF mRNA in mineralocorticoid target cells. The first such cell line, called M1/MR+, expresses MR constitutively and has been characterized previously (21). As shown in Fig. 1A, aldosterone at a physiological concentration significantly induced the expression of PLZF mRNA as early as 2 h after hormone treatment. Interestingly, the induction was not only maintained but further increased for up to 24 h, reaching a mean increase of ∼20-fold at 24 h.
Fig. 1.
A: Aldosterone induces promyelocytic leukemia zinc finger protein (PLZF) expression in M1-MR+ cells. Cells were grown for 48 hrs in steroid-free medium, and then medium was changed to the same (control) or supplemented with 1 nM aldosterone, for 1–24 h. PLZF and β-actin mRNA levels were determined by RT-PCR from the same samples. Values are RE (relative expression) of PLZF/β-actin mRNA, and are expressed as fold change. *, P < 0.05 vs. time 0, using 1-tailed unpaired t-test; n = 5 for time 0 and 2 h; n = 3 for other time points. B: Dose-response effect of aldosterone on PLZF mRNA levels in M1/TetON-MR cells. Cells were treated with 500 ng/ml dox for 2 days to induce MR expression, then with 0–10 nM aldosterone for an additional 24 h, and PLZF RNA levels determined as above. Values shown are RE *, P < 0.05 vs. untreated cells using 1-tailed unpaired t-test, n = 3. C: effect of aldosterone is mediated via the MR. Cells were incubated with or without 500 ng/ml dox for 2 days, then with 1 nM aldosterone for an additional 24 h. Aldosterone increased PLZF expression only when MR expression was induced by dox. n = 3 for each treatment. **, P < 0.01 vs. untreated cells using 2-tailed unpaired t-test.
To further validate results obtained with the M1/MR+ cell line, in this study we also used another M1 cell line that expresses a Tet-regulated MR. In this cell line, referred to as M1/TetON-MR cells, in the absence of tetracycline or its derivative, Dox, no specific aldosterone binding or MR transcriptional activity can be detected. However, after 24 h of induction with 500 ng/ml Dox, MRs are expressed as determined by [3H]aldosterone binding and functional studies (17).
Since aldosterone can also bind to the glucocorticoid receptor (GR), albeit with lower affinity than to the MR, it was important to examine the concentration dependency of the aldosterone effect on PLZF induction. As illustrated in Fig. 1B, aldosterone was effective at low physiological concentrations (0.1–1 nM), and the effect plateaued at 10 nM, strongly indicating that aldosterone acts via the MR. This conclusion was further strengthened by testing the effect of aldosterone on the levels of PLZF mRNA in the absence of an induced MR (i.e., in the absence of dox). As shown in Fig. 1C, neither dox alone, nor 1 nM aldosterone alone affected the levels of PLZF mRNA in the M1/TetON-MR cells.
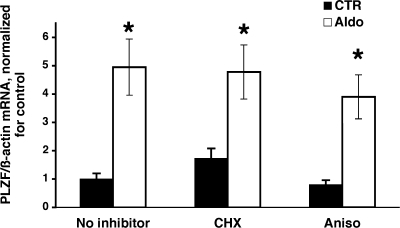
The rapid time course of PLZF induction by aldosterone suggests that this is a direct effect via the MR. To test this hypothesis experimentally, we pretreated M1/TETOn-MR cells with two structurally dissimilar protein synthesis inhibitors at concentrations that were shown previously to block protein synthesis effectively (14). As shown in Fig. 2, neither cycloheximide nor anisomycin treatment prevented the induction of PLZF by aldosterone. These data strongly indicate that de novo protein synthesis is not required for the steroid induction of PLZF expression, and thus it is likely to be due to binding of the MR to MRE/GRE in the PLZF promoter (7).
Fig. 2.
The effect of protein synthesis inhibition on the induction of PLZF by aldosterone. M1/TetON-MR cells were grown in steroid-free medium for 2 days, then expression of MR was induced with 500 ng/ml dox for 2 days. Cells were then preincubated for 30 min with vehicle (no inhibitor), or the protein synthesis inhibitors cycloheximide (CHX; 5 μg/ml) or anisomycin (aniso; 10 μM). Aldosterone (1 nM) was given (or not) at 30 min and cells were incubated for an additional 2 h before RNA was prepared. PLZF and β-actin mRNA levels were determined by RT-PCR from the same samples. Values are RE (relative expression) of PLZF/β-actin mRNA, and are expressed as fold change vs. untreated cells (no inhibitor, no aldosterone) *, P < 0.01, vs. control cells, using one-tailed unpaired t-test. The values of PLZF mRNA levels in the presence aldosterone alone were not significantly different from those in cells incubated with aldosterone and CHX or anisomycin (using 2-tailed unpaired t-tests); n = 3 for each cell type.
Effects of PLZF on transepithelial sodium transport.
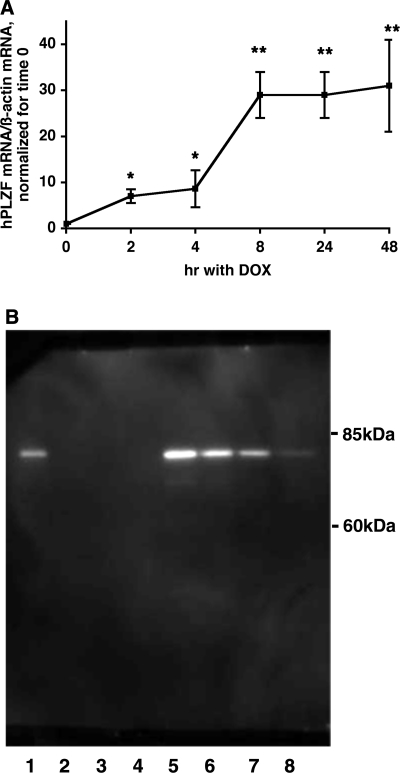
To determine whether changes in PLZF expression levels regulate ENaC function and thereby transepithelial sodium transport in renal epithelia, we first generated an M1 cell line with a stably integrated TET-inducible human PLZF cDNA (this cell line is referred to as M1/TETOn-hPLZF). Using real-time PCR, we tested the expression levels of hPLZF mRNA at different time points after induction with 0–1,000 ng/ml Dox. These experiments revealed that in the absence of Dox hPLZF levels were very low. However, following treatment with 50–1,000 ng/ml Dox, we observed a significant induction of hPLZF. The dose-response curve at 24 h indicated that optimal induction of hPLZF mRNA was observed at 500 ng/ml of Dox (data not shown). hPLZF mRNA levels increased ∼7-fold after 2 h and 30-fold after 24 h with this Dox concentration (Fig. 3A). Western blot analyses indicated that treatment with Dox was also accompanied by a robust accumulation of PLZF protein, while in the absence of Dox PLZF protein levels were undetectable (Fig. 3B).
Fig. 3.
Induction of PLZF expression by doxycycline (dox) in cells stably expressing hPLZF. Expression of hPLZF in M1/TetON-hPLZF cells was induced by 500 ng/ml dox treatment for different periods, and the levels of hPLZF transcript (A) or protein (B) were determined as described in the Experimental Procedures. A: Values shown are RE, and are expressed as fold increase in PLZF expression vs. time 0. *, P < 0.01; **, P < 0.005. B: Western blot analysis of expression of hPLZF. Cells were induced by dox at different concentrations (0–1,000 ng/ml) for 24 h, lysed and PLZF protein expression determined by immunoblotting using 1:1,000 dilution of a PLZF-specific mouse monoclonal antibody from Calbiochem. Forty micrograms of total protein was loaded in each lane; Lane 1: + control (HL-60 cell extract); Lanes 2–4, no dox treated cell lysate; lane 5–8: cells were treated with 1,000, 500, 250, 125 ng/ml dox, respectively.
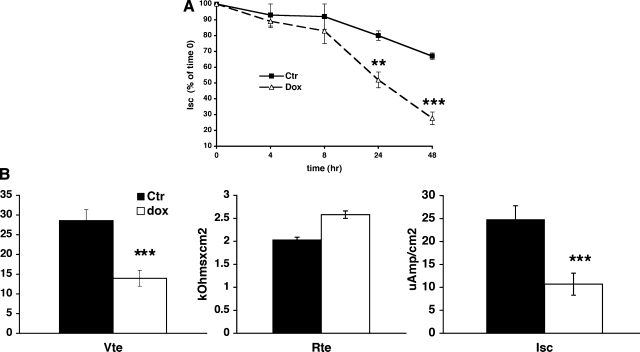
Next, we examined the functional consequences of inducing hPLZF expression on transepithelial sodium transport in CCD cells. Previous studies from our laboratory (20) established that amiloride-sensitive current in M1 cells corresponds to transepithelial sodium transport via the ENaC. We induced hPLZF expression by Dox and followed the time course of sodium current in Dox-treated and control M1/hPLZF cells for 48 h. To our surprise, induction of PLZF expression resulted in a significant decline of transepithelial Na transport. Na current started to diminish after 8 h of Dox treatment (Fig. 4A), that is with an ∼6-h delay (Fig. 3A), and decreased further by ∼50 and 70% at 24 and 48 h of dox treatment, respectively. This decrease in Isc represents a genuine inhibition of transcellular sodium transport rather than loss of integrity of the monolayer, as transepithelial resistance of the monolayer was not decreased. In fact, there was a tendency for Rte to increase with Dox treatment (Fig. 4B), as expected with diminished ENaC activity.
Fig. 4.
Stable expression of hPLZF decreases transepithelial Na+ current in M1 cells. A: Time course of PLZF effect on transepithelial Na current (Isc). M1/TETOn-hPLZF cells were grown in complete growth medium (PC1) to confluence on Millicell membranes. Cells were then maintained in steroid-free medium for 2 days, then 500 ng/ml dox (dashed line) or vehicle (solid line) was added to the cells, and transepithelial voltage (Vte) and resistance (Rte) determined at different times and Isc calculated as described in materials and methods. Values are expressed as % of time 0 value of the same culture. Note spontaneous decrease in Isc values of control cells, which is due to maintenance on steroid-free medium. Isc in cells in which PLZF expression was induced by dox is significantly reduced at 24 and 48. **, P < 0.01; ***, P < 0.001, vs. time 0 using 2-tail unpaired t-test. n = 16 for times 0, 24 and 48 h, n = 8 for times 4 and 8 h. B: Effects of PLZF on Vte, Rte and Isc. PLZF decreases Vte (left) and Isc (right) but has no significant effect on Rte (middle). PLZF expression was induced for 24 h with 500 ng/ml dox. ***, P < 0.001, n = 16.
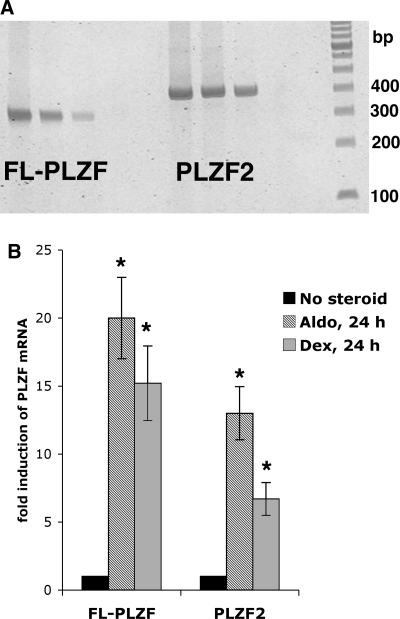
Transcription of the hPLZF gene generates several splice variants (27). In theory, such splice variants might compete for binding sites on target genes, and might even exert opposite effects on transcription. To test whether the unexpected reduction in Na current following increased expression of hPLZF is due to competition of the full-length hPLZF with other splice variants expressed endogenously in M1 cells, we first tested whether M1 cells indeed express different PLZF transcripts. Our results revealed that M1 cells express mPLZF2 (GenBank AK040211), a truncated PLZF splice variant (Fig. 5A) that encodes a protein missing the COOH-terminal 75 amino acids. Using isoform-specific primers and real-time PCR, we next tested whether the expression of PLZF isoforms is differentially regulated by corticosteroids. These experiments revealed that that while there are quantitative differences in the magnitude of steroid regulation, both the full-length and the truncated PLZF isoforms are induced by aldosterone and dexamethasone in M1 cells (Fig. 5B).
Fig. 5.
A: Expression of the full-length (FL) and C-terminally truncated (mPLZF2) PLZF isoforms in mouse cortical collecting duct cells. RNA was prepared from parent M1 cells, reverse transcribed, and splice variant primers pairs were used in RT-PCR as described in the materials and methods. The expected size of the FL-PLZF-specific amplicon is 315 bp, while that of the mPLZF2-specific amplicon is 399 bp. B: Effect of corticosteroids on the expression of PLZF isoforms in M1 cells. M1/TetON-MR cells were treated or not (filled bars) with 1 nM aldosterone (aldo, hatched bars) or 10 nM dexamethasone (grey bars) for 24 h and expression of PLZF isoforms determined with RT-PCR as above. n = 3 in each group. *P < 0.05 vs. no steroid group.
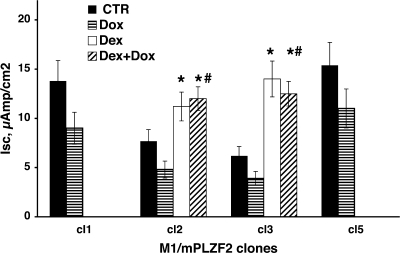
To test whether changes in the level of the COOH terminally truncated mPLZF2 affect Na current differently than the full-length hPLZF, we generated stable M1 cell lines expressing mPLZF2 in a TET-regulated manner. These experiments revealed that the effect of mPLZF2 on Na transport is similar to that of hPLZF; i.e., Isc decreased in four separate clonal cell lines expressing mPLZF2 (Fig. 6), albeit that the decrease was somehat smaller than that observed following induction of hPLZF. Thus increasing the expression levels of either hPLZF or the truncated mPLZF2 isofrom resulted in a reduced transepithelial Na transport in CCD cells.
Fig. 6.
Stable expression of mouse PLZF2 decreases transepithelial Na+ current in M1. Individual clonal lines (clones 1 -3 and 5) of M1/TetOn-mPLZF2 cells were grown in complete growth medium (PC1) to confluence on Millicell membranes, then maintained in steroid-free medium for 2 days, and then mPLZF2 expression was induced by 500 ng/ml dox (striped bars) or not (vehicle; filled bars) added to the cells. In clones 2 and 3 separate cultures received 100 nM dexamethasone (dex, open bars) or dox plus dex (hatched bars). Isc values shown are derived from Vte and Rte determined 48 h after treatment. For each treatment group n = 2 for clones 1 and 5, n = 3 for clones 2 and 3. *, P < 0.05 vs. no steroid group; #, P < 0.05 vs. dox-treated cells.
After establishing that mPLZF2 also inhibits basal Na transport, we asked the question whether this protein also modulates steroid-induced Na transport in M1 cells. Since the M1 cells do not express functional MRs, we used the GR agonist dexamethasone to stimulate Na transport since engagement of the GR mimics the effect of aldosterone in CCD cells (20). As expected, dexamethasone in itself stimulated Na current, while induction of mPLZF2 by Dox alone inhibited it. On the other hand, overexpression of mPLZF2 did not inhibit steroid-induced Na transport (Fig. 6, middle).
head3PLZF decreases β- and γ-ENaC mRNA levels.
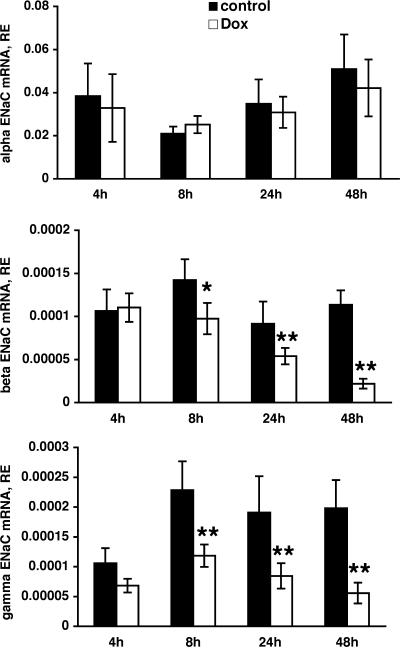
Next, we examined the cellular mechanism that might underlie the decreased Na transport following enhanced PLZF expression. Since PLZF is a transcriptional repressor, and ENaC is the main contributor to transepithelial Na transport in CCD cells, we hypothesized that PLZF might decrease transcription of ENaC subunits. To test this idea, we determined the expression levels of mRNAs encoding the three ENaC subunit using real-time RT-PCR following induction of PLZF expression by Dox. The levels of α-ENaC subunit mRNA were not altered by increased expression of hPLZF (Fig. 7). However, we observed a marked decrease in the levels of β- and γ-ENaC mRNAs that reached statistical significance at 24 h following Dox and continued to decrease at 48 h (Fig. 7). These changes correlate with the changes observed in transepithelial Na current following induction of PLZF (Fig. 4). Interestingly, P-Match analysis (www.gene-regulation.com) revealed that the mouse γ-ENaC promoter contains a putative PLZF-binding site (gtacagtac) 859 nt downstream from the ATG codon, suggesting that this might be a direct transcriptional repression by PLZF.
Fig. 7.
Effect of PLZF on the expression of epithelial Na channel (ENaC) subunit mRNAs. M1/TETOn-hPLZF cells were grown as above, and hPLZF expression induced with 500 ng/ml dox for 4–48 hrs. Cells were then lysed and the levels of alpha (upper panel), beta (middle panel) and gamma (lower panel). ENaC mRNA determined as described in the materials and methods. *, P < 0.05; **, P < 0.01; n = 5 for each time point.
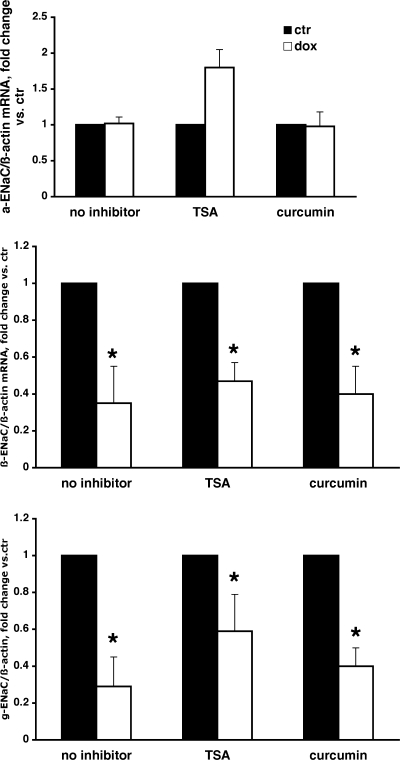
Previous studies showed that transcriptional repression by PLZF involves recruitment of corepressors and histone deacetylases (HDAC) (10). Interestingly, PLZF-mediated repression is also dependent on its own acetylation by p300, a well-known histone acetyltransferase (HAT) (11). To investigate whether pharmacological inhibition of either HDAC activity (by TSA) or p300 HAT activity (by curcumin) affects PLZF's ability to repress the transcription of β- and γ-ENaC, we tested the effects of these inhibitors in the presence or absence of Dox on ENaC mRNA levels. Our data indicate that neither TSA nor curcumin prevented or reduced the inhibitory effect of PLZF on β- and γ-ENaC mRNA expression (Fig. 8).
Fig. 8.
Inhibition of histone deacetylase and p300 does not prevent repression of β- and γ-ENaC by PLZF. M1-PLZF cells were grown in complete medium until reaching confluence. Cells were then steroid-starved for 48 h prior to an 24-h treatment with 500 ng/ml dox (open bars or vehicle (filled bars). Where indicated, 100 ng/ml trichostatin A (TSA) or 100 μM curcumin was added and cells incubated for 24 h. ENaC and β-actin mRNA levels were determined as above. Values are RE of ENaC/β-actin mRNA, normalized for control (no dox) in each group. *, P < 0.05 control vs. dox treated cells; n = 3 in each group.
DISCUSSION
In this study, we established that the gene encoding the transcriptional repressor PLZF is a direct target of aldosterone regulation in renal epithelial cells. Interestingly, and surprisingly, our results also demonstrate that inducible overexpression of PLZF in CCD cells reduces transepithelial Na current with a concomitant decrease in the mRNA levels of two ENaC subunits. This effect is unlikely to be a consequence of expressing PLZF at levels much higher than can be achieved by physiological regulators, since our data also demonstrate that the degree of PLZF induction by aldosterone (or dexamethasone) treatment is comparable to those achieved by the Tet-induced expression.
Almost all aldosterone-regulated genes identified thus far are positive regulators of ENaC activity (5, 19, 21, 26), and very little is known about negative transcriptional regulators of ENaC. Zhang and coworkers (31) reported that Dot1a, a histone methyltransferase, under basal conditions, represses ENaC transcription but aldosterone downregulates Dot1a expression thereby relieving the inhibition. On the other hand, we show here that PLZF, which itself is induced by aldosterone, significantly reduces ENaC-mediated Na transport in CCD cells. A possible explanation for these findings is that PLZF is part of a negative feedback loop that limits aldosterone's stimulatory effects to prevent an uncontrolled increase in Na reabsorption by the collecting duct. Indeed, the negative effect of PLZF on transepithelial Na transport is slower than activation of ENaC/Na transport in the CCD cells, which takes a few hours in cultured cells but only 45 min-1 h in vivo (28). An analogous feedback loop involving PLZF has been described in the prostate. In this tissue, androgens upregulate PLZF expression, while PLZF inhibits the biological effect of androgens on cell growth and proliferation (15).
Apparently, the repressive effect of the full-length PLZF on Na current and ENaC expression is not due to competition with the endogenously expressed truncated mPLZF2, as inducible overexpression of this isoform had a similar effect on Na current to full-length PLZF. However, since in humans there are at least five PLZF splice variants, it is possible that mouse CCD cells express additional PLZF variants (in addition to of FL-PLZF and mPLZF2) that might be induced by aldosterone and involved in the regulation of Na transport. Thus our data do not exclude the possibility that the mPLZF2 might have a dominant negative function by competing with these yet unidentified splice variants. Since the truncated mPLZF is missing the COOH-terminal 75 amino acids present in full-length PLZF, which includes the last two zinc fingers, our data suggest that for the inhibitory effect on Na current these particular zinc fingers are not necessary.
The function of PLZF is closely linked to protein acetylation. PLZF was shown to bind to consensus PLZF binding sites in the promoter of target genes and to recruit corepressor complexes with HDAC activity (6). Consequently, the transcriptional repression by PLZF can be relieved by HDAC inhibitors such as TSA (6, 10). To explore the role of histone deacetylation in the repression of ENaC transcription by PLZF, we tested the effect of TSA. However, we found that this HDAC blocker did not prevent PLZF's inhibition of ENaC mRNA expression.
In addition to histone deacetylation, it seems that acetylation of PLZF itself via p300 HAT is critical for its repressor function (11). Therefore, the negative outcome of the HDAC inhibition could have been due to a concurrent increase in the acetylation (and thereby activation) of PLZF itself, which would mask a potential decrease in transcriptional repression of ENaC. To test this possibility, we examined the effect of p300 inhibition. However, this intervention did not prevent the inhibitory effect of PLZF on ENaC mRNA levels, suggesting that ENaC repression seems to be independent of acetylation.
Another interesting question is whether repression of ENaC transcription is a “direct” effect of PLZF. Negative regulation by transcription factors is often indirect, via suppression of the effect of other (stimulatory) transcription factors. In theory, PLZF might interfere with steroid-induced upregulation of ENaC subunits. However, in M1 cells steroids upregulate the α-subunit of ENaC while having only a marginal effect on the β- and no effect on the γ-subunit (4). In contrast, our present data show a strong repression of β- and γENaC by PLZF while no repression of α-ENaC, making it unlikely that PLZF inhibits a steroid hormone-mediated induction of ENaC subunits.
A possible mechanism by which PLZF could inhibit ENaC transcription is interfering with dimerization of the MR and/or GR. PLZF was shown to interfere with dimer formation by other nuclear receptors and inhibit their transcriptional activity (18). However, in our case such a mechanism seems unlikely, since we found that corticosteroids are still capable of inducing expression of SGK1 (data not shown), as well as Na current (Fig. 7) in cells stably overexpressing mPLZF2. These results suggest that the action of PLZF on Na current probably involves interaction with other steroid-regulated gene products in addition to the reduction of β- and γ-ENaC mRNA expression.
It is interesting to note that in another system, i.e., the rat heart, PLZF and another component of the renin-angiotensin system, i.e., the angiotensin II receptor act synergistically in the development of cardiac hypertrophy (23). In addition, PLZF was recently shown to interact with the renin/prorenin receptor, and to activate two signal transduction pathways with opposing final effects. On one hand, PLZF represses transcription of the renin/prorenin receptor itself; on the other hand it activates the p85a subunit of PI3K, which mediates the cell proliferative effects of renin on cardiomyocytes (22). These data, together with our current observations, suggest a complex interaction between PLZF and the components of the renin-angiotensin system.
In summary, our data indicate that PLZF is a negative regulator of ENaC activity in CCD cells and this inhibitory action most likely involves transcriptional repression of the β- and γ-subunit of ENaC. We speculate that PLZF provides a negative feedback to limit aldosterone-stimulated sodium transport, and this way it might be involved in the “fine tuning” of the regulation of ENaC subunits via aldosterone.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41841, DK-55845, and DK-58898.
Acknowledgments
We thank Drs. Zhou Wand and Anthony Saporita (Northwestern University, Chicago IL) for the human PLZF constructs, Dr. Francis Kern (Southern Research Institute, Birmingham, AL) for providing the pBTE construct, and Dr. Saimak Agha-Mohammadi (University of Pittsburgh, Pittsburgh, PA) for the SG-TRE construct. We would also like to thank to Donna Michael, Natalia Plotnikova and Elena Martinez for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alvarez de la Rosa D, Canessa CM. Role of SGK in hormonal regulation of epithelial sodium channel in A6 cells. Am J Physiol Cell Physiol 284: C404–C414, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet 25: 166–172, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA 95: 9424–9429, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd C, Naray-Fejes-Tóth A. Gene regulation of ENaC subunits by serum- and glucocorticoid-inducible kinase-1. Am J Physiol Renal Physiol 288: F505–F512, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David G, Alland L, Hong SH, Wong CW, DePinho RA, Dejean A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene 16: 2549–2556, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Fahnenstich J, Nandy A, Milde-Langosch K, Schneider-Merck T, Walther N, Gellersen B. Promyelocytic leukaemia zinc finger protein (PLZF) is a glucocorticoid- and progesterone-induced transcription factor in human endometrial stromal cells and myometrial smooth muscle cells. Mol Hum Reprod 9: 611–623, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084–1092, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Fejes-Tóth G, Chen WR, Rusvai E, Moser T, Náray-Fejes-Tóth A. Differential expression of AE1 in renal HCO3-secreting and -reabsorbing intercalated cells. J Biol Chem 269: 26717–26721, 1994. [PubMed] [Google Scholar]
- 10.Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara FF, Zamir I, Seiser C, Grignani F, Lazar MA, Minucci S, Pelicci PG. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391: 815–818, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Guidez F, Howell L, Isalan M, Cebrat M, Alani RM, Ivins S, Hormaeche I, McConnell MJ, Pierce S, Cole PA, Licht J, Zelent A. Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein. Mol Cell Biol 25: 5552–5566, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidez F, Ivins S, Zhu J, Soderstrom M, Waxman S, Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood 91: 2634–2642, 1998. [PubMed] [Google Scholar]
- 13.Helms MN, Fejes-Tóth G, Náray-Fejes-Tóth A. Hormone-regulated transepithelial Na+ transport in mammalian CCD cells requires SGK1 expression. Am J Physiol Renal Physiol 284: F480–F487, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Itani OA, Cornish KL, Liu KZ, Thomas CP. Cycloheximide increases glucocorticoid-stimulated α-ENaC mRNA in collecting duct cells by p38 MAPK-dependent pathway. Am J Physiol Renal Physiol 284: F778–F787, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Jiang F, Wang Z. Identification and characterization of PLZF as a prostatic androgen-responsive gene. Prostate 59: 426–435, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Li JY, English MA, Ball HJ, Yeyati PL, Waxman S, Licht JD. Sequence-specific DNA binding and transcriptional regulation by the promyelocytic leukemia zinc finger protein. J Biol Chem 272: 22447–22455, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Martel JA, Michael D, Fejes-Tóth G, Náray-Fejes-Tóth A. Melanophilin, a novel aldosterone-induced gene in mouse cortical collecting duct cells. Am J Physiol Renal Physiol 293: F904–F913, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Martin PJ, Delmotte MH, Formstecher P, Lefebvre P. PLZF is a negative regulator of retinoic acid receptor transcriptional activity. Nucl Recept 1: 6, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G. SGK is an aldosterone-induced kinase in the renal collecting duct: effects on epithelial Na+ channels. J Biol Chem 274: 16973–16978, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Náray-Fejes-Tóth A, Fejes-Tóth G. Glucocorticoid receptors mediate mineralocorticoid-like effects in cultured collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 259: F672–F678, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Náray-Fejes-Tóth A, Snyder PM, Fejes-Tóth G. The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Na+ transport. Proc Natl Acad Sci USA 101: 17434–17439, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355–1366, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Senbonmatsu T, Saito T, Landon EJ, Watanabe O, Price E Jr, Roberts RL, Imboden H, Fitzgerald TG, Gaffney FA, Inagami T. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J 22: 6471–6482, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shigaev A, Asher C, Latter H, Garty H, Reuveny E. Regulation of sgk by aldosterone and its effects on the epithelial Na+ channel. Am J Physiol Renal Physiol 278: F613–F619, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Snyder PM The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280: 39970–39981, 2005. [DOI] [PubMed] [Google Scholar]
- 27.van Schothorst EM, Prins DE, Baysal BE, Beekman M, Licht JD, Waxman S, Zelent A, Cornelisse CJ, van Ommen GJ, Richard CW, 3rd, Devilee P. Genomic structure of the human PLZF gene. Gene 236: 21–24, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Verrey F Early aldosterone action: toward filling the gap between transcription and transport. Am J Physiol Renal Physiol 277: F319–F327, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Verrey F, Pearce D, Pfeiffer R, Spindler B, Mastroberardino L, Summa V, Zecevic M. Pleiotropic action of aldosterone in epithelia mediated by transcription and post-transcription mechanisms. Kidney Int 57: 1277–1282, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D. Impaired renal Na+ retention in the sgk1-knockout mouse. J Clin Invest 110: 1263–1268, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Xia X, Jalal DI, Kuncewicz T, Xu W, Lesage GD, Kone BC. Aldosterone-sensitive repression of ENaC α transcription by a histone H3 lysine-79 methyltransferase. Am J Physiol Cell Physiol 290: C936–C946, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]