Abstract
Indoleamine 2,3-dioxygenase (IDO) catabolizes tryptophan to N-formyl kynurenine and has a proapoptotic role in renal tubular epithelial cells (TEC) in response to IFN-γ and TNF-α in vitro. TEC produce abundant amounts of IDO in vitro in response to inflammation but a pathological role for IDO in renal injury remains unknown. We investigated the role of IDO in a mouse model of renal ischemia-reperfusion injury (IRI). IRI was induced by clamping the renal pedicle of C57BL/6 mice for 45 min at 32°C. Here, we demonstrate upregulation of IDO in renal tissue at 2 h after reperfusion which reached maximal levels at 24 h. Inhibition of IDO following IRI prevented the increase in serum creatinine observed in vehicle-treated mice (86.4 ± 25 μmol/l, n = 11) compared with mice treated with 1-methyl-d-tryptophan, a specific inhibitor of IDO (33.7 ± 8.7 μmol/l, n = 10, P = 0.031). The role of IDO in renal IRI was further supported by results in IDO-KO mice which maintained normal serum creatinine levels (32.5 ± 2.0 μmol/l, n = 6) following IRI compared with wild-type mice (123 ± 30 μmol/l, n = 9, P = 0.008). Our data suggest that attenuation of IDO expression within the kidney may represent a novel strategy to reduce renal injury as a result of ischemia reperfusion.
Keywords: tubular epithelial cell, apoptosis, renal function
ischemia-reperfusion injury (IRI) is invariably associated with solid organ transplantation. As therapeutic agents that completely or predictably prevent IRI are currently unavailable, IRI remains a major problem in renal transplantation. The degree of injury sustained under such conditions influences both the immediate and potentially the long-term outcome of a transplant (17, 36, 44). One of the deleterious consequences of renal IR involves tubular epithelial cell (TEC) injury and death (25, 26, 42). Kidney TEC undergo apoptosis as well as necrosis as a result of IRI (26, 37, 43). As TEC are central to the function of the nephron and thus the kidney, strategies that limit or prevent TEC death may improve long-term renal allograft function.
Indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme in the kynurenine enzymatic pathway and is expressed in various tissues and cells including dendritic cells and macrophages in response to IFN-γ, TNF-α, and LPS (3, 8, 21, 38, 46–50). IDO expression has been shown to cause activation-induced cell death (AICD) of T cells by enhancing Fas-mediated apoptosis (24). The activity of the enzyme has also been demonstrated to augment anti-Fas-mediated cell death of human tumor cell lines (19). Previous studies associated IDO expression in the kidney with renal acute rejection and chronic failure (5, 39, 45). Specifically, the expression of IDO in renal tubules and the presence of high concentration of kynurenine, found in sera and urine of recipients, during acute rejection and chronic renal failure suggest IDO may participate in graft injury (5, 39, 45). Recently, we demonstrated that IDO augments Fas/FasL-mediated self injury and death of TEC in response to IFN-γ and TNF-α in vitro (32). However, a role for IDO in promoting renal injury following IRI has not been determined in vivo. The aim of this study was to determine whether renal expression of IDO could promote IRI by augmenting tubular cell death and worsening function. Using IDO-KO mice as well as the IDO-specific inhibitor 1-methyl-d-tryptophan (1-MT) in wild-type (WT) mice, we tested for renal injury following renal artery clamping in uninephrectomized mice. Our studies demonstrate that renal IDO expression is deleterious during renal IR and elimination or inhibition of IDO prevented injury with maintenance of normal histology and renal function. Therapeutic inhibition of IDO is clinically feasible in the time frame of IRI, and as such these results may be considerably significant in renal transplantation.
METHODS
Animals.
C57BL/6 (B6) mice (male, 8–10 wk old) were obtained from the Jackson Laboratory (Bar Harbor, ME). Both WT B6 and IDO-KO mice (31) (C57BL/6 background) were maintained in the animal facility at the University of Western Ontario (London Ontario, Canada). Animal experiments were conducted in accordance with the Canadian Council on Animal Care guidelines under protocols approved by the Animal Use Subcommittee at our institution.
Renal IRI induction.
Ischemic injury was induced by clamping the renal pedicle of the left kidney for 45 min at 32°C. After the clamps were released, reperfusion of the kidneys was confirmed visually, and the right kidney was removed. For the IDO inhibitor treatment, WT B6 mice received intraperitoneal injections of 3 mg of 1-MT (Sigma-Aldrich, Mississauga, ON) dissolved in PBS or PBS alone (vehicle) twice a day for a total period of 48 h following reperfusion. Some mice received 1-MT 1 h before ischemia as well as following reperfusion. After 48 h of reperfusion, the mice were killed at which point serum and tissue samples were collected. Uninephrectomized mice that did not undergo IR were also included in the study as sham-operated controls. Renal TEC were subjected to ischemic conditions in vitro. NG 1.1 (32) TEC (2.0 × 106) were seeded to each well of six-well plates overnight. Medium was replaced in confluent monolayer cultures with K1 medium without serum or growth factors to arrest cell division. To mimic ischemia injury in vitro, sterile mineral oil (Sigma) was added to the wells to cover the medium, followed by continued culture at 37°C. At various time points, oil was removed and TEC RNA was collected by using TRIzol at the different time points (Baseline, 0, 1, 2, 4, 8, 24 h).
RNA isolation and RT-PCR.
Kidney tissues pooled from three mice were added to 1–2 ml of TRIzol (Invitrogen GIBCO, Burlington, ON) and ground up using a tissue sonicator. RNA was isolated according to the manufacturer's instructions. RT-PCR was carried out using total isolated RNA. Briefly, 5 μg of RNA from each sample were used to carry out reverse transcription with Superscript II (Invitrogen GIBCO). Subsequently, PCR amplification of IDO cDNA was carried out using a specific pair of primers 5′-CATAAGACAGAATAGGAGGC (sense) and 5′-GAAGATGTGGGCTTTGCTCTA (anti-sense) for 32 cycles. Glyceraldehyde-3-phospate dehydrogenase (GAPDH) mRNA levels were used as an internal control for the amount of RNA loaded in each sample. PCR products were visualized on a 1% agarose gel with 0.5 μg/ml ethidiumbromide. Although semiquantitative, comparisons of expression of IDO mRNA levels between sample times were made using densitometry with GAPDH mRNA expression levels.
Western blot analysis.
Kidney tissue samples pooled from three different mice were added to lysis buffer [10 mM HEPES (pH 7.9), 10 mM KCl, 0.10 mM EDTA, 0.10 mM EGTA, 0.10% NP 40, 1.0 mM DTT, and a protease cocktail (Roche Diagnostics, Mannheim, Germany)] and ground up using a sonicator. An equal amount of 4× SDS sample buffer [20 mM Tris·HCl (pH 6.8), 5.0% (wt/vol) SDS, 10% vol/vol beta-mercaptoethanol, 2.0 mM EDTA, and 0.020% bromophenol blue] was added to the ground up tissue. The samples were run on a 12% SDS-polyacrylamide gel and subsequently transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was blocked using 5.0% milk in TBS-T (20 mM Tris·HCl, pH 7.6, 0.14 M NaCl, 0.10% Tween 20) for 1 h. The IDO protein (∼45 kDa) was detected using a primary polyclonal rabbit anti-IDO antibody (31) (1:3,000), in TBS-T containing 2.5% milk and a goat anti-rabbit peroxidase-conjugated secondary antibody (Sigma). The bound protein on the membrane was visualized by an enhanced chemiluminescence assay (ECL, Amersham Pharmacia Biotech, Buckinghamshire, UK). Blots were reprobed using total ERK antibody (Sigma-Aldrich) as a loading control. Quantification of IDO protein levels at various sample times was made using densitometry and comparing IDO levels to total ERK levels.
Densitometry analysis.
RT-PCR or Western blot images were scanned using a GS700 Imaging densitometry scanner (BioRad, Mississauga, Ontario). Analysis of the images was carried out with BioRad Densitometry software (BioRad). Individual blot/gel images were examined by analyzing the intensity of each band within a fixed volume. The density ratio was determined by dividing the intensity of the target mRNA/protein by the respective GAPDH/total ERK band intensities.
Tissue immunohistochemical staining.
Paraffin-embedded tissue sections were immersed for 5 min in 100% xylene twice, 1:1 (xylene:ethanol), 100, 90, 75% ethanol, and washed three times in Tris buffer saline (TBS; pH 7.4). The slides were transferred into already boiling 10 mM sodium citrate buffer (pH 6.0) for 40 min and then left to cool in room temperature for 20 min. Three 5-min washes in TBS were carried out and the sections were treated with 100 μl of 2.0% H2O2 for a period of 10 min followed by permeabilization with 0.20% Triton X-100 for 10 min at room temperature. The sections were washed two times with TBS and blocked for 1 h using 4.0% rabbit and 2.0% goat serum. Subsequently, the slides were incubated with 1:100 of primary anti-IDO antibody overnight at 4°C. Three 10-min TBS washes were carried out and the samples were incubated with 1:200 of secondary biotin-conjugated antibody for 2 h. Three TBS washes were carried out and the slides were incubated with 1:200 of avidin solution for 45 min at room temperature. Antibody binding was visualized using 3,3′-diaminobenzidine (DAB) as substrate (Sigma Aldrich) followed by counterstaining with hematoxylin for 5 min. All slides contained duplicate sections from which one served as a control for secondary antibody binding specificity.
Determination of renal function, injury, and cellular infiltration.
Kidney function was determined through measurement of serum creatinine and blood urea nitrogen (BUN), by a Jaffe reaction method with an automated CX5 clinical analyzer (Beckman Instruments, Fullerton, CA). Histological evaluation of tubular injury in kidney sections was performed in a blinded fashion. Formalin-fixed and paraffin-embedded sections (5-μm thickness) were stained by hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) method. These sections were examined in a blinded fashion by a pathologist. Kidney injury was assessed as percentage of tissue involved with histological changes in the cortex and medulla using a semiquantitative scale and an evaluation of area involved with tubular injury and neutrophil infiltration on a 5-point scale as follows: 0, no change; 1, 10–25%; 2, 25–50%; 3, 50–75%; and 4, 75–100%. Scores were averaged for at least 10 nonoverlapping fields for each kidney. Neutrophil (PMN) infiltration was assessed by direct counting of sections at ×400. Results are expressed as PMN ± SE per 5 high-power fields (hpf).
TUNEL assay.
Buffered formalin-fixed sections were processed with proteinase K (30 min at 37°C), followed by quenching endogenous peroxidase in 3.0% H2O2 in TBS (pH 7.4) for 30 min at room temperature, then permeabilizing with 0.20% Triton X-100 for 10 min at room temperature. After being washed with TBS, the sections were incubated with terminal transferase and FITC-conjugated dUTP (Roche Diagnostics) for 60 min at 37°C. FITC-dUTP-labeled nuclei were detected using anti-FITC antibody conjugated with phosphatase (Sigma-Aldrich) and visualized with phosphatase substrate Sigma Fast Red (Sigma-Aldrich). The number of TUNEL-positive cells representing apoptotic/necrotic cells in the section of each kidney was counted in at least 10 nonoverlapping hpf under ×400 magnification and averaged in a blinded fashion.
Statistical analysis.
Two-way ANOVA and t-tests (2-tailed distribution) were used for comparisons between groups using Statview software (SAS Institute, Cary, CA). A P value of ≤0.05 was considered significant.
RESULTS
Upregulation of IDO following renal IR.
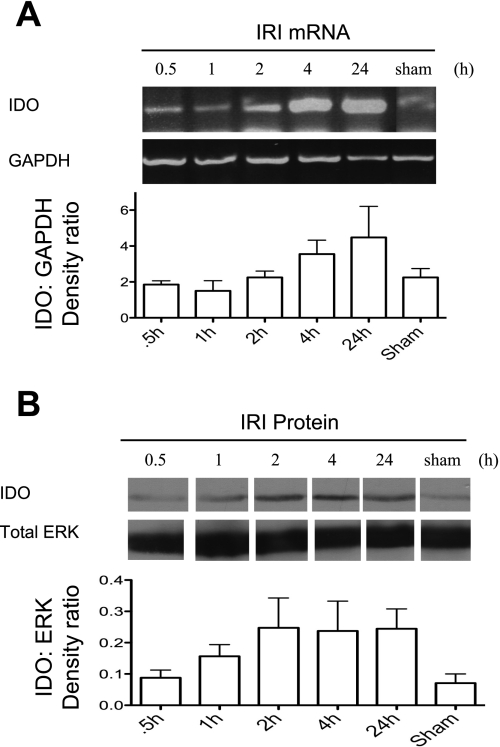
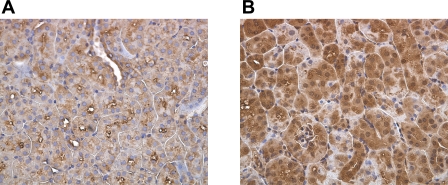
The activity of IDO has diverse physiological functions including suppression of activated T cells by placental tissues in fetal-maternal tolerance, tryptophan starvation of intracellular pathogens, and UV protection in the cornea (33, 41, 46). Recently, we demonstrated the ability of IDO to enhance renal TEC fratricide mediated through TEC expression of Fas/FasL in response to inflammatory cytokines (32). Therefore, we hypothesized that if IDO is expressed in the kidney in response to inflammation following IRI, it may promote this form of TEC self-injury. To determine whether the expression of IDO is upregulated in response to renal IRI, WT mice were subjected to renal IRI and killed at various times points. Sham-operated mice, used as controls, displayed basal expression levels of IDO mRNA and those levels remained unchanged in mice that were subjected to 45 min of ischemia and 0.5 and 1 h of reperfusion (Fig. 1A). However, at 2 h postreperfusion there was a noticeable increase in the level of IDO mRNA that became most pronounced at 4 and 24 h (Fig. 1A). Similar to IDO mRNA expression kinetics, the level of IDO protein initially increased at 2 h postreperfusion and remained upregulated at 4 and 24 h compared with sham control (Fig. 1B). IDO mRNA was detected in quiescent TEC but was not increased following ischemic conditioning in vitro (not shown), suggesting enhanced IDO expression in TEC is related to the reperfusion-associated inflammation, rather than ischemia alone. Although RT-PCR and Western blot analysis are sensitive in detecting mRNA and protein levels of the desired target, these methods do not distinguish location of expression within the kidney. Immunostaining was performed on sections obtained from sham-operated and IR-treated mice that were killed 24 h following reperfusion. IDO staining was detected very weakly within TEC of control mice (Fig. 2A). In contrast, IR-subjected mice had intense and diffuse IDO staining within the TEC of the renal cortex (Fig. 2B). These results suggest that tubular cells appear to be primarily responsible for the elevated expression of IDO noted, but do not distinguish proximal or distal tubules. IDO expression in TEC was not clearly localized to specific cellular compartments in these tissue sections, although expression appeared to be primarily cytoplasmic in cultured TEC (not shown). It is unknown whether IDO can translocate in epithelial cells to possibly facilitate immune modulation given its reported role in promoting T cell apoptosis.
Fig. 1.
Ischemia reperfusion induces renal indoleamine 2,3-dioxygenase (IDO) expression. The renal pedicle of uninephrectomized C57BL/6 mice was clamped for 45 min at 32°C and mice were killed at 0.5, 1, 2, 4, and 24 h following reperfusion. Renal tissue was collected and pooled from 3 separate mice for each time point. A: IDO mRNA levels were detected by RT-PCR. GAPDH was used to control for the amount of RNA used in each sample. mRNA changes by this method are semiquantitative and densitometry is provided as a ratio of IDO:GAPDH to demonstrate increases following ischemia-reperfusion injury (IRI). B: IDO protein (∼45 kDa) was detected using a rabbit-anti-IDO antibody in Western blot. Total ERK was used as a protein loading control. Densitometry of IDO:ERK levels is shown. Data are representative of 2 separate experiments.
Fig. 2.
Immunohistochemical staining displays tubular expression of IDO in response to IR. Paraffin-embedded fixed kidney sections were probed with anti-IDO antibody to pinpoint IDO expression by a specific cell type due to IR. A: sham-operated mice. B: mice exposed to 45 min of ischemia at 32°C and killed 24 h following reperfusion. Data are representative of 3 separate experiments.
IDO-deficient kidneys have improved function following IRI.
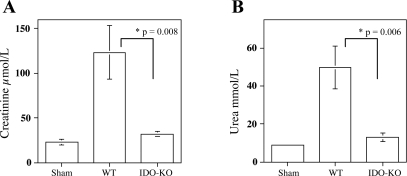
Previous studies of renal IDO expression established an association with acute rejection, decreased glomerular filtration, and renal insufficiency. However, these studies have not directly linked renal IDO to acute or chronic injury (5, 39, 45). To address this, we carried out IRI studies using IDO-KO mice (31). Following 48 h of reperfusion, sera from the mice were collected and creatinine and BUN levels were measured to assess kidney function. Clamped IDO-KO mice had markedly lower level of creatinine (32.5 ± 2.0 μmol/l, n = 6) compared with 123 ± 30 μmol/l (n = 9, P = 0.008) in WT mice (Fig. 3A) reflecting protection of these kidneys from IRI. Clamped IDO-KO mice also had improved kidney function reflected by preserved sera BUN concentrations. IDO-KO mice had significantly lower levels of urea (13.0 ± 2.0 mmol/l, n = 6) vs. WT mice (49.6 ± 11 mmol/l, n = 9, P = 0.006; Fig. 3B). Collectively, these data demonstrate that enhanced IDO expression occurs with IRI and that renal expression of IDO can have a deleterious effect on kidney function.
Fig. 3.
IDO-KO mice have improved kidney function subsequent to IR. C57BL/6 IDO-KO and wild-type (WT) age-matched C57BL/6 mice were exposed to 45 min of ischemia at 32°C and killed 48 h postreperfusion. A: serum creatinine levels of IDO-KO mice compared with WT mice, P = 0.008. B: serum urea levels of IDO-KO mice compared with WT mice, P = 0.006. Data are represented as means ± SE, n = 6 for IDO-KO, n = 9 for WT, and n = 4 for sham-operated mice.
Administration of IDO-specific inhibitor 1-MT improves renal function following IRI.
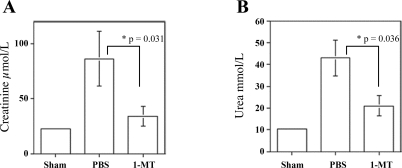
Downregulation of IDO expression might be useful as a strategy to prevent renal injury including transplantation. 1-MT is a potent inhibitor of IDO (7) and hence we used this agent to substantiate data obtained with IDO-KO mice. WT mice subjected to IR and treated with 1-MT pre- and postischemia had creatinine levels (33.7 ± 8.7 μmol/l, n = 10) substantially lower than vehicle-treated mice (86.4 ± 25 μmol/l, n = 11, P = 0.031; Fig. 4A) and were equivalent to that observed in IDO-null mice. Similarly, BUN concentrations in 1-MT-treated mice (20.9 ± 4.6 mmol/l, n = 10) were lower than those from WT mice (43.4 ± 8.3 mmol/l, n = 11, P = 0.036; Fig. 4B). Therefore, pharmacological inhibition of IDO with 1-MT is consistent with the benefit observed using IDO-KO mice when administered 1 h before ischemia induction. However, no benefit was observed with 1-MT treatment postischemia as creatinine levels (111.7 ± 30 μmol/l, n = 4) were not different from control mice. Higher levels and greater delivery of 1-MT postischemia were limited by its low solubility and its rapid clearance, and the relatively large volumes required for injection.
Fig. 4.
IDO expression contributes to renal IRI. C57BL/6 WT mice were pretreated with 3 mg of 1-methyl-d-tryptophan (1-MT) dissolved in PBS or PBS alone 1 h before 45 min of ischemia at 32°C. Following reperfusion, the mice were injected intraperitoneally with the same dosage, 1-MT or PBS alone, twice a day for 48 h. A: serum creatinine of 1-MT-treated compared with PBS-treated mice, P = 0.031. B: serum urea levels of 1-MT-treated compared with PBS-treated mice, P = 0.036. Data are presented as means ± SE (n = 10 for 1-MT, n = 11 for WT, and n = 4 for sham-operated mice).
IDO expression contributes to TEC damage.
Histological assessments were carried out to establish a correlation between the functional protection observed and the degree of injury sustained by the kidney. Tissue sections were paraffin embedded, and stained with H&E or PAS for scoring, which was in a blinded fashion as previously described (11). WT kidney sections were characterized by extensive damage that included disrupted tubules, loss of normal architecture, flattening of cells with loss of tubular brush border, noncellular cast formation, and changes consistent with acute tubular necrosis (ATN; Fig. 5B). In contrast, sections obtained from IDO-KO mice showed preservation of normal architecture of cells with subtle injury compared with WT mice. Accordingly, tubular necrosis and vacuolization (%) scoring confirmed a considerable difference in tissue involved in WT kidneys (51 ± 5.1%, n = 9) vs. IDO-KO kidneys (23 ± 2.1%, n = 6, P = 0.001; Fig. 5D). Histology was also improved in mice treated with 1-MT before ischemia (Fig. 6C) with less tubular damage and minimal cast formation compared with vehicle-treated mice (Fig. 6B). Scoring of the sections and subsequent comparison confirmed that WT-PBS-treated mice had higher amounts of tubular necrosis and vacuolization (45 ± 3.0%, n = 11) compared with pre- and post-1-MT-treated mice (29 ± 3.3%, n = 10, P = 0.002; Fig. 6D). As IRI has been associated with cellular infiltration, we also carried out H&E staining to assess this. WT PBS-treated kidney sections had greater neutrophil infiltration (10.4 ± 9/5 hpf, n = 8) than preischemia 1-MT-treated mice (2.4 ± 5, n = 7, P = 0.02 vs. WT control) or IDO-null kidneys (0 ± 0.5/5 hpf, n = 6, P = 0.007 vs. WT control; Fig. 7, A, B, C). Hence, the functional improvement in IDO-KO and 1-MT mice following IRI is consistent with a reduction in histological kidney injury compared with their respective controls.
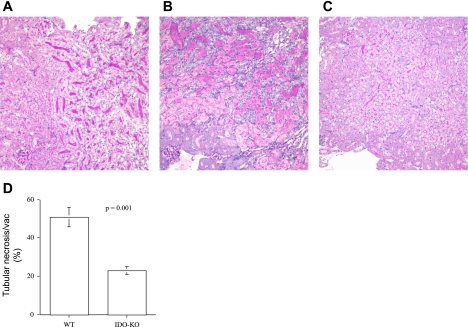
Fig. 5.
IDO-KO mice are resistant to renal IRI. C57BL/6 IDO-KO and WT mice were exposed to 45 min of ischemia at 32°C and killed 48 h postreperfusion. Kidneys were formalin-fixed, paraffin-embedded and sections (5 μm) were PAS-stained. A: sham-operated mice. B: WT. C: IDO-KO. D: tubular necrosis and vacuolization score. Blinded scoring of 10 nonoverlapping fields (×400) was performed and averaged for WT and IDO-KO sections, P = 0.001. Images are representative of each group. Data are presented as means ± SE (n = 6 for IDO-KO, n = 9 for WT, and n = 4 for sham-operated mice).
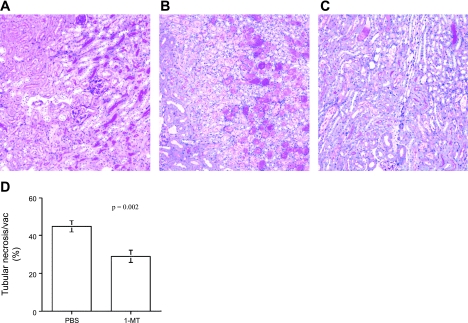
Fig. 6.
IDO expression contributes to renal IRI. C57BL/6 WT mice were pretreated with 3 mg of 1-MT dissolved in PBS or PBS alone 1 h before 45 min of ischemia at 32°C. Following reperfusion, the mice were injected intraperitoneally with the same amount of 1-MT or PBS alone, twice a day for 48 h. Kidneys were formalin-fixed, paraffin-embedded and sections (5 μm) were PAS- or H&E-stained. A: sham-operated mice. B: PBS control. C: 1-MT-treated mice. D: tubular necrosis and vacuolization score. Blinded scoring of 10 nonoverlapping fields (×400) was performed for PBS control and 1-MT-treated mice, P = 0.002. Data are presented as means ± SE (n = 10 for 1-MT, n = 11 for WT, and n = 4 for sham-operated mice).
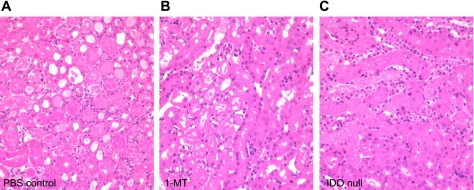
Fig. 7.
IDO-KO kidneys have reduced infiltrate following IRI. A, B, C: H&E staining of PBS control, 1-MT preischemia-treated, and IDO-KO kidney sections were carried out to detect infiltration. Images are representative for each group (n = 8 for WT, n = 7 for 1-MT, n = 6 for IDO-KO).
IDO-KO kidneys have reduced cellular apoptosis/necrosis following IRI.
TUNEL staining of the fixed sections was performed to study the effect of IDO elimination on kidney apoptosis and necrosis, in relation to the observed protection in IDO-null kidneys. WT mice had significantly higher numbers of TUNEL-positive cells (6.5 ± 0.9/hpf, n = 9) in the tubular compartment, than IDO-KO mice (2.1 ± 0.6/hpf, n = 6, P = 0.03; Fig. 8). These data suggest IDO expression contributes to apoptosis/necrosis within the kidney following IR. As control kidneys had significantly more infiltrate than either 1-MT or IDO-null kidneys, it is difficult to unequivocally identify which cells were undergoing apoptosis in tissue sections. However, the absence of diffuse and prominent infiltrates in control sections and morphology of cells would suggest that apoptosis was largely occurring within TEC. Apoptosis was also largely absent in ischemic IDO-null kidneys, consistent with reduced TEC injury and correlation to improved function.
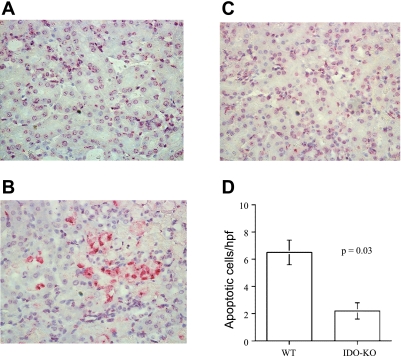
Fig. 8.
IDO-KO kidneys have reduced apoptosis in response to IRI. Kidney sections from sham (A), WT (B), and IDO-KO (C) mice were fixed, and cell death was determined by TUNEL staining. D: blinded scoring of 10 nonoverlapping fields was performed and averaged for WT and IDO-KO (×400), P = 0.03. Images are representative for each group. Data are presented as means ± SE (n = 6 for IDO-KO, n = 9 for WT).
DISCUSSION
IRI in kidneys represents an orchestrated series of inflammatory events leading to organ injury in which there is rapid and significant upregulation of numerous proinflammatory factors that are relevant to renal injury including IFN-γ, TNF-α, and MHC I, II, as well as Fas/FasL (9, 14, 15, 18, 29, 30). The degree of injury sustained to the kidney as a result of IR may not only influence graft acceptance through links between innate and adaptive immunity but also the longevity of the graft as nephrons continue to be injured by inflammation (1–3).
There are several key observations that suggest IDO may be in a position to affect renal IRI. TEC express basal levels of both Fas and FasL and increase Fas expression in response to LPS, inflammatory cytokines such as IFN-γ and TNF-α, as well as oxidative stress, which may lead to injury (1, 4, 10, 20, 22, 23, 28, 34, 35, 40). IRI and transplant studies carried out with MRL-lpr and MRL-gld mice have established that loss of functional Fas/FasL can limit IRI and consequently be beneficial to the organ (34). Recently, we showed that IDO promotes Fas/FasL-mediated TEC death in response to IFN-γ/TNF-α in vitro (32). Moreover, the expression and activity of IDO associated with renal acute rejection and chronic failure indirectly implicate a role for IDO in immune injury following transplantation. In the present study, we found that increased IDO expression is part of the acute response of IR as both mRNA and protein levels of the enzyme noticeably increased within 2 h postreperfusion and peaked between 4 and 24 h. The expression of IDO appears to be in response to reperfusion-associated inflammatory cells and the cytokines that they produce, rather than ischemia per se. TEC augment IDO expression in response to proinflammatory cytokines (32) but IDO mRNA did not change with ischemic in vitro conditioning in the present study (data not shown). The in vivo results in the present study examining kidneys of IDO WT mice therefore are consistent with our results in cytokine-exposed TEC, although IDO expression may differ in non-TEC cells.
This study is the first to describe the induction of IDO in response to renal IR. In addition, we elucidated that both IDO-KO and WT mice treated with the IDO inhibitor had improved kidney function and decreased tissue injury with reduced numbers of TEC undergoing apoptosis/necrosis. As a result, our studies suggest that therapeutic inhibition of IDO may be beneficial in the early stages of transplant injury that involve IR, particularly as this is when TEC express high levels of both IDO and Fas/FasL. Although our data would suggest that inhibition of IDO expression in kidneys is required before the onset of ischemia, IDO inhibition may be therapeutically relevant to some forms of renal injury. With renal transplantation, the addition of 1-MT or alternate pharmacological agents to block IDO or downstream metabolites to organ preservation solutions may be of greater benefit than treating recipients posttransplant.
Traditionally, IDO has been described to be beneficial to tissues expressing the enzyme as it is able to prevent the growth of tryptophan-dependent pathogens and suppress T cells by inhibiting proliferation in addition to inducing Fas/FasL-mediated cell death (3, 6, 8, 46). Several groups have been able to prolong survival of transplanted lungs, pancreatic beta cells, and skin (2, 13, 27, 33). More recently, Liu et al. (27) demonstrated that IDO plays a protective role in lung IR. Although our findings are not consistent with the above studies, we believe the disparity may be explained by the differential expression of Fas/FasL in parenchymal cells of the various organs. While overexpression of IDO may have potential beneficial effects to promoting long-term graft survival by evading the immune system, we suggest that caution should be taken in cells such as TEC in which Fas and FasL are coexpressed. Future studies examining this strategy are needed to determine potential immune protective effects of IDO bestowed to TEC following transplantation.
The mechanism(s) by which IDO augments injury in TEC have not yet been clearly delineated. Interestingly, the expression of IDO was associated with increased numbers of infiltrating cells as well as greater apoptosis in tissue sections. Our previous results using cultured TEC support a role for IDO in promoting tubular cell self-injury through Fas-FasL expression in response to inflammatory cytokines (32) and may hold true in vivo as our data do not exclude the possibility that IDO-associated renal injury in IRI is related to enhanced cytokine release by infiltrated cells. While we cannot exclude that apoptosis was also occurring in the infiltrating cells, the worsened function in WT IDO kidneys and improved function in the absence of IDO would suggest that the beneficial effect was on TEC and renal parenchymal cells. As neutrophils are observed with severe IRI, the reduction of neutrophil infiltration with attenuation of IDO was likely due to reduced tissue injury. However, the reduction of inflammatory infiltrates may have had a further benefit in limiting renal injury. Several studies associated an increase in IDO-related, downstream kynurenine pathway metabolites with acute rejection and chronic renal failure (5, 39, 45). Accordingly, our previous studies demonstrate a potentiating effect of cytokine-mediated TEC death, upon exposure to downstream metabolites picolinic acid and quinolinic acid (32). These findings are substantiated by previous reports of the ability of quinolinic acid to directly activate caspase-8 and cause the release of cytochrome c (12, 16). Strategies that limit downstream kynurenine pathway enzymes more specifically than 1-MT in donor kidneys before transplantation may suppress TEC injury while allowing IDO expression to persist and provide immune protection.
Conclusions.
In conclusion, IDO expression following IR has a deleterious effect on kidney injury and function. Our data suggest that attenuation of IDO may represent a novel strategy to reduce renal IRI and thereby potentially improve kidney graft function and survival.
GRANTS
This work was supported by a grant from the Canadian Institutes of Health Research (A. M. Jevnikar and C. Du) and the Multi-Organ Transplant Program at the London Health Sciences Centre.
DISCLOSURES
A. L. Mellor has intellectual property interests in the therapeutic use of IDO and IDO inhibitors and receives consulting income from NewLink Genetics.
Acknowledgments
We thank P. Gardner for secretarial assistance and Drs. J. Madrenas, E. Cairns, and the late Dr. R. Zhong for invaluable advice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akasaka Y, Ishikawa Y, Kato S, Ishii T, Masuda T, Fujita K, Yamada T, Kawamura S. Induction of Fas-mediated apoptosis in a human renal epithelial cell line by interferon-gamma: involvement of Fas-mediated apoptosis in acute renal rejection. Mod Pathol 11: 1107–1114, 1998. [PubMed] [Google Scholar]
- 2.Alexander AM, Crawford M, Bertera S, Rudert WA, Takikawa O, Robbins PD, Trucco M. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes 51: 356–365, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon mediated chlamydial persistence. Infect Immun 62: 3705–3711, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra J, van der Woude FJ, Wever PC, Laterveer JC, Daha MR, van Kooten C. Expression and function of Fas (CD95) on human renal tubular epithelial cells. J Am Soc Nephrol 8: 1517–1524, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Brandacher G, Cakar F, Winkler C, Schneeberger S, Obrist P, Bosmuller C, Werner-Felmayer G, Werner ER, Bonatti H, Margreiter R, Fuchs D. Noninvasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int 70: 60–67, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism in the mechanism for gamma-interferon mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun 53: 347–351, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys 291: 326–333, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Currier AR, Ziegler MH, Riley MM, Babcock TA, Telbis VP, Carlin JM. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced anti-chlamydial indoleamine dioxygenase activity independently. J Interferon Cytokine Res 20: 369–376, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dannahoo K, Meng X, Ayala A, Cain MP, Harken AA, Medrum DR. Early kidney TNF-α expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol Regul Integr Comp Physiol 277: R922–R929, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Du C, Jiang J, Guan Q, Yin Z, Masterson M, Parbtani A, Zhong R, Jevnikar AM. Renal tubular epithelial cell self-injury through Fas/Fas ligand interaction promotes renal allograft injury. Am J Transplant 4: 1583–1594, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Du C, Jiang J, Guan Q, Diao H, Yin Z, Wang S, Zhong R, Jevnikar AM. NOS2 (iNOS) deficiency in kidney donor accelerates allograft loss in a murine model. Am J Transplant 7: 17–26, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Fallarino F, Grohnmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ 9: 69–77, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Ghahary A, Li Y, Tredget EE, Kilani RT, Iwashina T, Karami A, Lin X. Expression of Indolemaine 2,3-dioxygenase in dermal fibroblasts functions as a local immunosuppressive factor. J Invest Dermatol 122: 953–964, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Goes N, Urmson J, Ramassar V, Halloran P. Ischemic acute tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation 59: 565–572, 1995. [PubMed] [Google Scholar]
- 15.Goes N, Sims T, Urmson J, Vincent D, Ramassar V, Halloran PF. Disturbed MHC regulation in the IFN-y knockout mouse, evidence for three states of MHC expression with distinct roles for IFN-gamma. Immunology 155: 4559–4566, 1995. [PubMed] [Google Scholar]
- 16.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 24: 242–248, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int 66: 523–527, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Halloran PF, Autehried P, Ramassar V, Urmson J, Cockfiled S. Local T cell responses induce widespread MHC expression evidence that IFN-y induces its own expression in remote sites. Immunology 148: 3837–3846, 1992. [PubMed] [Google Scholar]
- 19.Hassan M, Mirmohammadsadegh A, Selimovic D, Nambiar S, Tannapfel A, Hengge UR. Identification of functional genes during Fas-mediated apoptosis using a randomly fragmented cDNA library. Cell Mol Life Sci 62: 2015–2026, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo S, Cha D, Cho W, Kim H, Chang K, Yun S, Won NH. Inflammatory cytokines and lipopolysaccharide induce Fas mediated apoptosis in renal tubular cells. Nephron 91: 4112–4118, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Kallio RE, Berg CP. Tryptophan metabolism: tryptophan, kynurenine, and related compounds as precursors of nicotinic acid. J Biol Chem 181: 333–341, 1949. [PubMed] [Google Scholar]
- 22.Kato S, Akasaka Y, Kawamura S. Fas antigen expression and its relationship with apoptosis in transplanted kidney. Pathol Int 47: 230–237, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Koide N, Narita K, Kato Y. Expression of Fas and Fas ligand on mouse renal tubular epithelial cells in the generalized schwartzman reaction and its relationship to apoptosis. Infect Immun 67: 4112–4118, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee G, Park H, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107: 452–460, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol Renal Fluid Electrolyte Physiol 271: F477–F488, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Liberthal W, Menza SA, Levine J. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol Renal Physiol 274: F315–F327, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Liu L, Fletcher BS, Visner GA. Novel action of indoleamine 2,3-dioxygenase attenuating acute lung allograft injury. Am J Respir Crit Care Med 173: 566–572, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Lorz C, Ortiz A, Justo P. Pro-apoptotic Fas ligand is expressed by normal kidney tubular epithelium and injured glomeruli. J Am Soc Nephrol 11: 1266–1277, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Meldrum K, Meldrum D, Meng X, Ao L, Harken A. TNF-α-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ Physiol 282: H540–H546, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Meldrum KK, Meldrum DR, Hile KL, Yerkes EB, Ayala A, Cain MP, Rink RC, Casale AJ, Kaefer MA. p38 MAPK mediates renal tubular cell TNF-α production and TNF-α-dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol 281: C563–C570, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol 171: 1652–1655, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Mohib K, Guan Q, Diao H, Du C, Jevnikar AM. Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am J Physiol Renal Physiol 293: F801–F812, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Nogae S, Miyazaki M, Koayahsi N, Saito T, Abe K, Saito H, Nakane PK, Nakanishi Y, Koji T. Induction of apoptosis in ischemia-reperfusion model of mouse kidney: possible involvement of Fas. J Am Soc Nephrol 9: 620–631, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz-Arduan A, Dannoff TM, Kalluri R, Gonzalez-Cuadrado S, Karp SL, Elkon K, Egido J, Neilson EG. Regulation of Fas and Fas ligand expression in cultured murine renal cells in the kidney during endotoxemia. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1193–F1207, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Pagtalunan ME, Olson JL, Tilney N, Meyer TW. Late consequences of acute ischemic injury to a solitary kidney. J Am Soc Nephrol 10: 366–373, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Preuss HG, Tourkantonis A, Hsu CH, Shim PS, Barzyk P, Tio F, Schreiner GE. Early events in various forms of experimental acute tubular necrosis in rats. Lab Invest 32: 286–294, 1975. [PubMed] [Google Scholar]
- 38.Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res 25: 20–30, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito K, Fujigaki S, Heyes M, Shibata K, Takemura M, Fujii H, Wada H, Noma A, Seishima M. Mechanism of increases in l-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol 279: F565–F572, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Schelling J, Cleveland R. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int 56: 1313–1316, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Serbecic N, Beutelspacher SC. Indoleamine 2,3-dioxygenase protects corneal endothelial cells from UV mediated damage. Exp Eye Res 82: 416–423, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu A, Yamanaka N. Apoptosis and cell desquamation in repair process of ischemic tubular necrosis. Virchows Arch 64: 171–180, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Solez K, Morel-Maroger L, Sraer JD. The morphology of acute tubular necrosis in man; analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine 58: 362–376, 1979. [PubMed] [Google Scholar]
- 44.Takada M, Nadeau KC, Shaw GD, Tilney NL. Prevention of late renal changes after initial ischemia/reperfusion injury by blocking early selectin binding. Transplantation 64: 1520–1525, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Tankiewicz A, Pawlak D, Topczewska-Bruns J, Buczko W. Kidney and liver kynurenine pathway enzymes in chronic renal failure. Adv Exp Med Biol 527: 409–414, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Thomas SM, Garrity LF, Brandt CR, Schobert CS, Feng GS, Taylor MW, Carlin JM, Byrne GI. IFN-gamma-mediated anti-microbial response. Indoleamine 2,3-dioxygenase deficient mutant host cells no longer inhibit intracellular Chlamydia ssp Or Toxoplasma growth. J Immunol 150: 5529–5534, 1993. [PubMed] [Google Scholar]
- 47.Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of of bacterial lipoplysaccharide. Proc Natl Acad Sci USA 75: 3998–4000, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida R, Urade Y, Nakata K, Watanabe Y, Hayaishi O. Specific induction of indoleamine 2,3-dioxygenase by bacterial lipopolysaccharide in the mouse lung. Arch Biochem Biophys 212: 629–637, 1981. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida R, Urade Y, Tokuda M, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci USA 78: 129–132, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor beta. J Biol Chem 272: 14899–14907, 1997. [DOI] [PubMed] [Google Scholar]