Abstract
The role of carbonate in the binding of cis-diamminedichloroplatinum(II) to DNA was investigated in order to understand the potential involvement of carbonato-cisplatin species in the mechanism of action of platinum anticancer agents. Cisplatin was allowed to react with both double- and single-stranded DNA in carbonate, phosphate, and HEPES buffers, and the products were analyzed by agarose gel electrophoresis and enzymatic digestion/mass spectrometry, respectively. The data from these experiments demonstrate (1) that carbonate, like other biological nucleophiles, forms relatively inert complexes with platinum that inactivate cisplatin, and (2) that the major cisplatin-DNA adduct formed is a bifunctional cross-link. These results are in accord with previous studies of cisplatin-DNA binding and reveal that the presence of carbonate has no consequence on the nature of the resulting adducts.
The activation of the anticancer drug cisplatin, cis-[Pt(NH3)2Cl2], for binding to biological targets involves replacement of chloride ligands with water molecules.1 Aquated cisplatin can bind to purine bases on DNA, forming intrastrand cross-links that destroy cancer cells.2 The intermediacy in this process of platinum complexes with other biologically abundant anions such as acetate,3 phosphate,3 and thiolate,4 has been explored in depth. Recent work has drawn attention to the reaction of cisplatin with carbonate ion and to the potential role of platinum carbonato species in mediating cellular uptake, DNA binding, and anti-tumor properties.5 It was specifically suggested that carbonate alters the binding of cisplatin to DNA, leading to monofunctional platinum-DNA adducts rather than bifunctional cross-links that form with the aqua complexes.6 Because of the potential implications of these findings to the field of platinum-based anticancer agents, we have investigated the effects of carbonate on cisplatin binding to DNA in vitro. Our results, which are fully consistent with early immunochemical studies of platinum anticancer drugs,7 demonstrate that at physiological concentrations of carbonate the major DNA adducts are bifunctional cross-links. Like phosphate ion and other biological nucleophiles, the major function of carbonate is to reduce the amount of platinum available for DNA modification.
We investigated cisplatin binding to both double- and single-stranded DNA in carbonate, phosphate, and HEPES buffer. In 24 mM buffer at pH 7.4, and 5 mM NaCl, cisplatin was allowed to react with closed circular pBR322 plasmid DNA. After 24 h in the dark at 37°C, unreacted cisplatin was removed by dialysis, and the amount of platinum on the DNA was measured by atomic absorption (AA) spectroscopy. The DNA was then analyzed by agarose gel electrophoresis. Cisplatin intrastrand cross-links unwind duplex DNA and concomitantly wind the superhelix, dramatically altering its mobility on agarose gels. This behavior can be used to probe the binding of cisplatin to DNA in a sensitive manner.8
Analysis of the samples showed that, in HEPES buffer, unwinding of closed circular DNA occurs with increasing concentrations of cisplatin. When the plasmid is completely unwound, such that it co-migrates with DNA containing a single-strand break, increased platinum binding winds the DNA into a positive superhelix. In phosphate buffer, DNA unwinding occurs only at the highest cisplatin concentrations and the coalescence point is not reached. No DNA unwinding occurs for samples treated in carbonate. These data (Fig. S1, Supporting Information) are identical to previously published results, the interpretation of which was that cisplatin binds to DNA monofunctionally in carbonate buffer.6 An alternative explanation, however, is that DNA treated with cisplatin in carbonate does not form a sufficient number of adducts to alter the supercoiling of plasmid DNA under these conditions.
To evaluate the latter possibility, we directly measured rb, the amount of platinum bound per nucleotide, as a function of added cisplatin (Fig. 1). In carbonate and phosphate buffers, the amount of cisplatin on pBR322 DNA is substantially less than in the reaction in HEPES buffer. Slightly less platinum binds to DNA in carbonate than in phosphate buffer. These results clearly reveal that the decreased unwinding of superhelical DNA by cisplatin in phosphate or carbonate buffers is not due to monofunctional adduct formation, but to significantly lower levels of bound platinum. Even at the highest rf, binding of cisplatin to DNA in carbonate buffer does not attain the rb value at which changes in DNA supercoiling occurs (Fig. S1).
Figure 1.
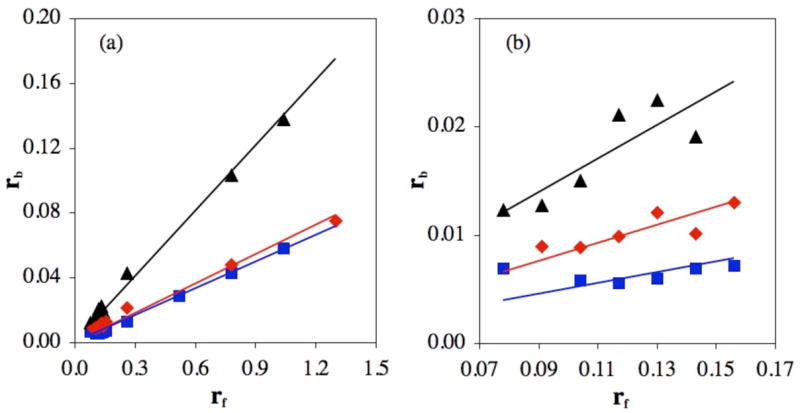
(a) Bound Pt/nt (rb) values of platinated pBR322 DNA as a function of added Pt/nt (rf) for reactions in HEPES (black triangles), phosphate (red diamonds) or carbonate (blue squares) buffer, pH 7.4. (b) Expansion of low range rf values, 0.07 – 0.17.
The reaction of cisplatin with a single-stranded 14mer oligonucleotide, 5′-TTCACCGGAATTCC-3′, was also investigated in 24 mM carbonate, phosphate, and HEPES buffers at pH 6.8 and 7.4, containing 5 mM NaCl (Figs. S2, S3). The DNA contains a single reactive GG site, allowing for both mono- and bifunctional platinum adducts to form. Cisplatin concentrations from 10 to 200 μM were investigated, and the DNA concentration was adjusted to maintain a fixed Pt:DNA ratio of 1.2:1. After 24 h of incubation in the dark at 37°C, reaction products were purified by ion exchange high performance liquid chromatography and characterized by UV and AA spectroscopy, enzymatic digestion with nuclease P1 and calf intestinal phosphatase (CIP), and electrospray mass spectrometry (ESI-MS).
In all buffer systems, the major product of the reaction was determined to be the 1,2-{Pt(NH3)2}2+-d(GpG) cross-link. The mass of this product, as revealed in the negative ion ESI spectrum, is 4424.9 ± 1.7 Da (Fig. S4), in agreement with the expected value of the 14mer bearing a {Pt(NH3)2}2+ lesion (4425.8 Da). After enzymatic digestion of the products with nuclease P1 and CIP, and separation of the resulting deoxynucleosides by LC-MS, the 1,2-{Pt(NH3)2}2+-d(GpG) adduct was directly observed (Fig. S5). A minor product detected by the same methods was a DNA strand containing a 1,3-{Pt(NH3)2}2+-d(CpGpG) cross-link. Cytosine is not very reactive toward cisplatin, although binding of platinum to the cytosine N3 position has been observed in an oligonucleotide.9 Identification of the 1,3-cross-link was confirmed by platinating the corresponding trinucleotide d(CpGpG) under the same conditions and analyzing the products by LC/MS. Peaks appeared at the same retention time with identical mass spectra. UV and AA analyses confirmed that all products contain one Pt atom per 14mer.
The product profile of the reaction of cisplatin and the 14mer oligonucleotide is unaffected by the choice of HEPES, phosphate, or carbonate buffer. Bifunctional platinum-DNA cross-links are the dominant product of all three reactions. In both carbonate and phosphate buffers, however, the reaction is significantly inhibited relative to the reaction in HEPES buffer, particularly at concentrations of cisplatin < 40 μM. These results are consistent with the data in Figure 1 obtained with pBR322 plasmid DNA.
At pH 6.8, the yield of platinated 14mer in carbonate and phosphate buffer increases slightly relative to the yield at pH 7.4. The yield of platinated DNA in HEPES buffer is unaffected by pH. The pKa of carbonic acid (for the equilibrium CO2 + H2O ⇌ HCO3− + H+) is 6.1,11 and the second pKa of phosphate (H2PO4− ⇌ HPO42− + H+) is 7.09. Therefore at lower pH, a larger fraction of both carbonate and phosphate ligands will be protonated, making them better leaving groups. This pH dependence provides strong evidence that inhibition by these anions is due to complexation with the platinum(II) center.
Aquated cisplatin complexes are cations; binding to DNA, a polyanion, is thus assisted by electrostatic attractions. Cisplatin carbonato or phosphato complexes, however, are either neutral or anionic species, depending on the binding mode and protonation state of the ligand, which have not been conclusively established. The altered charge may therefore explain why carbonate and phosphate inhibit binding to DNA. Carbonate is a better ligand for platinum than phosphate,12 which renders it a stronger inhibitor of the reaction.
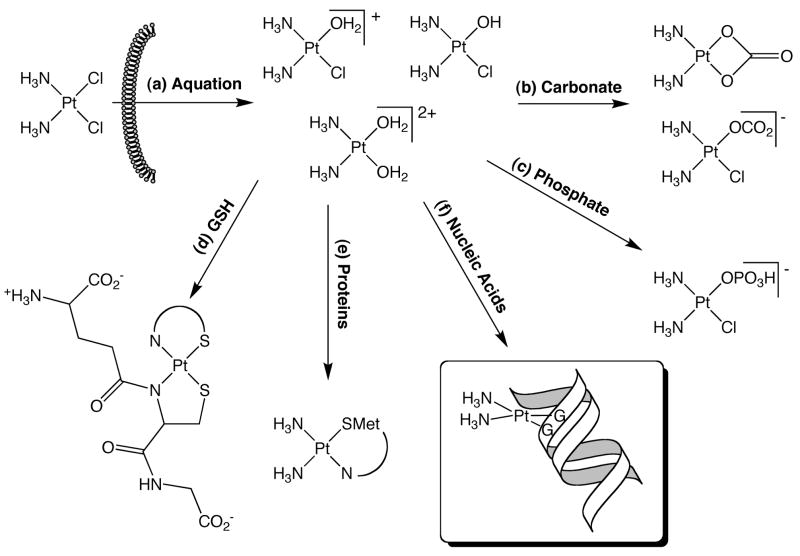
The data presented here show that the presence of physiological concentrations of carbonate and phosphate inhibit the binding of cisplatin to both pBR322 DNA and single-stranded oligonucleotides through formation of cisplatin carbonato and phosphato complexes, respectively. Interactions between cisplatin and other biological nucleophiles such as glutathione4 or hydroxide ion13 “deactivate” cisplatin through formation of relatively unreactive platinum complexes. Our experiments suggest that carbonate and phosphate ions may perform a similar function. Physiological concentrations of phosphate and carbonate in interstitial fluid are approximately 5 mM and 24 mM, respectively, and the concentrations of these species in the cell are approximately 80 mM and 12 mM, respectively.14 Along with proteins and nucleic acids, these biological anions provide numerous options for binding cisplatin once it is aquated inside the cell (see Fig. 2).
Figure 2.
Routes for biological processing of cisplatin. Upon entering the cell, cisplatin is aquated (a). The activated platinum species can then react with carbonate (b) or phosphate (c) in a mono- or bidentate manner. Reaction with glutathione results in the formation of a bis-(glutathionato)- platinum complex4 (d). Platinum can also bind sulfur-containing amino acids, such as Met in human albumin10 (e). Finally, cisplatin can react with purine bases of both DNA and RNA, forming intra- and interstrand crosslinks (f).
Through use of enzymatic digestions and electrospray mass spectrometry, we also demonstrate that cisplatin binds to DNA in a bifunctional manner in the presence of both coordinating (carbonate, phosphate) and weakly coordinating (HEPES) buffers. These results reveal that cisplatin forms primarily bifunctional adducts under physiologically relevant conditions. Intrastrand cross-links with adjacent guanines have previously been assigned as the primary cisplatin-DNA adduct in experiments conducted both in vitro and in vivo with immunochemical detection, accounting for 65% of total platinum-DNA lesions.7 Our findings demonstrate that the presence of carbonate does not affect the preference of cisplatin to form such intrastrand adducts.
Supporting Information Available
Experimental details as cited in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the National Cancer Institute under grant CA34992. We thank Engelhard Corporation (Iselin, NJ, now BASF) for a generous gift of potassium tetrachloroplatinate, which was used to synthesize cisplatin for these experiments. K.S.L. acknowledges financial support from a National Science Foundation Graduate Research Fellowship.
References
- 1.Bancroft DP, Lepre CA, Lippard SJ. J Am Chem Soc. 1990;112:6860–6871. [Google Scholar]
- 2.Jamieson ER, Lippard SJ. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 3.Segal E, Le Pecq JB. Cancer Res. 1985;45:492–498. [PubMed] [Google Scholar]
- 4.Ishikawa T, Ali-Osman F. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- 5.Centerwall CR, Goodisman J, Kerwood DJ, Dabrowiak JC. J Am Chem Soc. 2005;127:12768–12769. doi: 10.1021/ja053353c. [DOI] [PubMed] [Google Scholar]
- 6.Binter A, Goodisman J, Dabrowiak JC. J Inorg Biochem. 2006;100:1219–1224. doi: 10.1016/j.jinorgbio.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 7.(a) Terheggen PMAB, Floot BGJ, Scherer E, Begg AC, Fichtinger-Schepman AMJ, den Engelse L. Cancer Res. 1987;47:6719–6725. [PubMed] [Google Scholar]; (b) Fichtinger-Schepman AMJ, van Oosterom AT, Lohman PHM, Berends F. Cancer Res. 1987;47:3000–3004. [PubMed] [Google Scholar]; (c) Fichtinger-Schepman AMJ, van der Veer JL, den Hartog JHJ, Lohman PHM, Reedijk J. Biochemistry. 1985;24:707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- 8.Cohen GL, Bauer WR, Barton JK, Lippard SJ. Science. 1979;203:1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- 9.Comess KM, Costello CE, Lippard SJ. Biochemistry. 1990;29:2102–2110. doi: 10.1021/bi00460a020. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov AI, Christodoulou J, Parkinson JA, Barnham KJ, Tucker A, Woodrow J, Sadler PJ. J Biol Chem. 1998;273:14721–14730. doi: 10.1074/jbc.273.24.14721. [DOI] [PubMed] [Google Scholar]
- 11.Gross E, Kurtz I. Am J Physiol Renal Physiol. 2002;283:F876–F887. doi: 10.1152/ajprenal.00148.2002. [DOI] [PubMed] [Google Scholar]
- 12.(a) Howe-Grant ME, Lippard SJ. Metal Ions in Biological Systems. 1980;11:63–125. [Google Scholar]; (b) Mahtani HK, Chang SC, Ruble JR, Black INL, Stein PB. Inorg Chem. 1993;32:4976–4978. [Google Scholar]
- 13.Lippert B. Coord Chem Rev. 1999;182:263–295. [Google Scholar]
- 14.Van Wynsberghe D, Noback CR, Carola R. Human Anatomy and Physiology. 3. McGraw-Hill: New York; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details as cited in the text. This material is available free of charge via the Internet at http://pubs.acs.org.