Abstract
The effect of cocaine exposure during early postnatal ages on coupling of dopamine (DA) D1- and D2-like receptors to their respective Gs/olf and Gi was examined in striatum and medial frontal cortex. Sprague-Dawley rats were subcutaneously injected with either 50mg/kg cocaine or vehicle during postnatal day (PnD) 11–20 and dopaminergic D1- and D2-like receptor signaling was evaluated at PnD 60. Results showed that cocaine exposure did not affect the magnitude of both DA D1- and D2-like receptor coupling to their respective Gs/olf and Gi in striatum. However, in the medial-frontal cortex, the basal and the DA D1-like receptor and Gs association were reduced in cocaine-exposed brains. However, there was no change in basal or DA D2-like receptor – Gi linkage in medial frontal cortex. Since frontal cortex plays a critical role in regulating cognition and working memory, disruption of DA-modulated circuits or alteration of dopaminergic activity resulting from postnatal cocaine exposure may result in abnormal responses to environmental challenges leading to long-term behavioral changes.
Cocaine abuse among childbearing women in the United States remains a public health issue [1]. Evidence from both clinical [2, 3] and animal experiments suggests that use of cocaine during pregnancy may cause long-lasting behavioral abnormalities [4–7]. Even though the exact mechanism through which cocaine exposure produces its long-lasting behavioral effects is largely unknown, dysfunction of dopamine (DA) transmembrane signaling has been implicated [8–12]. While prenatal cocaine exposure reduces Gs/olf – DA D1 receptor coupling [9, 12], effects of cocaine on DA receptor signaling have not been studied following early postnatal exposure in the rat, a period brain development similar to late third trimester brain development in human. Since this period is characterized by synaptic pruning and functional development of multiple forebrain systems, we hypothesized that cocaine administration during this period may alter DA receptor signaling. In the present study, cocaine was injected subcutaneously during postnatal day (PnD) 11–20 and the coupling of the DA D1- and D2-like receptors to their respective Gs/olf and Gi proteins, the key step in signaling, was assessed in striatum and medial-frontal cortex (MFC) at adulthood (PnD 60).
All animal protocols were approved by SUNY’s Institutional Animal Care and Use Committee. Adult female Sprague-Dawley rats (VAF, Charles River, Wilmington, ME) were mated in our AAALAC-accredited vivarium (20–22° C with 12 h light-dark cycles, lights on 7AM) with males of the same strain. Starting from the morning of a sperm-positive smear, referred to as gestation day 1(G1), they were housed individually with ad lib food and water and left undisturbed until day of birth in 44 × 24 × 20 cm plastic cages with wood chip bedding. On the day of birth (PnD 1), the litter was culled to 12 pups maintaining equivalent gender representation, if possible, and the pups were toe-clipped for identification. Litters were randomly placed into one of two treatment groups: 50 mg/kg cocaine HCl (Sigma, St Louis, MO) or vehicle (sterile water, 5 µl/g body weight, Baxter). Subcutaneous injections were administered daily from days 11–20 between 11:00 and 13:00. On PnD 21 the pups were weaned into same-sex cages, ear-clipped for identification and weighed every 4 days thereafter until they were 60 days of age. At 60 days, rats were weighed, taken to the necropsy room one at a time and placed in a CO2 chamber until lightly anesthetized and then decapitated. The brains were rapidly removed and put in −50° C methylbutane for 30 sec to solidify. Then the slice containing the striatum (−0.4mm to 1.6mm relative to Bregma) and medial-frontal cortex (5.5mm to 4.2mm relative to Bregma) was dissected from the remaining piece and then frozen in methylbutane at −20° C for 1 minute. The methylbutane was evaporated and the brain sections were put in labeled bags and frozen at −80°C until thawed for preparation of membranes.
To assess the effect of cocaine exposure on the linkage between DA D1 receptors and Gs/olf proteins as well as the coupling between DA D2 receptors and Gi proteins, crude neuronal membranes were prepared from rat brain striata and medial-frontal cortices as described previously [13, 14]. Tissues were thawed on ice and then homogenized in 10 volumes of 25 mM HEPES (pH 7.5) buffer containing 2 mM MgCl2, 1 mM EDTA, 0.2% 2-mercaptoethanol, 50 µg/ml leupeptin, 25 µg/ml pepstatin A, 0.01 U/ml soybean trypsin inhibitor, 0.04 mM phenylmethylsulphonyl fluoride (PMSF) using glass/glass homogenizer. Homogenate was centrifuged at 800 g for 5 min and the supernatant obtained was then centrifuged for 10 min at 48,200 g. Membranes were washed twice, resuspended in 500 µl of oxygenated Kreb’s-Ringer solution: 25 mM HEPES, pH 7.4; 118 mM NaCl, 4.8 mM KCl, 25 mM NaHCO3, 1.3 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM glucose, 100 µM ascorbic acid, 50 µg/ml leupeptin, 10 µg/ml aprotinin, 2 µg/ml soybean trypsin inhibitor, 0.04 mM PMSF. The concentration of membrane proteins was determined by the method of Bradford according to manufacturer’s instruction. Membrane proteins (200 µg) were incubated in Kreb’s-Ringer solution with or without 1 µM of dopamine for 5 min (total incubation volume of 500 µl). The reaction was terminated by addition of Ca2+-, Mg2+- free Kreb’s-Ringer solution containing 1 mM EDTA and centrifuged for 10 min at 48,200 g (at 4°C). Tissues were then resuspended by sonicating for 10 sec on ice in 0.25 ml of the immunoprecipitation buffer containing 100 mM HEPES, pH7.5, 200 mM NaCl, 2 mM MgCl2, 1 mM EDTA, 0.02% 2- mercaptoethanol, 50 µg/ml leupeptin, 25 µg/ml pepstatin A, 0.01 U/ml soybean trypsin inhibitor and 0.04 mM PMSF and solubilized by 0.5% digitonin, 0.2% Sodium cholate, 0.5% vol/vol Nonidet P-40 at 4°C with end-over-end rotation for 1 hr. Following dilution with 0.75 ml immunoprecipitation buffer and then clearing by centrifugation at 48,200 g for 10 min, the solubilized membrane proteins were immunoprecipitated with antibodies directed against Gαs/olf (SC-383) or Gαi (SC-7276) proteins (Santa Cruz Biotechnology, Santa Cruz, CA) using the procedure described previously [13, 14]. The specificities of the anti-Gα antibodies were extensively characterized and described previously [14]. While anti-Gαi antibody cross-reacts mildly with Gαo, anti-Gαo did not precipitate appreciable D1- and D2 receptors [13] Solubilized tissues were incubated with 2 µg anti-Gαs/olf or -Gαi for 2 hr at 4°C followed by an 1 hr incubation with 25 µl of Agarose-conjugated protein A/G (Santa Cruz Biotechnology). The suspension was centrifuged and washed twice with 1 ml immunoprecipitation buffer, the pellet obtained from each tube is suspended in 500 µl of binding buffer (50 mM Tris-HCl, pH7.5; 5 mM MgCl2 and 1 µM mesulergine, a 5-HT2C receptor antagonist) since SCH23390 could also label the Gq-coupled 5-HT2C receptors and incubated for 30 min at 30°C with 1 nM [3H]SCH23390 (70.3 Ci/mmol, PerkinElmer, Boston) or 2 nM [3H]raclopride (60.5 Ci/mmol, PerkinElmer, Boston, MA) for determination of Gαs/olf-coupled DA D1-like receptors and Gαi-coupled DA D2-like receptors, respectively. Nonspecific binding was defined by the addition of 1 µM of unlabeled cis-(Z)-flupenthixol (for determination of the D1-like DA receptors) or l-sulpiride (for measurement of D2-like DA receptors). The reaction was terminated by addition of 9 ml of ice-cold binding buffer and immediately vacuum filtered over Whatman GF/C filters. The amount of radioactivity on filter was assessed by liquid scintillation spectrometry and specific [3H]SCH23390 or [3H]raclopride binding was determined.
To assess the expression levels of the D1AR, D2R and various Gα proteins, Western blotting was conducted as described previously [13, 14] using 25 µg MFC or striatal lysate with antibodies specific for D1AR (SC-33660), D2R (SC-5303), Gαs/olf (SC-383), Gαi (SC-7276), Gαo (SC-387) and Gαq/11 (SC-392), respectively.
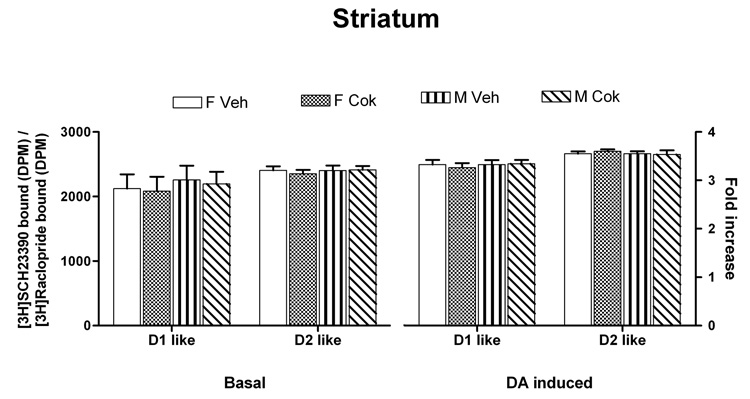
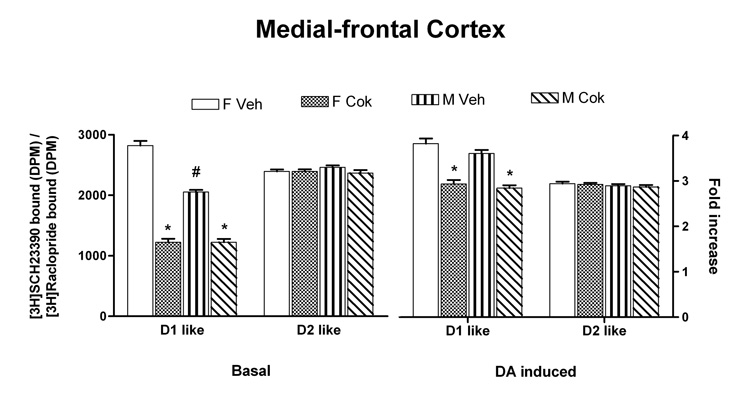
Data were analyzed with mixed linear models in SPSS with D1-like striatum, D2-like striatum, D1-like MFC, D2-like MFC as separate dependent variables, sex and treatment as fixed factors, litter as random factor. All variables failed to show any differences in striatum (Fig. 1). In MFC, however, basal SCH 23390 binding showed a significant decrease in adults treated with cocaine postnatally [F(1,30)=446, p<0.001] (Fig 2). In addition, control males showed a lower level of SCH binding than did the control females [F(1,14)=78.4, p<0.001]. There was also a main effect for treatment for the stimulated D1-like effect [F(1,30) = 107.24, p < 0.0001] within both sexes with the cocaine-exposed rats showing a lower D1-like effect. This cocaine-mediated effect was not the result of altered total receptor populations or G protein levels since our Western blot data showed comparable D1A and D2 –like receptor concentrations as well as various Gα protein levels in both striatum (data not shown) and medial-frontal cortex (Table 1).
Fig. 1.
Comparable association of the Gs/olf - DA D1-like receptors and Gi - D2-like receptors in striatum of PnD 60 male and female rats that have had exposed to cocaine during PnD 11–20. The effect of cocaine exposure during Pn11–20 on Gs/olf - D1-like receptor and Gi – D2-like receptor coupling in striatum from PnD60 male and female rats was assessed under basal (left) and 1 µM DA-stimulated (right) conditions. The Gs/olf-coupled D1-like receptors and Gi-linked D2-like receptors were isolated together with Gs/olf and Gi, respectively by anti-Gαs/olf and -Gαi and then assessed using [3H]SCH23390 and [3H]raclopride binding. No significant differences were observed in the levels of Gs/olf-coupled D1-like receptors and Gi-associated D2-like receptors under either basal or DA-stimulated conditions.
Fig. 2.
Cocaine exposure during PnD11–20 reduces coupling of Gs/olf to DA D1-like receptors but not Gi to D2-like receptors in medial frontal cortex of the PnD 60 male and female rats. Medial frontal cortices were obtained from PnD60 male and female rats that had been exposed to cocaine during PnD 11–20. Following incubation of brain slices with vehicle or 1 µM DA, of the levels of Gs/olf-associated DA D1-like and Gi-coupled DA D2-like receptors were assessed by [3H]SCH23390 and [3H]raclopride binding following immunoprecipitation with anti-Gαs/olf and -Gαi, respectively. Both basal (left) and DA stimulated D1-like effect (right) were significantly reduced in cocaine-treated rats in both sexes when compared with vehicle-treated rats (* denotes significant difference of treated group compared to controls [p<0.001]). Basal SCH23390 binding also showed a significant sex difference for the controls with males showing lower basal D1-like binding than the females (# denotes significant difference from female controls [p<0.001]).
Table 1.
Effect of cocaine administration during the early postnatal period on the abundance of dopamine receptors and Gα proteins.
| Optical Intensity (arbitrary units) | ||
|---|---|---|
| Saline | Cocaine | |
| D1AR | 1017.3 ± 55.0 | 954.0 ± 61.0 |
| D2R | 1036.5 ± 93.8 | 1104.8 ± 137.8 |
| Gαs-51-KDa | 902.8 ± 96.5 | 881.0 ± 92.3 |
| Gαs-45-KDa | 527.5 ± 42.2 | 545.8 ± 44.2 |
| Gαi | 1822.8 ± 138.0 | 1866.8 ± 110.7 |
| Gαo | 1570.8 ± 145.3 | 1505.8 ± 102.0 |
| Gαq/11 | 816.8 ± 35.1 | 865.3 ± 147.2 |
The expression levels of the D1AR, D2R and various Gα proteins were determined in 25 µg of MFC lysate from 2 female, 2 male rats in each of the saline- and cocaine-treated groups. Solubilized MFC lysates were analyzed by Western blotting using specific antibodies to each of the indicated proteins. The optical intensity of the protein bands was determined by densitometric scanning. The data are expressed as mean ± s.e.m. There are no statistical differences in the levels of any proteins assessed.
The reduced coupling of Gs to DA D1-like receptors in MFC observed in current study is compatible with that from our previous studies on prodynorphin mRNA expression [15] and rat brain glucose metabolism after postnatal cocaine exposure [16] as well as the observation of abnormal differentiation of cerebral cortical neurons in rabbit following cocaine exposure [17]. Since D2-like receptor coupling to Gi proteins in both striatum and MFC following cocaine exposure was unaffected, our data also match the observations on rabbit in which prenatal cocaine exposure impaired receptor-G protein coupling only in the D1- but not D2-like receptors [9]. Similar to these results [9], we also did not find changes in density of the D1A and D2 –like receptors as well as various Gα proteins (Data not shown). These methods used enabled us to differentiate the coupling status of a specific receptor system, in this case Gs/olf-coupled D1-like receptors or Gi-linked D2-like receptor system. However, these methods can not discern the possible effects on specific receptor subclasses. Our methods also do not allow elucidation of a possible alteration in the affinity of G protein as a mechanism underlying the reduced coupling between Gs and DA D1-like receptor in MFC. Our previous findings in the rabbit demonstrated that a sustained hyper-phosphorylation of the D1A receptor in prenatal cocaine-exposed brains mediates uncoupling of the D1A –like receptor from its associated Gs/olf protein without an effect on Gα protein [18]. Our present data agree with their previous report that in exposed rabbit brains there is an uncoupling of D1 receptor from its associated Gs/olf as indicated by reduction in dopamine-stimulated Gs – D1 receptor coupling [9, 17]. Collectively, these data suggest that cocaine exposure to developing brains attenuates sensitivity of the D1-like receptor to dopamine and/or reduces coupling efficiency between Gs and D1-like receptor. In contrast, prenatal cocaine-exposed brains showed no change in basal coupling but reductions in DA - Gs- D1A receptor coupling which were attributed to a persistent hyper-phosphorylation of D1A receptor [9, 17–19]. Our data show that cocaine exposure during early postnatal life persistently attenuated basal D1-like receptor – Gs coupling in MFC, reflecting reduced high affinity of D1-like receptors. Hence, the reduced basal coupling together with attenuated dopamine-stimulated Gs – D1-like receptor coupling would amass a dramatic reduction in D1-like receptor activities in animals that have been exposed to cocaine during the early postnatal period. Moreover, the differential effects of cocaine exposure during diverse stages of brain development on basal D1 receptor coupling suggest that different underling mechanisms are involved.
The striatum is the major target for midbrain dopaminergic neurons. The dopaminergic neurons are detected in rat striatum on gestation day 12–14 [20] and undergo rapid differentiation during the last 8 days of gestation [21] with subsequent increases in dopamine levels from gestation day 17 [22]. DA D1 receptors are present in stratum at birth and their overall density reaches adult levels approximately two weeks later [23]. By comparison, the development of the dopaminergic innervation in the medial-frontal cortex takes much longer. Even though the actual dopamine innervation can start as early as PnD 4 in rat, density of the dopaminergic fibers increases steadily until postnatal day 60 [24]. Therefore, cocaine exposure during postnatal days 11 to 20 could have a greater impact on medial frontal cortex dopaminergic circuits that are undergoing development at the time of drug administration. Dopaminoceptive neurons appear to reduce their sensitivity to dopamine when dopaminergic innervation is established and functional neurotransmission begins [25]. This diminished sensitivity of D1-like receptor after cocaine exposure may result from an uncoupling of the Gs protein to D1-like receptor [19, 26] that may be triggered by excessive dopamine in the synapse and/or selective D1-like receptor stimulation during functional maturation of the prefrontal cortex. In fact, the striking reduction in coupling of the DA D1 receptor to its G protein, which is sustained into adulthood, has been suggested as the primary factor for behavioral disturbances as well as dendritic changes caused by cocaine exposure during development [26].
In contrast to a reduced D1-like receptor – Gs coupling in medial-frontal cortex of the cocaine-treated rats, cocaine exposure did not affect the coupling levels of Gs/olf - D1-like and Gi - D2-like receptors in the striatum in the present study. These data however, could not exclude the possibility of an aberrant striatal dopaminergic circuitry because D1-like receptor was activated by phasic but not tonic DA release [27] and DA transporter binding exhibits biphasic alterations after cocaine exposure [28].
Frontal cortex is essential to higher cognition and plays a critical role in working memory and attention control [29]. The dopamine system is integrally involved with both motor control and reward and motivation [30]. Thus, one could predict that even a minimal disruption of DA-modulated circuits or alteration of dopaminergic activity resulting from cocaine exposure in medial frontal cortex may result in abnormal response to environmental or pharmacological challenges.
Taken together, our data presented here support the notion that cocaine exposure during the early postnatal period of the rat may critically affect normal monoaminergic receptor development in brain leading to long-term neurochemical and behavioral changes.
Acknowledgements
This work was supported by NIDA grants DA10990 (D D-E) and MIDARP (HYW). The authors would like to thank Mses. April Jackson and Stacy Stephenson for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ostrea EM, Jr, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89:107–113. [PubMed] [Google Scholar]
- 2.Singer LT, Garber R, Kliegman R. Neurobehavioral sequelae of fetal cocaine exposure. J. Pediatr. 1991;119:667–672. doi: 10.1016/s0022-3476(05)82426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe JJ. Effect of cocaine use on the fetus. N. Engl. J. Med. 1992;327:399–407. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- 4.Spear LP, Kirstein CL, Frambes NA. Cocaine effects on the developing central nervous system: behavioral, psychopharmacological, and neurochemical studies. Ann. N. Y. Acad. Sci. 1989;562:290–307. doi: 10.1111/j.1749-6632.1989.tb21027.x. [DOI] [PubMed] [Google Scholar]
- 5.Henderson MG, McMillen BA. Effects of prenatal exposure to cocaine or related drugs on rat developmental and neurological indices. Brain Res. Bull. 1990;24:207–212. doi: 10.1016/0361-9230(90)90207-g. [DOI] [PubMed] [Google Scholar]
- 6.Dow-Edwards DL. Cocaine effects on fetal development: a comparison of clinical and animal research findings. Neurotoxicol. Teratol. 1991;13:347–352. doi: 10.1016/0892-0362(91)90082-8. [DOI] [PubMed] [Google Scholar]
- 7.Richardson GA. Prenatal cocaine exposure. A longitudinal study of development. Ann. N. Y. Acad. Sci. 1998;846:144–152. [PubMed] [Google Scholar]
- 8.Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- 9.Friedman E, Wang HY. Prenatal cocaine exposure alters signal transduction in the brain D1 dopamine receptor system. Ann. N. Y. Acad. Sci. 1998;846:238–247. [PubMed] [Google Scholar]
- 10.Lidow MS. Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann. N. Y. Acad. Sci. 1998;846:182–193. [PubMed] [Google Scholar]
- 11.Simansky KJ, Baker G, Kachelries WJ, Hood H, Romano AG, Harvey JA. Prenatal exposure to cocaine reduces dopaminergic D1-mediated motor function but spares the enhancement of learning by amphetamine in rabbits. Ann. N. Y. Acad. Sci. 1998;846:375–378. [PubMed] [Google Scholar]
- 12.Harvey JA, Romano AG, Gabriel M, Simansky KJ, Du W, Aloyo VJ, Friedman E. Effects of prenatal exposure to cocaine on the developing brain: anatomical, chemical, physiological and behavioral consequences. Neurotox. Res. 2001;3:117–143. doi: 10.1007/BF03033234. [DOI] [PubMed] [Google Scholar]
- 13.Jin LQ, Wang HY, Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J. Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Dow-Edwards DL, Hurd YL. Perinatal cocaine decreases the expression of prodynorphin mRNA in nucleus accumbens shell in the adult rat. Brain Res. Mol. Brain Res. 1998;62:82–85. doi: 10.1016/s0169-328x(98)00218-6. [DOI] [PubMed] [Google Scholar]
- 16.Dow-Edwards DL, Freed-Malen LA, Hughes HE. Long-term alterations in brain function following cocaine administration during the preweanling period. Brain Res. Dev. Brain Res. 1993;72:309–313. doi: 10.1016/0165-3806(93)90198-j. [DOI] [PubMed] [Google Scholar]
- 17.Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang HY, Friedman E, Levitt P. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J. Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen XC, Torres C, Wang HY, Friedman E E. Protein phosphatase 1 regulates brain D1A dopamine receptor phosphorylation: role in dopaminergic dysfunction after in utero cocaine exposure. J Neurosci. 2001;21:9160–9167. doi: 10.1523/JNEUROSCI.21-23-09160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HY, Runyan S, Yadin E, Friedman E. Prenatal exposure to cocaine selectively reduces D1 dopamine receptor-mediated activation of striatal Gs proteins. J. Pharmacol. Exp. Ther. 1995;273:492–498. [PubMed] [Google Scholar]
- 20.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25:857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 21.Coyle JT, Henry D. Catecholamines in fetal and newborn rat brain. J. Neurochem. 1973;21:61–67. doi: 10.1111/j.1471-4159.1973.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 22.Santana C, Rodriguez M, Afonso D, Arevalo R. Dopaminergic neuron development in rats: biochemical study from prenatal life to adulthood. Brain Res. Bull. 1992;29:7–13. doi: 10.1016/0361-9230(92)90003-g. [DOI] [PubMed] [Google Scholar]
- 23.Murrin LC, Zeng WY. Dopamine D1 receptor development in the rat striatum: early localization in striosomes. Brain Res. 1989;480:170–177. doi: 10.1016/0006-8993(89)91579-5. [DOI] [PubMed] [Google Scholar]
- 24.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J. Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 25.Kim DS, Froelick GJ, Palmiter RD. Dopamine-dependent desensitization of dopaminergic signaling in the developing mouse striatum. J. Neurosci. 2002;22:9841–9849. doi: 10.1523/JNEUROSCI.22-22-09841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanwood GD, Levitt P. Drug exposure early in life: functional repercussions of changing neuropharmacology during sensitive periods of brain development. Curr. Opin. Pharmacol. 2004;4:65–71. doi: 10.1016/j.coph.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95 Suppl 2:S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- 28.Koff JM, Miller LG. Prenatal cocaine exposure: increased striatal dopamine transporter binding in offspring at 3 and 6 months of age. Brain Res. Bull. 1994;33:223–224. doi: 10.1016/0361-9230(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 29.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]