Abstract
Grainyhead transcription factors play an evolutionarily conserved role in regulating epidermal terminal differentiation. One such factor, the mammalian Grainyhead-like Epithelial Transactivator (Get1/Grhl3), is important for epidermal barrier formation. In addition to a role in barrier formation, Grainyhead genes play roles in closure of several structures such as the mouse neural tube and Drosophila wounds. Consistent with these observations, we found that Get1 knockout mice have an eye-open at birth phenotype. The failure of eyelid closure appears to be due to critical functions of Get1 in promoting F-actin polymerization, filopodia formation, and the cell shape changes that are required for migration of the keratinocytes at the leading edge during eyelid closure. The expression of TGFα, a known regulator of leading edge formation, is decreased in the eyelid tip of Get1−/− mice. Levels of phospho-EGFR and phospho-ERK are also decreased at the leading edge tip. Furthermore, in an organ culture model, TGFα can increase levels of phospho-EGFR and promote cell shape changes as well as leading edge formation in Get1−/− eyelids, indicating that in eyelid closure Get1 acts upstream of TGFα in the EGFR/ERK pathway.
Introduction
The transcription factor Grainyhead was first identified in Drosophila where it was demonstrated to play a key role in the formation of the normal larval cuticle (Bray and Kafatos, 1991). In addition to a role in epidermal barrier formation, Grainyhead is critical for wound healing in Drosophila (Mace et al., 2005). Studies in C. elegans and Xenopus also suggests a role for Grainyhead genes in cuticular formation and epidermal differentiation, respectively (Tao et al., 2005b; Venkatesan et al., 2003). In mammals, Grainyhead-like epithelial transactivator 1 (Get1; also referred to as Grainyhead-like 3, Grhl3) (Kudryavtseva et al., 2003; Ting et al., 2003), one of the three mammalian Grainyhead genes, has been most extensively studied. This gene regulates key aspects of the terminal differentiation program and barrier formation of the mouse epidermis (Ting et al., 2005; Yu et al., 2006). In addition, Get1/Grhl3 promotes epithelial closure of embryonic wounds as well as neural tube closure (Ting et al., 2005; Ting et al., 2003). Evolutionary sequence analysis suggests that the ancestral Grainyhead gene arose at the time of the first multicellular organisms over 500 million years ago (Venkatesan et al., 2003).
A subset of mice with a mutation in Get1 are born with eye-open phenotype (Yu et al., 2006), suggesting a defect in eyelid closure during embryonic development. Eyelid closure is a normal biological event in the development of all mammals; in the mouse the processes of eyelid development and closure are completed in five steps between embryonic days (e) 11.5 and 16.5 (Findlater et al., 1993; Mine et al., 2005; Tao et al., 2005a). First, the primitive eyelid starts forming at e11.5 with groove formation along with increased proliferation and morphological changes that generate protruding ridges of epithelium referred to as the eyelid root. This process is completed by e14.5 when in the second stage, a leading edge composed of groups of rounded cells starts extending from the tip of root epithelium. The third stage is marked by protrusion of the opposing leading edges, which extend over the cornea between e14.5 and e16. During the fourth stage, the leading edges meet together over the center of the cornea and fuse around e16. In the final stage, at e16.5, the eyelid roots appose, causing the epithelial cells of the leading edge to pile up at the center; these cells eventually slough off (Mine et al., 2005).
Several interacting signal transduction pathways have been shown to control eyelid formation. FGF signaling originating from the mesenchyme is important for the formation of the primitive eyelid as well as leading edge development (Li et al., 2001; Tao et al., 2005a). EGF ligands acting through the EGF receptor, and activin acting through JNK and c-Jun, are critical for leading edge migration (Xia and Kao, 2004). Eyelid closure defects are also common in mice with mutations in planar cell polarity genes (Wang and Nathans, 2007).
Similar to dorsal closure in Drosophila, eyelid closure in mammal is thought to reproduce many features of natural wound healing. Dorsal closure, eyelid closure and wound healing are all characterized by specific morphogenetic steps that include the formation of leading edge of epithelial cells with actin stress fibers, directed cell migration and re-epithelialization (Martin and Parkhurst, 2004). The eye-open phenotype of Get1 knockout mice is of interest because it provides an opportunity to investigate the role of Grainyhead-like genes, such as Get1, in closure of epithelial structures. While there is evidence for the role of Grainyhead genes in epithelial closure, both cellular and molecular mechanisms involved in Get1-dependent closure remain poorly defined. In this study, we have taken advantage of the eye-open phenotype to study the mechanisms of action for Get1-dependent epithelial closure. Our results indicate that Get1 promotes actin polymerization, filopodia formation, keratinocyte cell shape change, and the timely formation of the leading edge during eyelid closure. Furthermore, our data suggest that Get1 acts upstream of TGFα in the EGFR/ERK pathway to regulate migration of the keratinocyte leading edge.
Materials and Methods
Mice
The generation of Get-1−/− mice was previously described (Yu et al., 2006). Genotypes of mice were determined as previously described.
RT-PCR analysis
Semi-quantitative RT-PCR was performed with cDNA generated with the High Capacity cDNA Archive Kit (Applied Biosystems), using total RNA, which was prepared from back skin of WT and Get-1−/− embryos using Trizol Reagent (Invitrogen). Reactions were sampled after 25, 28 and 30 cycles at different PCR conditions to monitor product accumulation. TGFα forward primer: CTGAAGGGAAGCTGCTTG; reverse primer: CAACCCTTTGAGGTTCGTGT.
Histology, immunofluorescence, and BrdU staining
Embryonic mouse heads were fixed in 10% formalin and paraffin-embedded. Following sectioning, 6-μm sections were stained with hemotoxylin and eosin. For immunofluoresence staining, 6-μm-thick fresh frozen sections were air-dried and fixed in cold Acetone (−20°C) for 5 min, followed by a PBS wash. Sections were then soaked in blocking solution for 40 min, incubated with primary antibody for 2 h, washed three times with PBS, and incubated with FITC secondary IgG polyclonal antibody (Oncogene Research Products) and DAPI as a counterstain. Pregnant mice were injected with BrdU (100ug/g of body weight) 1 h before dissection. For BrdU detection, slides were pre-treated in 1 M HCl for 1 h at 37 °C. The primary antibodies were as follows: Rabbit anti-mouse Get-1, rabbit anti-mouse TGFα (sc-9043, Santa Cruz Biotechnology), rabbit anti-mouse EGFR (sc-03, Santa Cruz Biotechnology), goat anti-mouse phospho-EGFR (sc-12351, Santa Cruz Biotechnology), mouse monoclonal antibody for phospho-ERK (9106, Cell Signaling Technology), rabbit anti-mouse phospho-JNK (9251, Cell Signaling Technology) and anti-BrdU (Roche), rabbit anti-mouse keratin 6 (PRB-169p, Covance).
In-situ hybridization
In situ hybridization studies with 35S-labeled cRNA probes were performed on paraffin-embedded and frozen sections and counterstained with bisbenzamide as described previously (Andersen et al., 1995; Andersen et al., 1997).
Transmission electron microscopy
The eye region was dissected from e15.5 embryos and fixed by immersion in 4% paraformaldehyde and 1% gluteraldehye in 0.1 M PBS (pH 7.4) at room temperature followed by a PBS wash. The tissues were then postfixed with 0.2% Ruthenium tetroxide (RuO4) and dehydrated through graded ethanol series, and embedded in agar 100 resin. Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined on a transmission electron microscope.
Scanning electron microscopy
The eye region was dissected and fixed in 10% formalin overnight. After a PBS wash, tissues were postfixed in 1% Osmium for 1 h and dehydrated through graded ethanol series and HMDS. After gold coating, tissues were observed with scanning electron microscope.
Wholemount Phalloidin Staining
The dissected eyes were fixed in 4% PFA, washed with PBS and incubated in Texas Red -phalloidin (Molecular Probes) overnight. Tissues were scanned with a confocal microscope.
Generation of a Get1 antibody
Rabbit anti mouse Get-1 polyclonal antibody was generated by injecting a conjugated peptide corresponding to the N-terminus of Get1 into rabbits. The peptide used for generating the antibody was CSNEDEAWKTYLENPLT where cystein was added to the N-terminus to aid in conjugation. The fifth and the terminal bleeds from the two rabbits were affinity purified using the same peptide.
Explant cultures of mouse eyelids
Eyelids were cultured according to a previously described method (Tao et al., 2006; Tao et al., 2005a). Briefly, eyelids from e14.5 mouse embryos were dissected under a sterile condition, and placed on 0.4-μm Milli Cell-EM culture plate insert (PICM03050, Millipore) in 6-well plates containing DK-SFM (Invitrogen) with PBS or 20 ng/mL TGFα (Sigma). The organs were incubated at 37°C with 100% humidity, 95% air and 5% carbon dioxide for 10h.
Results
Mutation of Get1 leads to a delayed eyelid closure in mice
We previously generated a mutant allele of Get1 by deleting exons 4 to 7, which include regions encoding the critical DNA-binding domain. At birth (e18.5), approximately 10% of Get1−/− embryos displayed eye-open phenotype while none of wild type (WT) embryos had open eyes (Yu et al., 2006). To determine whether the eye-open phenotype was due to an effect of Get1 on eyelid closure, we investigated the eye phenotype of Get1−/− embryos during earlier stages. As expected, we found that eyelid closure was completed in 100% of WT mouse embryos by e16. In contrast, 100% and 25% of Get1−/− embryos had open eyes at e16 and e17, respectively (Figs. 1A–C). Thus, the eye-open phenotype of Get1−/− mice is fully penetrant when examined during embryogenesis. These results show that a mutation of Get1 leads to delayed eyelid closure, indicating that Get1 functions in normal eyelid closure. Eyelid closure defects are sometimes associated with a delay in other developmental fusion processes during late embryogenesis in the mouse, including fusion of the ear pinnae to the scalp, and digit fusion (Juriloff, 1987) (Maconnachie, 1979). Consistent with this notion, we found that at e15.5 the pinnae were fused to the cranium in WT embryos (Figs. 1D and F), while this fusion was incomplete in the Get1−/− mice (Figs. 1E and G). However, we did not detect delayed digit fusion (Supplementary Fig. 1).
Fig. 1. Delayed eyelid closure and delayed pinna fusion in Get1 mutant mice.
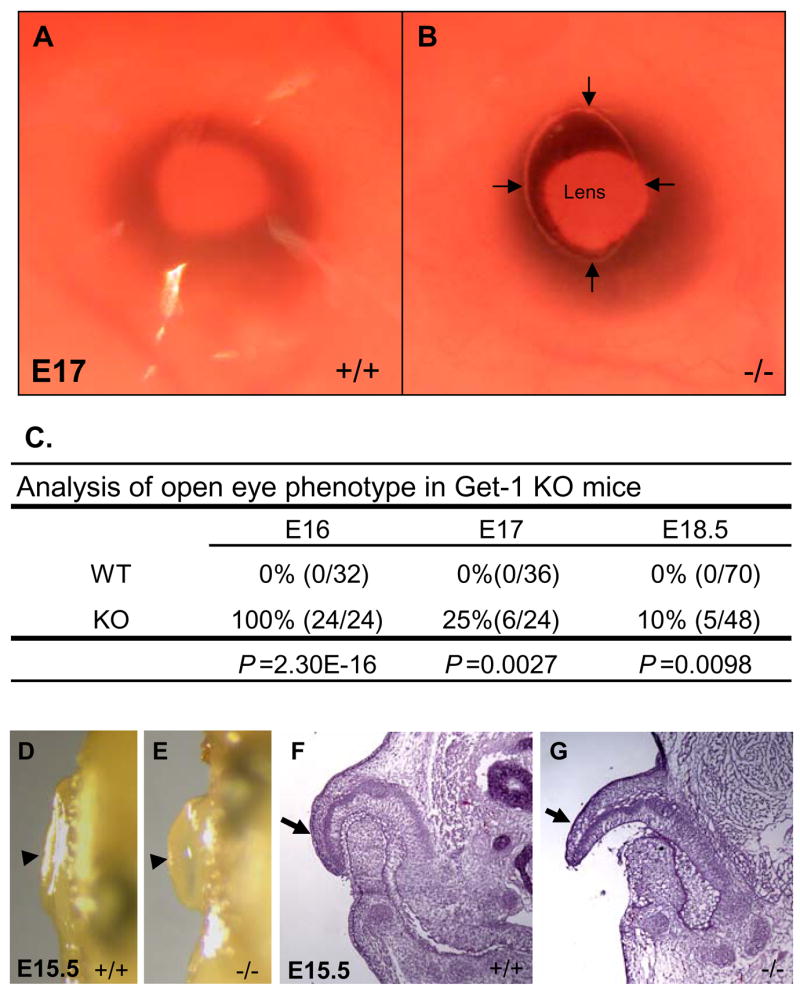
(A) Eyelid is closed in WT embryos at e17. (B) Open eye in Get1 mutant mice at e17. (C) Statistical analysis of the eye-open phenotype in WT and Get1 mutant mice. P-values were determined using fisher exact probability test (one tailed). (D) Ear pinna is fused to the scalp in WT embryos at e15.5. (D) Unfused ear pinna in Get1 mutant embryos at e15.5. Arrowheads point to the ear pinnae in panels D and E. (F and G) H&E staining of the ears from WT and Get1 mutant mice at e15.5. Arrows point to the tip of the pinnae in panels F and G.
Get1 expression is upregulated in keratinocytes of the leading edge
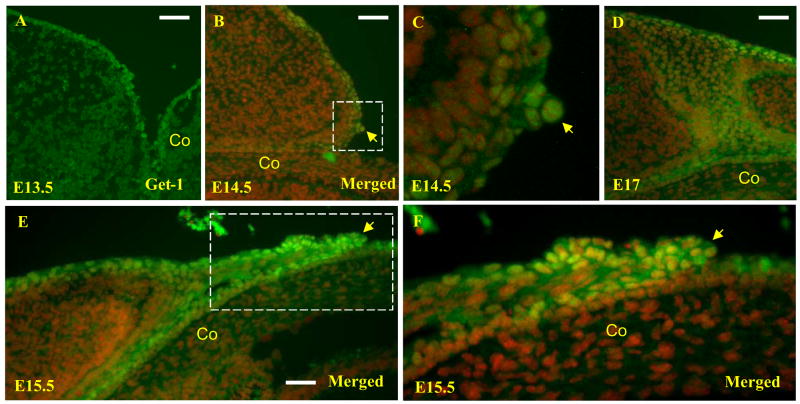
To start investigating the cause of the eye-open phenotype of Get1−/− mice, we performed immunofluorescence studies to determine the expression pattern of Get1 during eyelid development. Get1 transcripts are expressed throughout the developing epidermis from at least as early as e11.5 (Auden et al., 2006; Kudryavtseva et al., 2003), and as expected, uniform Get1 protein expression was found in epidermal keratinocytes covering the eyelid at e13.5 (Fig. 2A). Get1 expression becomes highly upregulated in the cluster of rounded keratinocytes that initiate the formation of the leading edge at e14.5 (Figs. 2B–C). At e15.5, when the leading edge is extending towards the center of eye, Get1 continues to be highly expressed in keratinocytes of the outer layer of the migrating leading edge (Figs. 2E–F). At e17, when the leading edges have fused to close the eyelid, Get1 expression is downregulated along the fusion line but persists in the suprabasal epidermal keratinocytes covering the eyelids (Fig. 2D). Interestingly, Get1 mRNA expression is also highly upregulated in the epidermal wound front in adult mice (Supplementary Fig. 2A–B), suggesting that upregulation of Get1 in migrating epidermal keratinocytes could be a common feature. Together, these data suggest the possibility that Get1 may play roles in keratinocyte migration during epidermal closure.
Fig. 2. Get1 expression during eyelid development.
(A to F) Immunofluorescence analysis of Get1 expression in eyelids at e13.5 (A), e14.5 (B and C), e15.5 (E and F) and e17 (D). (B and C) The arrow points to the beginning of the leading edge at e14.5, where Get1 is highly expressed. (C) High magnification of the boxed region in panel B. (E and F) The arrow indicates the leading edge of the eyelid which expresses Get1 at high levels. (F) High magnification of the boxed region in panel E. Red represents DAPI staining. Co, cornea. Scale bar: 50 μm.
Impaired formation of the leading edge in Get1−/− mice
To investigate the potential role of Get1 in eyelid closure, we compared eyelid development in Get1−/− and WT embryos at various stages, using histological analysis. In WT embryos, the root of the primitive eyelid was fully formed at e13.5 (Figs. 3A and C), and a small leading edge protruded from eyelid root at e14.5 (Figs. 3E and G); the leading edge extended towards the center at e15.5 (Figs. 3I and K) and fused in the center of eye at e16 (Figs. 3M and O). In Get1−/− embryos, no obvious abnormality was found at e13.5, indicating that eyelid root formation is independent of Get1 (Figs. 3B and D). However, marked abnormalities were found in the Get1−/− mice at e14.5 (Figs. 3F and H) and e15.5 (Figs. 3J and L); no leading edge had formed at these time points. At e16, a rudimentary leading edge was observed (Figs. 3N and P), but it did not continue to extend. These results show that both initiation and progression of the leading edge are affected by Get1 gene deletion. At birth, eyelid closure was achieved in 90% of Get1−/− mice, perhaps due to compensatory mechanisms or through apposition of the eyelid roots. Statistical analysis on multiple embryos at e15.5 shows that whereas the eyelid root is of normal size, the length of leading edge is strikingly shorter in Get1−/− mice (Figs. 3Q–R). These results indicate that during eyelid closure, Get1 functions predominantly in the formation and extension of the leading edge.
Fig. 3. Defective formation of the eyelid leading edge in Get1−/− mice.
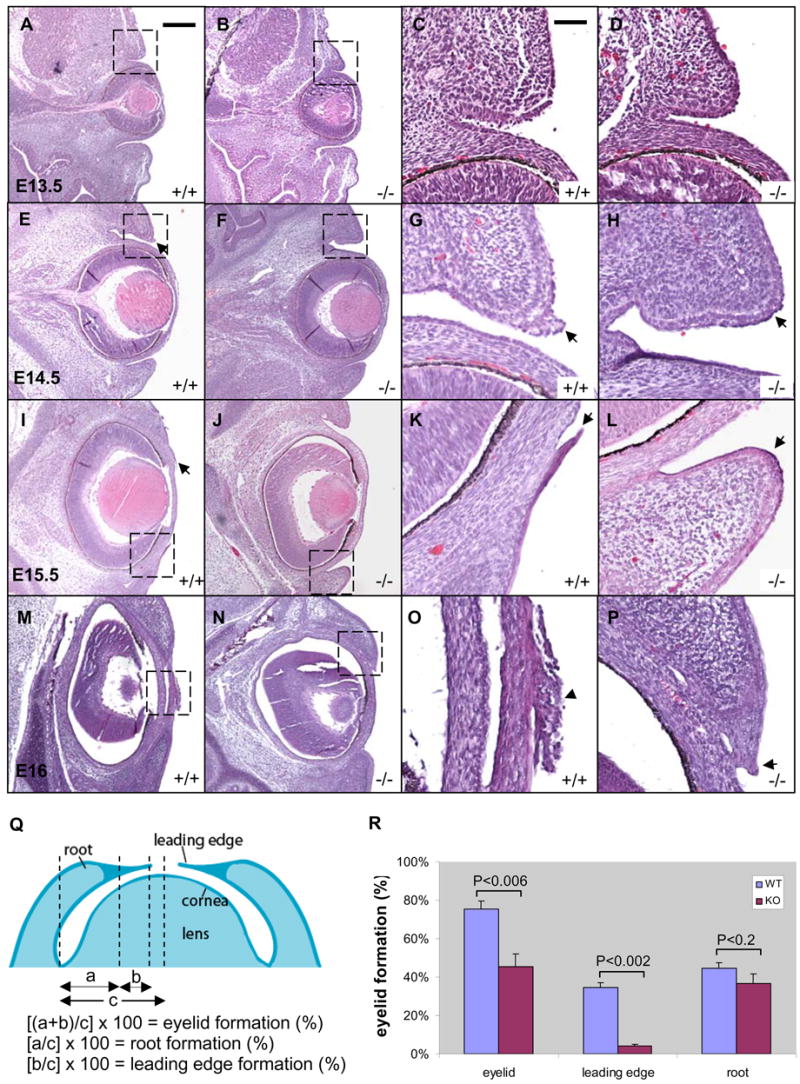
(A to P) H&E staining of the eyes from WT and Get1 mutant mice during eyelid closure at e13.5 (A to D), e14.5 (E to H), e15.5 (I to L) and e16 (M to P). The panels in the last two columns are high magnification pictures of the corresponding boxed regions in the panels in the two columns to the left. Arrows point to the tip of the eyelid. The arrowhead in panel O points round keratinocytes after eyelid fusion. (Q) Schematic diagram showing how eyelid root, leading edge and total eyelid were measured during eyelid closure at E15.5. Adapted from Mine et al. (Mine et al., 2005). (R) Quantification and statistics of eyelid closure in WT and Get1 mutant mice at e15.5. Results represent the mean and SEM for 5 embryos. P-values were determined using T-test (one tailed). Scale bars 200 μm for A, B, E, F, I, J, M, N; 50 μm for C, D, G, H, K, L, O, P.
Get1 is required for cell shape changes and filopodia formation in the leading edge
We used scanning electron microscopy (SEM) to study the cellular appearance of the eyelid in Get1−/− embryos. In WT mice at e15.5, we observed an accumulation of loosely associated surface keratinocytes with a rounded appearance at the eyelid margin (Figs. 4A, and C–F); the cell shape change is thought to relate to the migratory behavior of these cells. In Get1−/− mice at e15.5, this cell shape change was much less prominent, and of the rounded cells that were present, many extended from the epidermal surface at aberrant locations away from the eyelid tip (Figs. 4B and G–J). Compared with WT eyelids, the typical clump of rounded keratinocytes at the inner and outer canthus was absent in the Get1−/− mice (Figs. 4A, B, C, E, G, and I). Also, in the Get1−/− eyelids, many of the rounded keratinocytes appeared to have an axis parallel to the rim of the eyelid while in WT mice, rounded keratinocytes appeared to have an axis perpendicular to the edge of the eyelid, consistent with their migration towards the center of the cornea. By e16, WT eyelids were closed with a clump of degenerating keratinocytes located at the site of closure. In the Get1−/− mice, however, the eye remained open at e16 with disorganized and scattered rounded keratinocytes, many of them in aberrant locations (Fig. 4L). By e17, the Get1−/− eyelids closed, apparently through apposition of the eyelid roots and only occasional scattered rounded keratinoctes were observed; a typical cell clump does not form over the fused region (Figs. 4M and N).
Fig. 4. Defective cell shape changes during leading edge formation in Get1 mutant mice.
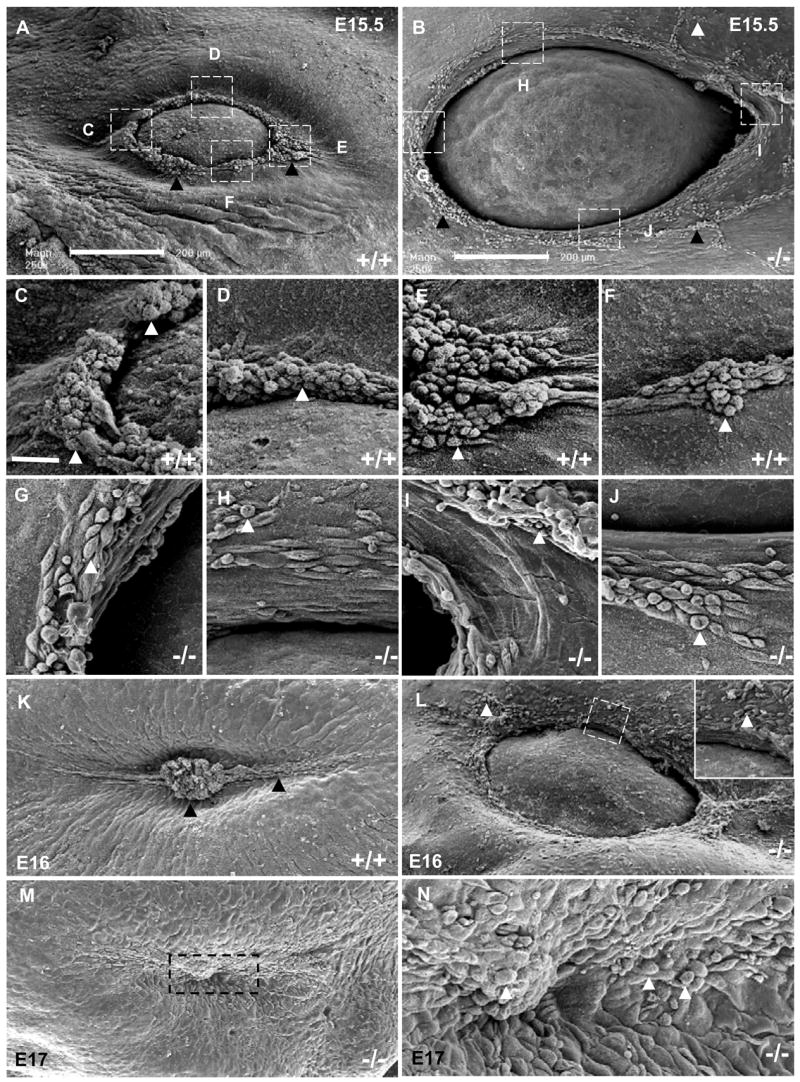
Scanning electron microscopy of the eyelid region of WT (A, C to F and K) and Get1−/− (B, G to J and L to N) mice during the indicated time points. (C to J) Higher magnification pictures of the corresponding boxed regions in panels A and B. The arrowheads point to rounded cells. The boxed region in panel L is shown in higher magnification in the insert. High magnification picture of the fusion region contained in the boxed region in panel M is shown in panel N. Scale bar is 200 μm for A, B, K, L, M; 25 μm for C-J.
The rounded keratinocytes at the leading edge are known to form filopodia, which are rod-like extensions of the cell surface filled with parallel bundles of actin filament that function in cell migration and epithelial fusion (Faix and Rottner, 2006; Martin and Parkhurst, 2004). Transmission electron microscopy (TEM) revealed abundant filopodia formation in keratinocytes of the WT leading edge. In contrast, the leading edge was immature with fewer and shorter filopodia in the Get1−/− mice (Figs. 5C–D). Consistent with the SEM findings in Get1−/− mice, rounded keratinocytes with filopodia were present in eyelid epithelia located away from the tip of eyelid (data not shown) in Get1 mutant mice. These data indicate that Get1 is required for the cell shape change and filopodia formation at the leading edge during eyelid closure.
Fig. 5. Defective filopodia formation in Get1 mutant mice.
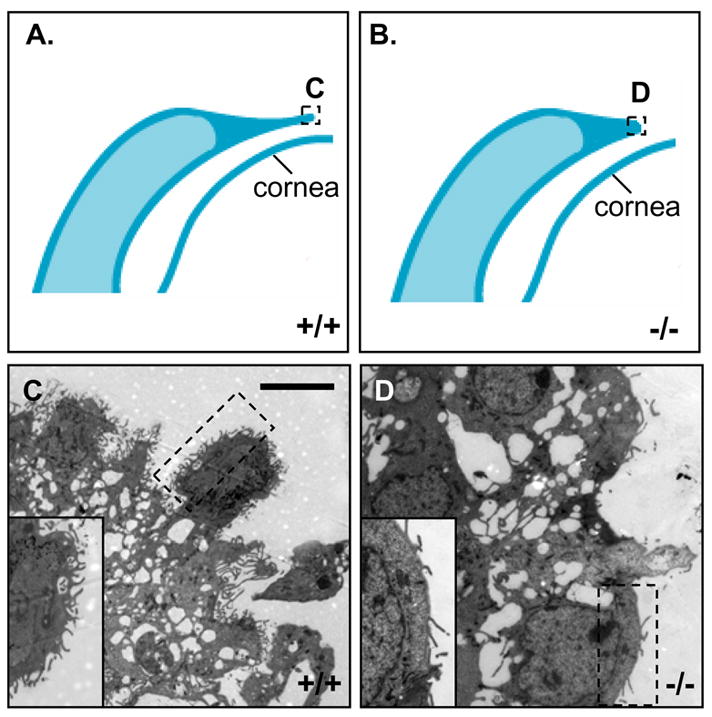
(A and B) Schematic diagram showing the tip region of WT and Get1 mutant eyelids at e15.5. The boxes C–F indicate the regions imaged by transmission electron microscopy and shown in the corresponding panels. (C) The tip of the WT leading edge. The high magnification insert of the boxed area shows numerous well developed filopodia. (D) The tip of the Get1 mutant eyelid. The insert shows high magnification picture of the boxed region, highlighting paucity of filapodia.
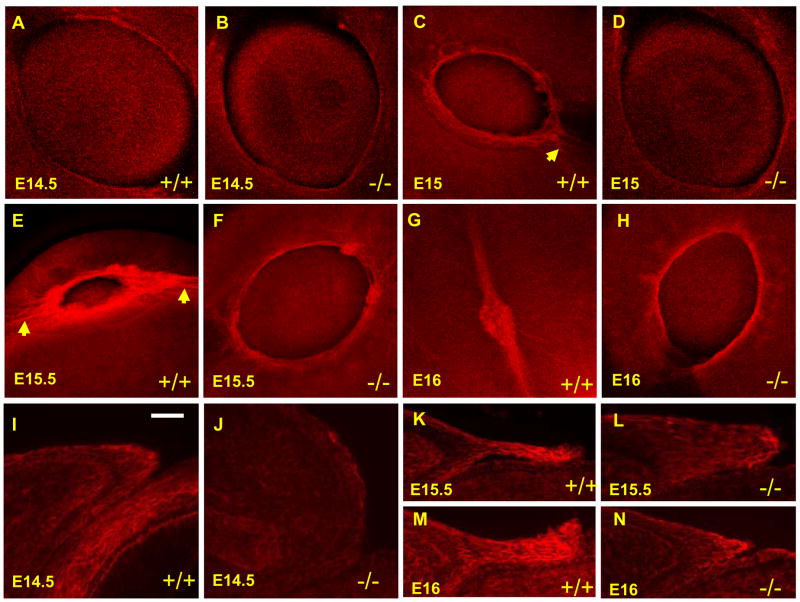
Get1 is required for normal actin bundle formation during leading edge development
During eyelid closure, actin filament polymerization in the leading edge generates the force required for formation of filopodia and cell migration. To test whether Get1 regulates actin filament polymerization in the eyelid epithelium, we examined the polymerization of F-actin at various time points using wholemount and section Phalloidin staining. In WT eyelids, F-actin first becomes prominent at e14.5. Subsequently, Phalloidin staining becomes progressively stronger at e15 and e15.5, eventually decreasing at e16 as the eyelids fuse (Figs. 6A, C, E and G). By contrast, in Get1−/− eyelids, F-actin formation is greatly decreased at all stages (Figs. 6B, D, F and H). These results are consistent with the relative absence of rounded keratinocytes, and fewer and shorter filopodia in the leading edge of Get1−/− eyelids as observed by SEM and TEM, respectively. Similar results were obtained when eyelid tissue sections were stained with Phalloidin (Figs. 6I–N). In contrast to the striking effects on F-actin formation, Get1 had no effect on cell proliferation (Supplementary Fig. 3A–D) or apoptosis (data not shown) in the eyelids during the same stages of eyelid development. We conclude that Get1 regulates genes that affect actin stress fiber polymerization in the leading edge of the developing eyelid.
Fig. 6. Get1 promotes actin filament polymerization in the developing eyelid epithelium.
(A to H) Wholemount phalloidin staining of WT and Get1 mutant eyes at e14.5 (A and B), e15 (C and D), e15.5 (E and F) and e16 (G and H). (I to N) Phalloidin staining of eyelid sections from WT and Get1 mutant mice at e14.5 (I and J), e15.5 (K and L) and e16 (M and N). Arrows in panel C and E indicate radial actin fibers. Scale bar: I–N, 50μm.
Get1 regulates the TGFα/EGFR/ERK signaling pathway during eyelid closure
Previous studies have shown that the EGFR/ERK signaling pathway plays a key role in eyelid closure (Xia and Kao, 2004). Mice lacking the EGFR ligand TGFα display impaired formation of the leading edge with occasionally eye-open phenotype at birth (Luetteke et al., 1993). Also, similar to Get1, TGFα is highly expressed in leading edge of the eyelid (Berkowitz et al., 1996) and in the suprabasal layers of the epidermis (Gibbs et al., 1998). Furthermore, we noticed in a previous microarray gene expression study that TGFα was downregulated in the epidermis of Get1 mutant mice (Yu et al., 2006). The similar expression patterns and eye phenotypes of these two mouse mutants prompted us to examine the potential role of Get1 in the TGFα pathway. TGFα transcript expression was decreased in the skin of Get1−/− mice at both e16.5 and e18.5 (Fig. 7A). Immunofluorescence studies showed that in WT mice, TGFα was most highly expressed in the most superficial keratinocytes of the epidermis, including the tip of the eyelids at e14 and e14.5 (Figs. 7B and D), and in the leading edge of eyelids at e15 and e15.5 (Figs. 7F and H). In Get1−/− eyelids, TGFα levels were lower and expression was especially decreased at the tip of the eyelid, which is the site where the normal leading edge forms (Figs. 7C, E, G and I).
Fig. 7. Impaired TGFα expression in Get1 mutant eyelids.
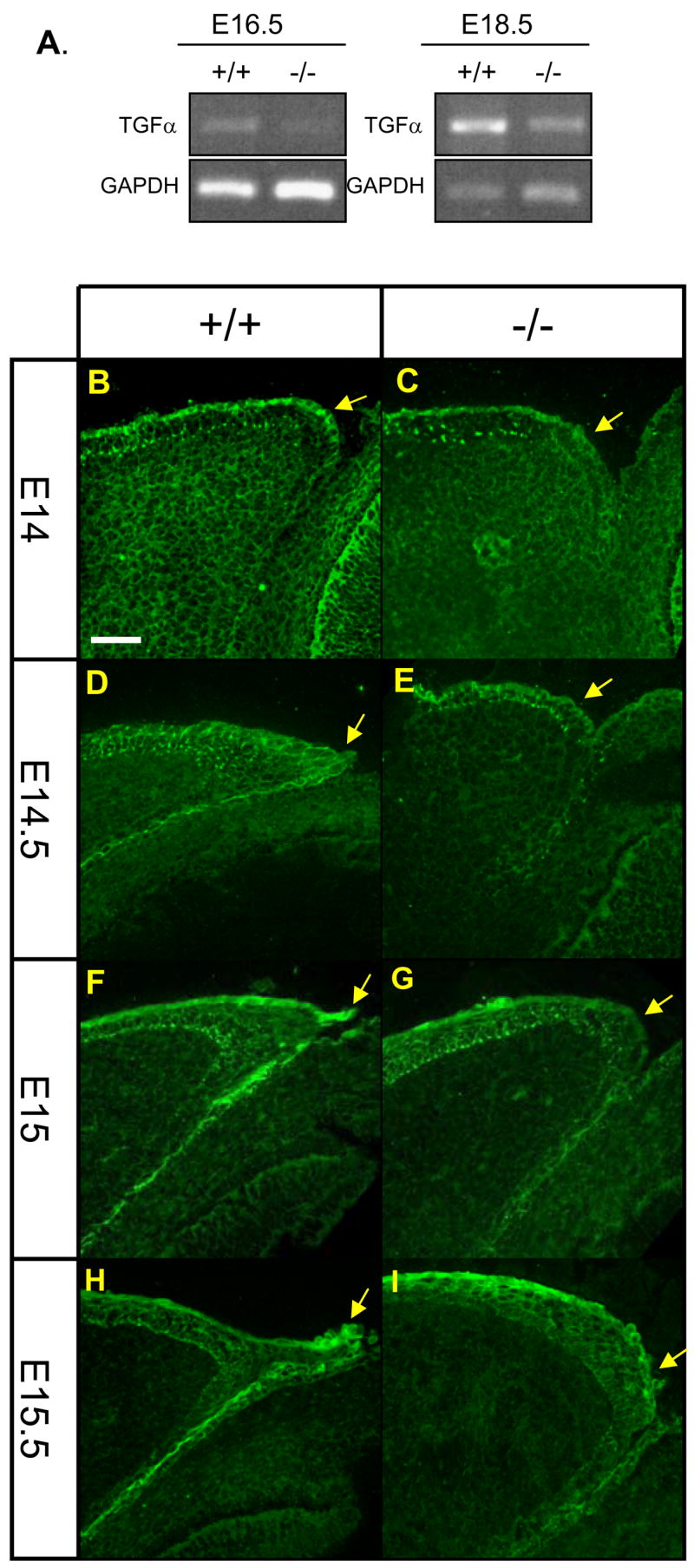
(A) RT-PCR analysis of TGFα expression in skin from WT and Get1−/− mice. (B to I) TGFα expression pattern in eyelid epithelium of WT and Get1 mutant mice at e14 (B and C), e14.5 (D and E), e15 (F and G) and e15.5 (H and I). Arrows point to the tip of the leading edge. Scale bar: 50μm.
While EGFR expression is similar in WT and Get1−/− eyelids at e14.5 and e15.5 (Figs. 8A–D), phosphorylated EGFR fails to concentrate in the tip and the leading edge of the Get1−/− eyelids, as is observed in the WT eyelids (Figs. 8E–H). Expression of phosphorylated EGFR in the suprabasal layers of the eyelid epidermis otherwise appears normal. Expression of phosphorylated ERK was also decreased at the tip of the developing eyelids of Get1−/− mice (Figs. 8I–L), but appeared to be normal in the basal layer of the epidermis and in the dermis at e15.5 (Figs. 8K–L). Keratin (K) 6, which is a marker gene for epithelialization (Paladini and Coulombe, 1998) in adult skin and in embryonic eyelid closure (Mazzalupo et al., 2003), plays a role in migration during wound healing (Wojcik et al., 2000; Wong and Coulombe, 2003). Also, a previous study showed that TGFα induces K6 and K16 expression (Jiang et al., 1993). Consistent with these studies, we found that K6 is markedly upregulated at the tip of the eyelid in WT mice (Figs. 8M,O). And consistent with the decreased TGFα/EGFR/ERK activation at the leading edge in Get1−/− mice, K6 expression fails to focus at the tip of the leading edge (Figs. 8M,P). Increased K6 expression, however, was observed in an aberrant location away from the rim of the eyelid, consistent with the presence of rounded keratinocytes observed in this location with SEM. In contrast to the defective EGFR signaling, there was no change in the expression of phosphorylated JNK (Figs. 8Q–T), a major downstream target for TGFβ/activin-MEKK1-JNK-cJun, which is also important for eyelid closure (Li et al., 2003; Weston et al., 2004; Xia and Kao, 2004; Zenz et al., 2003; Zhang et al., 2003). Taken together, these data indicate that Get1 participates in regulation of the TGFα/EGFR/ERK signaling pathway during leading edge formation in eyelid closure.
Fig. 8. Impaired EGFR/ERK signaling in Get1 mutant eyelids.
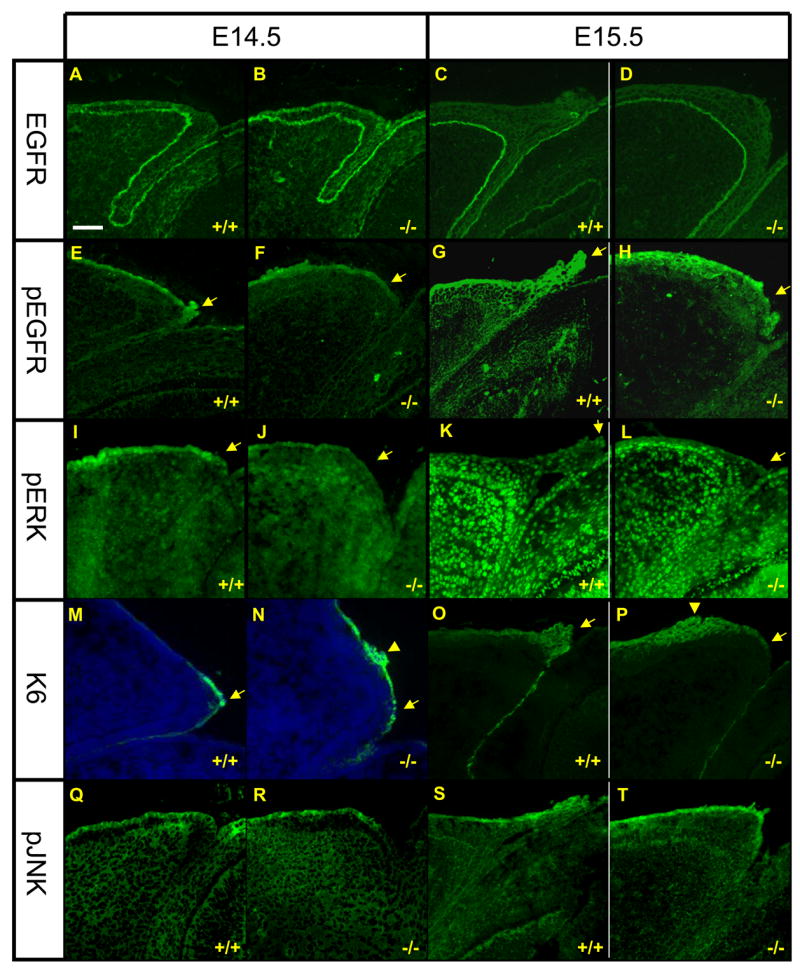
Expression of EGFR (A to D), pEGFR (E to H), pERK (I to L), K6 (M to P), and pJNK (Q to T) in WT and Get1−/− eyelids at e14.5 (n=3) and e15.5 (n=3). Arrows point to the tip of the leading edge. The arrow heads in panels N and P point to K6 upregulation in an aberrant location of the eyelid in Get1−/− mice. Scale bar: 50μm.
TGFα can rescue eye-open phenotype in Get1−/− eyes
If Get1 acts upstream of TGFα during eyelid closure, then TGFα should be able to promote eyelid closure in Get1−/− eyes. To test this prediction, we performed TGFα rescue experiments in Get1−/− mice, using eyelid explant culture methods previously developed by others (Tao et al., 2006; Tao et al., 2005a). TGFα added to the cultures significantly promotes eyelid closure in Get1−/− explant cultures after 10 hours (Figs. 9A–D). In response to TGFα treatment, we observed accelerated closure along both the short and long axis of the Get1−/− eyelids (Figs. 9E–F). TGFα does not affect WT eyelid closure significantly (Supplementary Fig. 4), indicating specificity of the TGFα effect. Histological analysis showed that while with PBS treatment, no leading edge formed over 10 hours (Fig. 9G), TGFα induced the formation of a leading edge in Get1−/− eyelids (Fig. 9H). Consistent with these findings, SEM showed limited accumulation of rounded cells in the rim of Get1−/− eyelids treated with PBS (Fig. 9I). In contrast, TGFα treatment induces accumulation of rounded cells at the eyelid rim (Fig. 9J), although the number of rounded cells is higher in WT eyelids (data not shown), suggesting incomplete rescue. These results indicated that TGFα can induce cell shape changes and the formation of the leading edge on a Get1−/− background. Furthermore, the downstream mediators of TGFα signaling, phospho-EGFR and phospho-ERK were upregulated in Get1−/− eyelids after addition of TGFα (Figs. 9K–N). Taken together, these results suggest that during leading edge formation in the eyelid, Get1 functions upstream of TGFα in the TGFα/EGFR/ERK signaling pathway.
Fig. 9. TGFα can rescue eyelid closure defect in Get1 mutant mice.
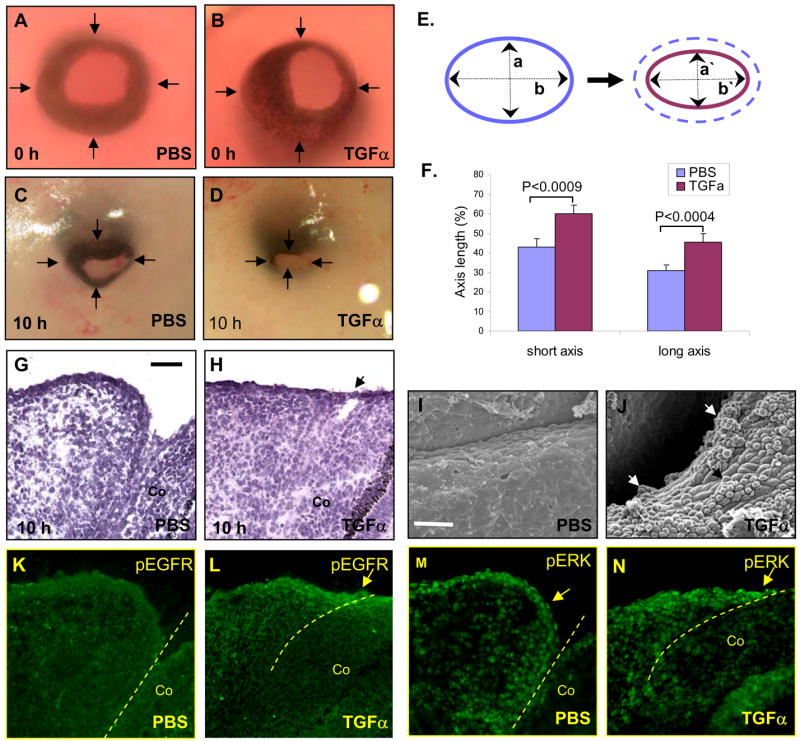
(A to D) Gross pictures of Get1−/− eyes before (A and B) and after (C and D) 10 h of PBS or TGFα treatment. Arrows point to the eyelid borders. (E) A schematic showing measurements of long and short axes, which was used to assess eyelid closure as previously described (Tao et al., 2005a), using the formula: % eyelid short axis closure = (1−a′/a) × 100, and % eyelid long axis closure = (1−b′/b) × 100 where “a” and “b” represent short axis and long axis of open eye, respectively, at time 0 h, and “a`” and “b`” represent short axis and long axis of open eye, respectively, at time 10 h. (F) Analysis of the effect of TGFα treatment on eyelid closure in Get1−/− eyes. The bars represent means and SEM, based on 15 experiments. (G and H) H&E staining of Get1−/− eyelids treated with PBS (G) and TGFα (H) for 10 h (n=4). The arrow points to the leading edge forming in response to TGFα treatment. (I and J) SEM analysis of Get1−/− eyelids after treatment with PBS (I) and TGFα (J) for 10 h (n=4). Arrows in panel J point to rounded cells forming in response to TGFα.. (K and L) pEGFR staining in Get1−/− eyelids after treatment with PBS (K) and TGFα (L) for 10 h (n=4). The arrow points to increased pEGFR staining in the leading edge in response to TGFα treatment. The broken line indicates the eyelid-cornea junction. (M and N) pERK staining in Get1−/− eyelids after treatment with PBS (M) and TGFα (N) for 10 h (n=4). The arrow points to increased pERK staining in the leading edge in response to TGFα treatment. The broken line indicates the eyelid-cornea junction. Co, cornea. Scale bars: G-L, 50μm; M-N, 25 μm.
Discussion
In this manuscript we report a novel role for Get1 in eyelid development; deletion of the Get1 gene leads to delayed eyelid closure. Get1 is strongly expressed in the leading edge of the developing eyelid and in the leading edge of wounds. Consistent with the expression pattern, the formation of the eyelid leading edge was impaired in Get1 mutant mice. The number of migrating round-shaped keratinocytes was greatly decreased and many of the rounded cells that were present were aberrantly localized to a region away from the eyelid tip in Get1 mutant mice. In addition, filapodia formation was impaired and actin bundles, which create the force for cell migration and formation of filopodia, are strikingly decreased in the eyelid tip of Get1 mutant mice. Actin fiber polymerization is known to be regulated by the TGFα/EGFR/ERK signaling pathway and TGFα and levels of its downstream targets, phosphorylated EGFR and phosphorylated ERK, were decreased in the tip of the eyelid in Get1 mutant mice. In explant cultures, the addition of TGFα to Get1−/− eyelids promoted cell shape changes and induced formation of a leading edge, leading to accelerated eyelid closure. These results indicate that Get1 acts upstream of TGFα in the EGFR/ERK signaling pathway to regulate eyelid closure.
Get1 regulates leading edge formation and keratinocyte sheet migration
Mammalian eyelid development and fusion follows a stereotypical stepwise pathway in which the primitive eyelid root forms first (Findlater et al., 1993; Mine et al., 2005; Tao et al., 2005a). Then, a leading edge of keratinocytes extends centripetally from the rim of the eyelid, ultimately covering the eye through keratinocyte sheet migration. After fusion of the eyelid, the eyelid roots appose and most of the leading edge epithelial sheet is shed. In addition to migration, keratinocyte proliferation in the basal layer of the eyelid root is required for normal eyelid closure. Our data shows that the eye-open phenotype of Get1−/− mice is due to a defect in the initiation of the leading edge and migration of the epithelial sheet (Figs. 3A–P). Get1 does not appear to affect eyelid root formation or keratinocyte proliferation (Supplementary Fig. 3). Therefore, we conclude that the likely role of Get1 in eyelid closure involves keratinocyte migration. This notion is also consistent with the upregulation of Get1 in wound front keratinocytes in adult skin (Supplementary Fig. 2). Interestingly, in Get1−/− mice, we observed the formation of rounded keratinocytes at the superficial surface of the eyelid, in an aberrant location away from the eyelid rim. These cells were observed with SEM (Fig. 4J) and with K6 upregulation (Figs. 8S and U). Therefore, Get1 may play a role in focusing the site of the initial leading edge.
Get1 deletion affects F-actin polymerization, keratinocyte shape change, and filopodia formation during eyelid closure
During eyelid closure, F-actin polymerizes to form a contractile cable at the leading edge (Zhang et al., 2003), which has been proposed to drive epithelial sheet movement in a purse string manner (Martin and Parkhurst, 2004; Shimizu et al., 2005). Similar actin cables are thought to generate the contractile force required for epithelial fusion during dorsal closure in Drosophila (Kiehart et al., 2000). In addition to actin cable formation, radial F-actin fibers form, which extend with an axis in direction of the leading edge (Zhang et al., 2003). Wholemount Phalliodin staining showed that both types of F-actin reorganization are greatly decreased in the developing eyelid of Get1−/− mice (Figs. 6A–H), indicating that Get1 regulates F-actin fiber formation. Actin reorganization is also thought to be required for the striking changes in keratinocyte cell-shape in the migrating leading edge (Kodama et al., 2004). During normal eyelid closure, keratinocytes take on a rounded appearance and in the Get1−/− mice this phenomenon is markedly decreased (Fig. 4). Most likely this defect relates to the lack of actin polymerization.
Filopodia are rod-like extensions of cell surface filled with parallel bundles of actin filaments (Faix and Rottner, 2006). Filopodia-mediated epithelial migration and fusion are common phenomena in vertebrate development fusion events (Martin and Parkhurst, 2004). Filopodia can be observed in the opposing eyelid epithelial cells, as the eyelids transiently fuse in late mammalian gestation (Zenz et al., 2003). Compared with WT mice, filopodia appear much fewer and shorter in the leading edge of Get1−/− mice (Figs. 5C–D). It is likely that this defect also relates to lack of F-actin polymerization, and contributes to decreased keratinocyte migration and delayed eyelid closure.
Get1 functions upstream of the TGFα/EGFR signaling pathway
While we have gained insights into the molecular mechanisms whereby Get1 regulates barrier formation, the molecular mechanisms underlying the role of Get1 in re-epithelialization remain unclear. An important regulator of eyelid closure is the EGFR signaling pathway. Targeted disruption of the EGFR gene leads to an eye-open phenotype at birth with 100% penetrance (Miettinen et al., 1995; Sibilia and Wagner, 1995; Threadgill et al., 1995). A critical event in EGFR activation during eyelid closure appears to be binding by its ligands TGFα and HB-EGF. Thus, both TGFα and HB-EGF are highly expressed in the leading edge of the mouse eyelid, and mice deleted for either TGFα or HB-EGF exhibit eye-open phenotype at birth, although with incomplete penetrance (Berkowitz et al., 1996; Luetteke et al., 1993; Mann et al., 1993; Mine et al., 2005). EGFR signaling activates ERKs, leading to the induction of actin filament polymerization (Xia and Kao, 2004; Zhang et al., 2003). The actin stress fibers form actin purse-string at the leading edge of the migrating epithelial sheet of eyelid, which drives epithelial cell closure (Martin and Parkhurst, 2004). Furthermore, TGFα/EGFR induces expression of K6 and K16 (Jiang et al., 1993), keratins which are markers of activated keratinocytes and facilitate their migration in the developing eyelid and wound healing (Paladini and Coulombe, 1998) (Mazzalupo et al., 2003) (Wong and Coulombe, 2003).
The decreased TGFα expression (Figs. 7B–I), as well as decreased EGFR and ERK activation (Figs. 8E–L) in the tip of Get1−/− eyelids suggests that Get1 may act upstream of TGFα in this pathway. Consistent, with this model, the severity of the eye-open phenotype of Get1 and TGFα mutated mice is similar, and TGFα was able to rescue the phenotype in Get1−/− eyelids (Figs. 9A–F). Under these conditions, TGFα was able to increase EGFR (Figs. 9I–J) and ERK (Figs. 9K–L) activation, and induce cell shape changes (Figs. 9M–N) and leading edge formation (Figs. 9G–H). These data lend further support for our model that Get1 acts upstream of TGFα. Since decreased TGFα expression in Get1−/− mice is observed prior to formation of the leading edge in WT mice, it is unlikely that this decrease is due to the absence of TGFα expressing cells (Figs. 7B–E).
We also noticed that the rescue of TGFα in Get1−/− eyelids was incomplete. While this may be due to limitations of the organ culture model, we cannot rule out the possibility that Get1 also acts downstream in the EGFR pathway. In this respect, it is important to note that in Drosophila wound healing, Grainyhead acts downstream of ERK signaling. Other pathways important for eyelid closure include the TGFβ/activin-MEKK1-JNK-cJun signaling pathway (Weston et al., 2004; Xia and Kao, 2004; Zhang et al., 2003), which crosstalk with the TGFα/EGFR/ERK pathway because c-Jun can induce EGFR and HB-EGF expression (Li et al., 2003; Zenz et al., 2003). Our data showing normal JNK activation in the Get1−/− eyelids argues against a major role for Get1 in the activation of the TGFβ/activin-MEKK1-JNK-cJun signaling pathway.
The eye open at birth phenotype is also common in mice with mutations in core planar cell polarity genes and it has been argued that eyelid closure represents a planar cell polarity process (Wang and Nathans, 2007). Interestingly, inhibition of WNT signaling in the developing epidermis leads to an eye open phenotype (Andl et al., 2002). However, it is unclear whether this relates to activation of the canonical WNT pathway or the planar cell polarity pathway. In Drosohpila, Grainyhead affects planar polarity in the wing (Lee and Adler, 2004), and the Get1−/− mice (Ting et al., 2005; Yu et al., 2006) have many features in common with mice mutated for core planar cell polarity genes (Jones and Chen, 2007; Seifert and Mlodzik, 2007; Wang and Nathans, 2007). Therefore, if the planar cell polarity pathway is important in eyelid closure, Get1 might modulate this pathway. However, the link between planar cell polarity genes and eyelid closure remains to be defined.
Get1 has sequential distinct roles in epithelial closure
Previous studies have demonstrated that Grainyhead genes play important roles in epithelial barrier formation and that this function is conserved in evolution across multiple species. In Drosophila, Grainyhead, activates the Dopa Decarboxylase (Ddc) gene, which is required for hardening of the larval cuticle (Bray and Kafatos, 1991). In the mouse, the Grainyhead homologue Get1 activates a broad set of terminal differentiation genes that are required for epidermal barrier formation. The genetic program activated by Get1 during terminal differentiation of the epidermis includes lipid-producing enzymes, adhesion molecules, structural genes and protein crosslinking enzymes (Fig. 10) (Ting et al., 2005; Yu et al., 2006).
Fig. 10. The sequential distinct roles of Get1 in eyelid development.
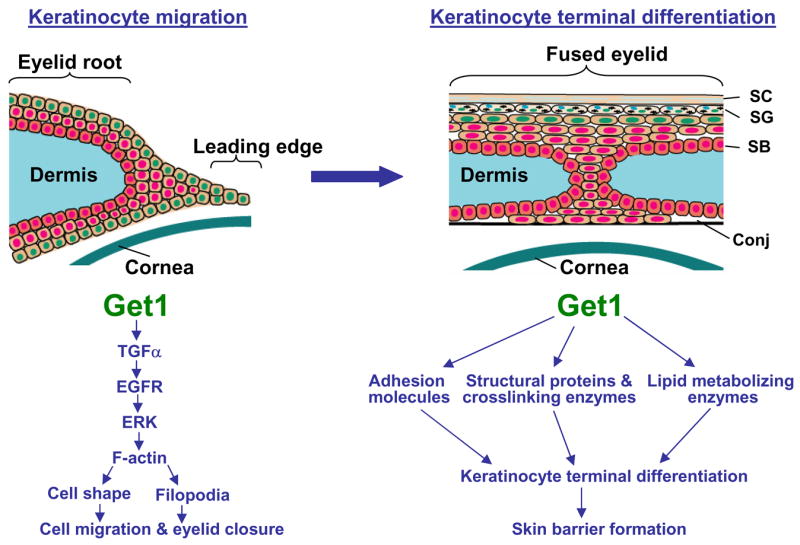
A schematic representation of the distinct roles of Get1 in eyelid development. Get1 plays an early role in formation and migration of the leading edge keratinocyte sheet (left panel), and a later role in the formation of the epidermal barrier after eyelid closure (right panel). During leading edge formation, Get1 activates F-actin polymerization through the TGFα/EGFR/ERK signaling pathway. F-actin fibers further regulate cell shape change and filopodia formation to promote keratinocyte migration and eyelid closure. Get1 promotes skin barrier formation by directly or indirectly regulating genes encoding structural proteins and crosslinking enzymes, adhesion molecules, and lipid metabolizing enzymes (Ting et al., 2005; Yu et al., 2006). SC, cornified layer; SG, granular layer; SB, basal layer; Conj, Conjunctiva.
In addition to a role in epidermal barrier formation, there is previous evidence indicating a role for Get1 in re-epithelialization. In Drosophila, Grainyhead is required for wound healing (Mace et al., 2005), and in mice, Get1 mutation impairs embryonic wound healing (Ting et al., 2005). Consequently, similar to the barrier function, the wound-healing role of Grainyhead also appears to be evolutionarily conserved. Our experiments strongly indicate that Get1 regulates eyelid closure by affecting F-actin polymerization through the TGFα/EGFR/ERK signaling pathway (Fig. 10). These observations suggest that in mice Get1 employs distinct mechanisms to execute the two functions in re-epithelialization and barrier formation. Therefore, during the epithelial closure process in mice, Get1 is likely to act at two distinct steps: early in epithelial migration to close the epithelial defect, and later after completion of re-epithelialization, in barrier formation of the epithelial sheet (Fig. 10). It has been proposed that during evolution Grainyhead genes arose in the first epithelia (Venkatesan et al., 2003). Since the early epithelia may not have evolved complex barrier mechanisms, it is tempting to speculate that the re-epithelialization function of Grainyhead may be more ancient than its barrier role.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AR44882 and EY016425 to BA. We thank Kevin Lin for help with statistics; James Jester for help with wholemount Phalloidin staining; and Liz Rugg for reading the manuscript and helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen B, et al. The Ames dwarf gene is required for Pit-1 gene activation. Dev Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- Andersen B, et al. Functions of the POU domain genes Skn-1a/i and Tst-1/Oct-6/SCIP in epidermal differentiation. Genes Dev. 1997;11:1873–84. doi: 10.1101/gad.11.14.1873. [DOI] [PubMed] [Google Scholar]
- Andl T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Auden A, et al. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr Patterns. 2006;6:964–70. doi: 10.1016/j.modgep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Berkowitz EA, et al. Characterization of the mouse transforming growth factor alpha gene: its expression during eyelid development and in waved 1 tissues. Cell Growth Differ. 1996;7:1271–82. [PubMed] [Google Scholar]
- Bray SJ, Kafatos FC. Developmental function of Elf-1: an essential transcription factor during embryogenesis in Drosophila. Genes Dev. 1991;5:1672–83. doi: 10.1101/gad.5.9.1672. [DOI] [PubMed] [Google Scholar]
- Faix J, Rottner K. The making of filopodia. Curr Opin Cell Biol. 2006;18:18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Findlater GS, et al. Eyelid development, fusion and subsequent reopening in the mouse. J Anat. 1993;183(Pt 1):121–9. [PMC free article] [PubMed] [Google Scholar]
- Gibbs S, et al. Temperature-sensitive regulation of epidermal morphogenesis and the expression of cornified envelope precursors by EGF and TGF alpha. Cell Tissue Res. 1998;292:107–14. doi: 10.1007/s004410051040. [DOI] [PubMed] [Google Scholar]
- Jiang CK, et al. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci U S A. 1993;90:6786–90. doi: 10.1073/pnas.90.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Chen P. Planar cell polarity signaling in vertebrates. Bioessays. 2007;29:120–32. doi: 10.1002/bies.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff DM. Maternal treatment with cortisone accelerates eyelid closure and other developmental fusion processes in fetal mice. Development. 1987;100:611–8. doi: 10.1242/dev.100.4.611. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, et al. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–90. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A, et al. Coordinating cytoskeletal tracks to polarize cellular movements. J Cell Biol. 2004;167:203–7. doi: 10.1083/jcb.200408047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva EI, et al. Identification and characterization of Grainyhead-like epithelial transactivator (GET-1), a novel mammalian Grainyhead-like factor. Dev Dyn. 2003;226:604–17. doi: 10.1002/dvdy.10255. [DOI] [PubMed] [Google Scholar]
- Lee H, Adler PN. The grainy head transcription factor is essential for the function of the frizzled pathway in the Drosophila wing. Mech Dev. 2004;121:37–49. doi: 10.1016/j.mod.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Fibroblast growth factor receptor 2 (Fgfr2) plays an important role in eyelid and skin formation and patterning. Dev Dyn. 2001;222:471–83. doi: 10.1002/dvdy.1205. [DOI] [PubMed] [Google Scholar]
- Li G, et al. c-Jun is essential for organization of the epidermal leading edge. Dev Cell. 2003;4:865–77. doi: 10.1016/s1534-5807(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, et al. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–78. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Mace KA, et al. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–5. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- Maconnachie E. A study of digit fusion in the mouse embryo. J Embryol Exp Morphol. 1979;49:259–76. [PubMed] [Google Scholar]
- Mann GB, et al. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73:249–61. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Mazzalupo S, et al. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev Dyn. 2003;226:356–65. doi: 10.1002/dvdy.10245. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–41. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Mine N, et al. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005;132:4317–26. doi: 10.1242/dev.02030. [DOI] [PubMed] [Google Scholar]
- Paladini RD, Coulombe PA. Directed expression of keratin 16 to the progenitor basal cells of transgenic mouse skin delays skin maturation. J Cell Biol. 1998;142:1035–51. doi: 10.1083/jcb.142.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–53. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–8. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Tao H, et al. Exogenous FGF10 can rescue an eye-open at birth phenotype of Fgf10-null mice by activating activin and TGFalpha-EGFR signaling. Dev Growth Differ. 2006;48:339–46. doi: 10.1111/j.1440-169X.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- Tao H, et al. A dual role of FGF10 in proliferation and coordinated migration of epithelial leading edge cells during mouse eyelid development. Development. 2005a;132:3217–30. doi: 10.1242/dev.01892. [DOI] [PubMed] [Google Scholar]
- Tao J, et al. BMP4-dependent expression of Xenopus Grainyhead-like 1 is essential for epidermal differentiation. Development. 2005b;132:1021–34. doi: 10.1242/dev.01641. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–4. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Ting SB, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–3. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- Ting SB, et al. The identification and characterization of human Sister-of-Mammalian Grainyhead (SOM) expands the grainyhead-like family of developmental transcription factors. Biochem J. 2003;370:953–62. doi: 10.1042/BJ20021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan K, et al. Functional conservation between members of an ancient duplicated transcription factor family, LSF/Grainyhead. Nucleic Acids Res. 2003;31:4304–16. doi: 10.1093/nar/gkg644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–58. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Weston CR, et al. The c-Jun NH2-terminal kinase is essential for epidermal growth factor expression during epidermal morphogenesis. Proc Natl Acad Sci U S A. 2004;101:14114–9. doi: 10.1073/pnas.0406061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, et al. Delayed wound healing in keratin 6a knockout mice. Mol Cell Biol. 2000;20:5248–55. doi: 10.1128/mcb.20.14.5248-5255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163:327–37. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Kao WW. The signaling pathways in tissue morphogenesis: a lesson from mice with eye-open at birth phenotype. Biochem Pharmacol. 2004;68:997–1001. doi: 10.1016/j.bcp.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Yu Z, et al. The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev Biol. 2006;299:122–36. doi: 10.1016/j.ydbio.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Zenz R, et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–89. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure. Embo J. 2003;22:4443–54. doi: 10.1093/emboj/cdg440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.