Abstract
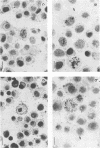
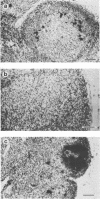
Strand-specific hybridization probes were used in in situ hybridization studies to localize cells containing mink enteritis virus (MEV) virion DNA or MEV replicative-form DNA and mRNA. Following the experimental MEV infection of 3-month-old unvaccinated mink, a significant increase in serum antibodies to MEV was detected at postinfection day (PID) 6, 2 days after the onset of fecal shedding of virus. Prior to the appearance of virus in feces, viral DNA could be detected in the mesenteric lymph node and intestine. The largest percentage of cells positive for virion DNA was 10% and was detected in the intestine on PID 6. However, replication of the virus apparently peaked at PID 4. The number of MEV replicative-form DNA and mRNA molecules was found to be approximately 250,000 copies per infected lymph node cell or crypt epithelial cell. The localization, levels, and time course of viral replication have important implications for the pathogenesis of MEV-induced disease. The data presented on MEV are correlated with earlier results on the other mink parvovirus, Aleutian mink disease parvovirus, and a possible explanation for the remarkable differences in pathogenesis of disease caused by these two parvoviruses is discussed.
Full text
PDF
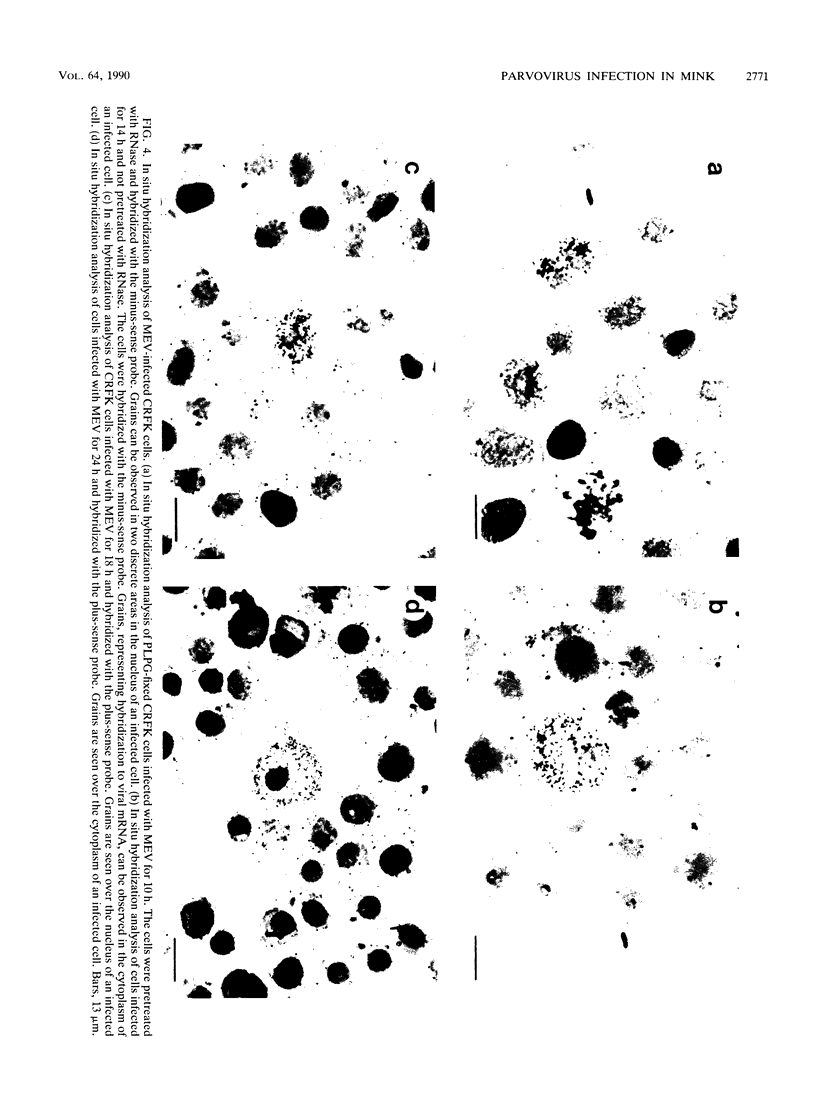


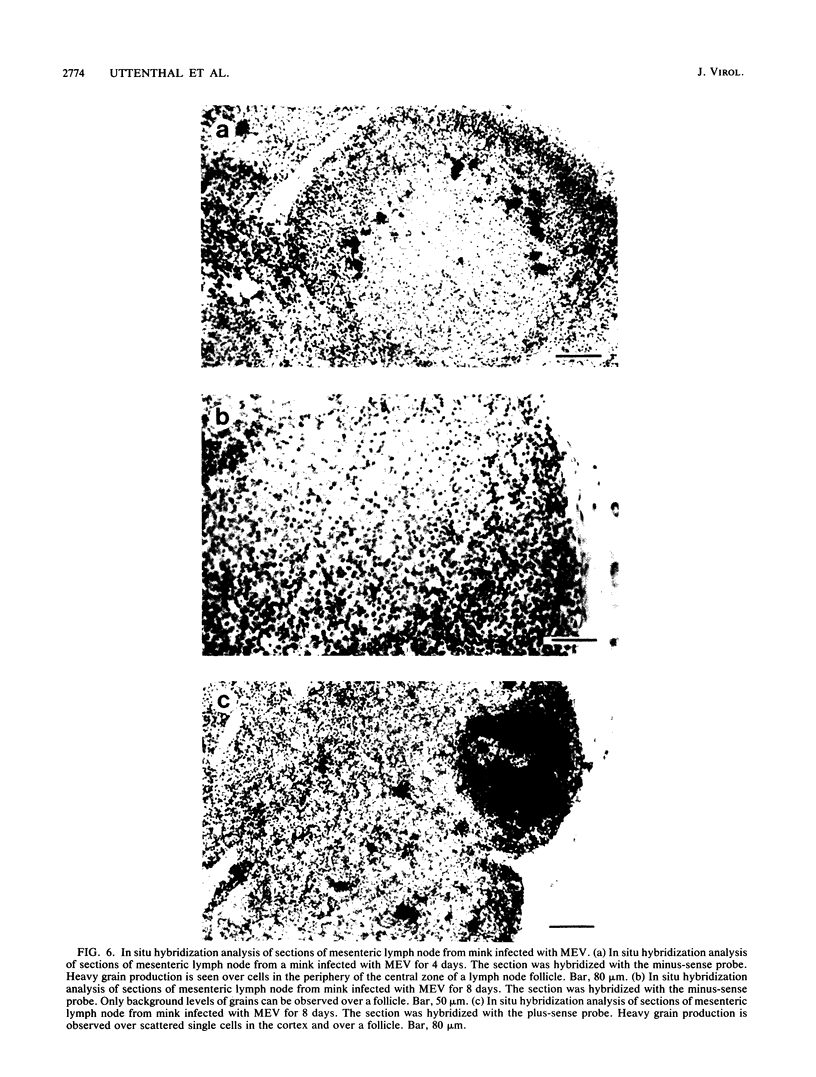


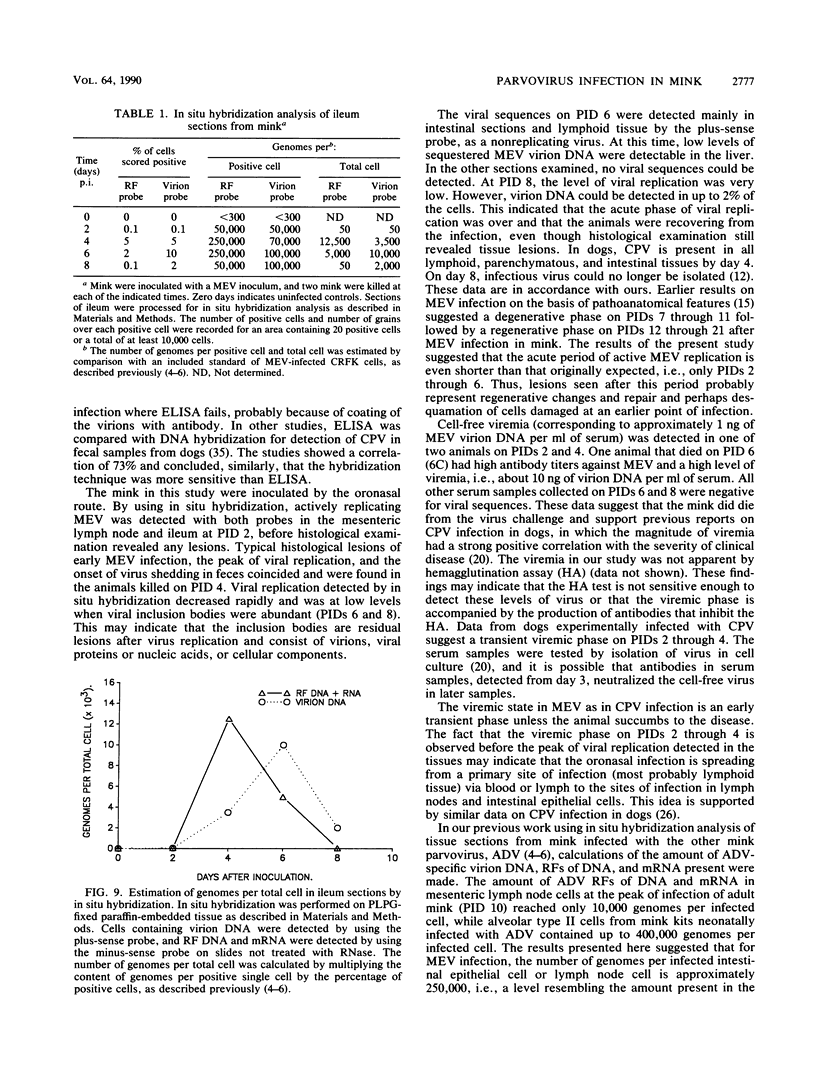


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B., Cohn A. Inhibition of precipitation in counter current electrophoresis. A sensitive method for detection of mink antibodies to Aleutian disease virus. Acta Pathol Microbiol Immunol Scand C. 1982 Feb;90(1):15–19. doi: 10.1111/j.1699-0463.1982.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987 Jan;61(1):81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Larsen S., Cohn A., Uttenthal A., Race R. E., Aasted B., Hansen M., Bloom M. E. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J Virol. 1989 Jan;63(1):9–17. doi: 10.1128/jvi.63.1.9-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker I. K., Povey R. C., Voigt D. R. Response of mink, skunk, red fox and raccoon to inoculation with mink virus enteritis, feline panleukopenia and canine parvovirus and prevalence of antibody to parvovirus in wild carnivores in Ontario. Can J Comp Med. 1983 Apr;47(2):188–197. [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Mori S., Wolfinbarger J. B. Analysis of parvovirus infections using strand-specific hybridization probes. Virus Res. 1989 Sep;14(1):1–25. doi: 10.1016/0168-1702(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Carman P. S., Povey R. C. Pathogenesis of canine parvovirus-2 in dogs: haematology, serology and virus recovery. Res Vet Sci. 1985 Mar;38(2):134–140. [PubMed] [Google Scholar]
- Cho H. J., Ingram D. G. Antigen and antibody in Aleutian disease in mink. I. Precipitation reaction by agar-gel electrophoresis. J Immunol. 1972 Feb;108(2):555–557. [PubMed] [Google Scholar]
- Johnson R. H., Siegl G., Gautschi M. Characteristics of feline panleucopaenia virus strains enabling definitive classification as parvoviruses. Arch Gesamte Virusforsch. 1974;46(3-4):315–324. doi: 10.1007/BF01240073. [DOI] [PubMed] [Google Scholar]
- Krunajevic T. Experimental virus enteritis in mink. A pathologic-anatomical and electron microscopical study. Acta Vet Scand Suppl. 1970;30(Suppl):1–88. [PubMed] [Google Scholar]
- Macpherson L. W. Feline Enteritis Virus - Its Transmission To Mink Under Natural And Experimental Conditions. Can J Comp Med Vet Sci. 1956 Jun;20(6):197–202. [PMC free article] [PubMed] [Google Scholar]
- McAdaragh J. P., Eustis S. L., Nelson D. T., Stotz I., Kenefick K. Experimental infection of conventional dogs with canine parvovirus. Am J Vet Res. 1982 Apr;43(4):693–696. [PubMed] [Google Scholar]
- McMaster G. K., Tratschin J. D., Siegl G. Comparison of canine parvovirus with mink enteritis virus by restriction site mapping. J Virol. 1981 Apr;38(1):368–371. doi: 10.1128/jvi.38.1.368-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P. C., Cooper B. J., Appel M. J., Lanieu M. E., Slauson D. O. Pathogenesis of canine parvovirus enteritis: sequential virus distribution and passive immunization studies. Vet Pathol. 1985 Nov;22(6):617–624. doi: 10.1177/030098588502200617. [DOI] [PubMed] [Google Scholar]
- Meunier P. C., Cooper B. J., Appel M. J., Slauson D. O. Pathogenesis of canine parvovirus enteritis: the importance of viremia. Vet Pathol. 1985 Jan;22(1):60–71. doi: 10.1177/030098588502200110. [DOI] [PubMed] [Google Scholar]
- Myers W. L., Fritz T. E. Histopathologic Changes in Virus Enteritis of Mink. Can J Comp Med Vet Sci. 1959 Aug;23(8):246–249. [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Carmichael L. E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988 Oct;166(2):293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology. 1986 Jan 15;148(1):121–132. doi: 10.1016/0042-6822(86)90408-3. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Gorham J. R., Schwartz T. M., Carmichael L. E. Characterization of antigenic variation among mink enteritis virus isolates. Am J Vet Res. 1984 Dec;45(12):2591–2599. [PubMed] [Google Scholar]
- Parrish C. R., Leathers C. W., Pearson R., Gorham J. R. Comparisons of feline panleukopenia virus, canine parvovirus, raccoon parvovirus, and mink enteritis virus and their pathogenicity for mink and ferrets. Am J Vet Res. 1987 Oct;48(10):1429–1435. [PubMed] [Google Scholar]
- Pollock R. V. Experimental canine parvovirus infection in dogs. Cornell Vet. 1982 Apr;72(2):103–119. [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. P., Jones E. V., Miller T. J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988 Jan;62(1):266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. A. Pathological changes in virus enteritis of mink. Can J Comp Med. 1970 Apr;34(2):155–163. [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Nucleotide sequence of the coat protein gene of canine parvovirus. J Virol. 1985 May;54(2):630–633. doi: 10.1128/jvi.54.2.630-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera E., Karlsson K. A. A solid-phase fluorescent immunoassay for detecting canine or mink enteritis parvoviruses in faecal samples. Vet Microbiol. 1987 Oct;15(1-2):1–9. doi: 10.1016/0378-1135(87)90123-4. [DOI] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Mildbrand M. M., Carlson J., Collins J. K., Winston S. Comparison of enzyme-linked immunosorbent assay, DNA hybridization, hemagglutination, and electron microscopy for detection of canine parvovirus infections. J Clin Microbiol. 1984 Sep;20(3):373–378. doi: 10.1128/jcm.20.3.373-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal A. Apparent lack of effect of vaccination against mink enteritis virus (MEV). A challenge study. Arch Virol. 1988;99(3-4):153–161. doi: 10.1007/BF01311066. [DOI] [PubMed] [Google Scholar]
- Witmer M. D., Steinman R. M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984 Jul;170(3):465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]