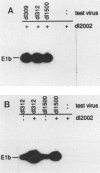
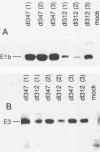
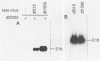
Abstract
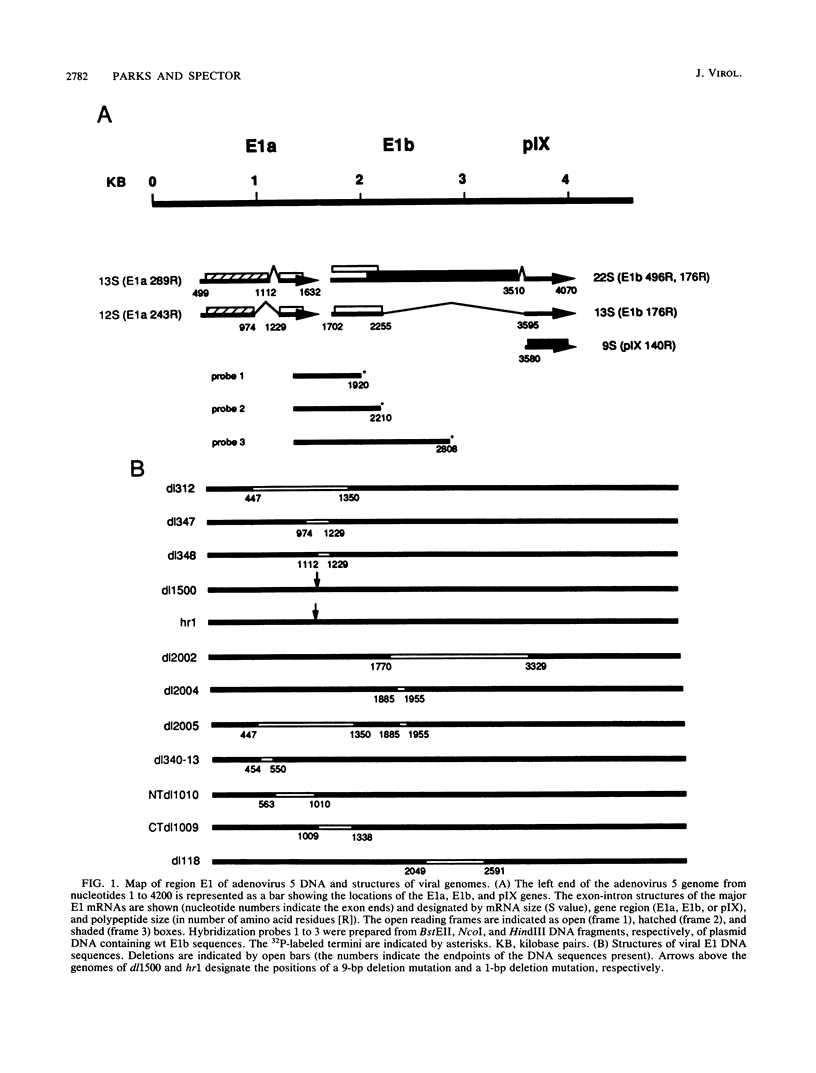
A cis-dominant mutation in the adjacent E1a gene disrupted the accumulation of adenovirus type 5 E1b mRNA during the early phase of infection. Steady-state levels of cytoplasmic and nuclear E1b RNAs in cells infected with dl312, a strain that lacks the E1a TATA box, cap site, and much of the coding sequence, were reduced 5- to 10-fold even when the E1a activator was provided in trans. The strain was defective for early E1b RNA synthesis but not for E1b RNA made late or during prolonged incubation in the presence of an inhibitor of DNA replication. The defect in E1b RNA synthesis could not be attributed to the E1a promoter sequences missing in dl312 DNA. If the E1a protein-coding region contains cis-acting regulatory sequences, they are not part of the previously mapped E1b transcriptional control region and may represent additional regulatory elements that ensure prompt and efficient E1b expression during the early phase of infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Fisher P. B., Ginsberg H. S. Effect on transformation of mutations in the early region 1b-encoded 21- and 55-kilodalton proteins of adenovirus 5. J Virol. 1984 Nov;52(2):389–395. doi: 10.1128/jvi.52.2.389-395.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman E., Paule M. R. Promoter occlusion during ribosomal RNA transcription. Cell. 1988 Sep 23;54(7):985–992. doi: 10.1016/0092-8674(88)90113-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bhat G., SivaRaman L., Murthy S., Domer P., Thimmappaya B. In vivo identification of multiple promoter domains of adenovirus EIIA-late promoter. EMBO J. 1987 Jul;6(7):2045–2052. doi: 10.1002/j.1460-2075.1987.tb02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., ten Wolde-Kraamwinkel H. C. The E1b promoter of Ad12 in mouse L tk- cells is activated by adenovirus region E1a. EMBO J. 1983;2(1):73–76. doi: 10.1002/j.1460-2075.1983.tb01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979 Nov;32(2):623–628. doi: 10.1128/jvi.32.2.623-628.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Falck-Pedersen E., Logan J., Shenk T., Darnell J. E., Jr Transcription termination within the E1A gene of adenovirus induced by insertion of the mouse beta-major globin terminator element. Cell. 1985 Apr;40(4):897–905. doi: 10.1016/0092-8674(85)90349-6. [DOI] [PubMed] [Google Scholar]
- Flint J., Shenk T. Adenovirus E1A protein paradigm viral transactivator. Annu Rev Genet. 1989;23:141–161. doi: 10.1146/annurev.ge.23.120189.001041. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grand R. J. The structure and functions of the adenovirus early region 1 proteins. Biochem J. 1987 Jan 1;241(1):25–38. doi: 10.1042/bj2410025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B., Zimmerman S. B. T4 polynucleotide kinase: macromolecular crowding increases the efficiency of reaction at DNA termini. Anal Biochem. 1986 Nov 1;158(2):307–315. doi: 10.1016/0003-2697(86)90555-5. [DOI] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A enhancer contains two functionally distinct domains: one is specific for E1A and the other modulates all early units in cis. Cell. 1986 Apr 25;45(2):229–236. doi: 10.1016/0092-8674(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Sassone-Corsi P., Chambon P. An enhancer element is located 340 base pairs upstream from the adenovirus-2 E1A capsite. Nucleic Acids Res. 1983 Dec 20;11(24):8747–8760. doi: 10.1093/nar/11.24.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Jochemsen A. G., Peltenburg L. T., te Pas M. F., de Wit C. M., Bos J. L., van der Eb A. J. Activation of adenovirus 5 E1A transcription by region E1B in transformed primary rat cells. EMBO J. 1987 Nov;6(11):3399–3405. doi: 10.1002/j.1460-2075.1987.tb02663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Matsui T. Novel regulation of transcription initiation of the peptide IX gene of adenovirus 2. Mol Cell Biol. 1989 Oct;9(10):4265–4271. doi: 10.1128/mcb.9.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Species identification and genome mapping of cytoplasmic adenovirus type 2 RNAs synthesized late in infection. J Virol. 1977 Aug;23(2):240–249. doi: 10.1128/jvi.23.2.240-249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Control of adenovirus E1B mRNA synthesis by a shift in the activities of RNA splice sites. Mol Cell Biol. 1984 May;4(5):966–972. doi: 10.1128/mcb.4.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Regulation of early adenovirus gene expression. Microbiol Rev. 1987 Dec;51(4):419–430. doi: 10.1128/mr.51.4.419-430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Gaynor R. B., Berk A. J. The TATA homology and the mRNA 5' untranslated sequence are not required for expression of essential adenovirus E1A functions. Cell. 1982 May;29(1):139–148. doi: 10.1016/0092-8674(82)90098-8. [DOI] [PubMed] [Google Scholar]
- Parks C. L., Banerjee S., Spector D. J. Organization of the transcriptional control region of the E1b gene of adenovirus type 5. J Virol. 1988 Jan;62(1):54–67. doi: 10.1128/jvi.62.1.54-67.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Berk A. J. Multiple transcription factor binding sites mediate adenovirus E1A transactivation. J Virol. 1989 Aug;63(8):3499–3506. doi: 10.1128/jvi.63.8.3499-3506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. C., Zhou Q., Berk A. J. Sp1 activates transcription without enhancing DNA-binding activity of the TATA box factor. Mol Cell Biol. 1989 Aug;9(8):3299–3307. doi: 10.1128/mcb.9.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. J., McGrogan M., Raskas H. J. Regulation of the appearance of cytoplasmic RNAs from region 1 of the adenovirus 2 genome. J Mol Biol. 1978 Dec 15;126(3):395–414. doi: 10.1016/0022-2836(78)90048-7. [DOI] [PubMed] [Google Scholar]
- Spector D. J., Tevethia M. J. Identification of a human cytomegalovirus virus DNA segment that complements an adenovirus 5 immediate early mutant. Virology. 1986 Jun;151(2):329–338. doi: 10.1016/0042-6822(86)90053-x. [DOI] [PubMed] [Google Scholar]
- Spector D. J. Transcription of adenovirus 5 early region 1b is elevated in permissive cells infected by a mutant with an upstream deletion. J Virol. 1982 Nov;44(2):544–554. doi: 10.1128/jvi.44.2.544-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Functions of the adenovirus E1B tumour antigens. Cancer Surv. 1986;5(2):389–404. [PubMed] [Google Scholar]
- Stow N. D. Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol. 1981 Jan;37(1):171–180. doi: 10.1128/jvi.37.1.171-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Spector D. J. Complementation of an adenovirus 5 immediate early mutant by human cytomegalovirus. Virology. 1984 Sep;137(2):428–431. doi: 10.1016/0042-6822(84)90236-8. [DOI] [PubMed] [Google Scholar]
- Vales L. D., Darnell J. E., Jr Promoter occlusion prevents transcription of adenovirus polypeptide IX mRNA until after DNA replication. Genes Dev. 1989 Jan;3(1):49–59. doi: 10.1101/gad.3.1.49. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Pettersson U. Organization of early region 1B of human adenovirus type 2: identification of four differentially spliced mRNAs. J Virol. 1985 May;54(2):383–391. doi: 10.1128/jvi.54.2.383-391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert F. C., Young C. S. The genetic analysis of recombination using adenovirus overlapping terminal DNA fragments. Virology. 1983 Feb;125(1):175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Wasylyk B. Transcription elements and factors of RNA polymerase B promoters of higher eukaryotes. CRC Crit Rev Biochem. 1988;23(2):77–120. doi: 10.3109/10409238809088317. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Denton A., Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988 Sep;62(9):3445–3454. doi: 10.1128/jvi.62.9.3445-3454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Darnell J. E., Jr Control of messenger RNA concentration by differential cytoplasmic half-life. Adenovirus messenger RNAs from transcription units 1A and 1B. J Mol Biol. 1981 May 25;148(3):231–251. doi: 10.1016/0022-2836(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Berk A. J. Transcriptional activation by the pseudorabies virus immediate early protein requires the TATA box element in the adenovirus 2 E1B promoter. Virology. 1988 Nov;167(1):318–322. doi: 10.1016/0042-6822(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Wu L., Berk A. Constraints on spacing between transcription factor binding sites in a simple adenovirus promoter. Genes Dev. 1988 Apr;2(4):403–411. doi: 10.1101/gad.2.4.403. [DOI] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]
- Zimmer S. G., Carlson D. G., Johnson L. Genome distribution of adenovirus total and self-complementary nuclear RNA at early times. Virology. 1981 Jun;111(2):301–311. doi: 10.1016/0042-6822(81)90334-2. [DOI] [PubMed] [Google Scholar]