Abstract
Previously, we reported that PC12 cells with decreased Dp71 expression (antisense-Dp71 cells) display deficient nerve-growth-factor-induced neurite outgrowth. In this study, we show that disturbed neurite outgrowth of antisense-Dp71 cells is accompanied by decreased adhesion activity on laminin, collagen and fibronectin. In wild-type cells, the immunostaining of Dp71 and _1-integrin overlaps in the basal area contacting the substrate, but staining of both proteins decrease in the antisense-Dp71 cells.
Morphology of antisense-Dp71 cells at the electron microscopic level is characterized by the lack of filopodia, cellular projections involved in adhesion. Our findings suggest that Dp71 is required for the efficient PC12 cell attachment to b1-integrin-dependent substrata and that decreased adhesion activity of the anti-sense-Dp71 cells could determine their deficiency to extend neurites.
Keywords: Animals; Cell Adhesion; drug effects; physiology; Cell Differentiation; drug effects; physiology; Collagen; drug effects; physiology; Dystrophin; analogs & derivatives; physiology; Fibronectins; drug effects; physiology; Fluorescent Antibody Technique; methods; Integrin beta Chains; metabolism; Laminin; drug effects; physiology; Microscopy, Electron, Scanning; methods; Neurites; drug effects; physiology; Oligodeoxyribonucleotides, Antisense; pharmacology; PC12 Cells; drug effects; physiology; ultrastructure; Rats; Statistics, Nonparametric
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder causing progressive muscle degeneration and death. The DMD gene displays a complex transcriptional regulation, and encodes at least seven different dystrophins that differ in structure and expression patterns [1]. The dystrophin of 427 kDa is the predominant muscular isoform; it participates in the stability of the muscle cell membrane by binding to a group of transmembrane glycoproteins and cytoplasmic proteins collectively called dystrophin-associated protein complex (DAPC) [2]. Dystrophin Dp71, the smallest DMD gene product, is the major dystrophin isoform in the nervous system. It contains three different protein domains: a unique N-terminal domain composed of seven residues, a cysteine-rich region and a C-terminal domain [3]. As Dp427 does, Dp71 associates with DAPC via the cysteine-rich and C-terminal domains [4].
Several lines of evidence indicate that Dp71 plays a role in neuronal cells: (1) It has been observed that Dp71 is necessary for the stabilization of DAPC in the brain [5]. (2) Dp71 expression increased in parallel with brain development [6]. (3) C-terminal mutations in the DMD gene, which would adversely affect Dp71 expression, are associated with mental retardation [7]. Supporting these data, we have provided the first direct evidence implicating Dp71 in a neuronal function; by using an antisense strategy we revealed that PC12 cells with depletion of Dp71 protein levels (anti sense-Dp71 cells) exhibit a marked inhibition of neurite outgrowth induced by nerve growth factor (NGF) [8].
At the present stage, it is difficult to establish what event of the differentiation pathway is obstructed by Dp71 deficiency. However, since Dp71 and DAPC members have been related to adhesion activity in different cell types [9,10] and this cellular process is intimately linked to neurite outgrowth [11], it is possible that decreased Dp71 expression could alter primarily the adhesion activity of PC12 cells and, in consequence, their ability to extend neurites in response to NGF.
In the present study, we evaluated the adhesion activity and neurite outgrowth of antisense-Dp71 cells cultured on different extracellular matrices (ECMs). In addition, to evaluate the potential role of Dp71 in b1-integrin-mediated adhesion, we analyzed the subcellular distribution of Dp71 and b1-integrin in the antisense-Dp71 cells.
Cell culturing
PC12 cells were grown as described previously [8] and maintained at 371C in a humidified incubator with a 5% CO2 atmosphere. The antisense-Dp71 clone [8] was maintained with 500 mg/ml of G418 (Invitrogen, Carlsbad, California, USA), a neomycin analog.
Cell differentiation assay
To induce differentiation, 2 × 104 cells were seeded onto 24-well plates, coated with 20 mg/ml collagen (Sigma, St Louis, Missouri, USA), 20 mg/ml laminin (Sigma), 200 mg/ml poly-D-lysine (Sigma) or 10 mg/ml fibronectin (Sigma), and treated with 50 ng/ml 2.5S NGF (Alomone Labs, Jerusalem, Israel). Medium containing NFG was changed every third day. To determine the neurite outgrowth index, the number of neurites per cell and the relative length of the neurites were scored in 100 different cells at 6 days of differentiation [12].
Cell adhesion assay
Cells were seeded onto 96-well plates (a) coated with collagen, laminin, poly-D-lysine or fibronectin at 3.0 densities of 2 ×106. After a 2-h incubation period, wells were washed with phosphate-buffered saline (PBS) by shaking gently for 30 s at 15 Hz, and the supernatant with detached cells was removed and counted by flow cytometry with an argon laser at 488 nm (Fac’sCalibur, Beckton Dickinson, Franklin Lakes, New Jersey, USA). Adhesion index was calculated as follows: 100% - (number of detached cells × 100/total number of seeded cells).
Scanning electron microscopy
Cell samples were fixed in 2.5% glutaraldehyde, dehydrated in ethanol and dried at critical point with CO2 (maximum temperature 39°C; maximum pressure 110 psi) in a SAMDRY-780 dryer (b) (Tousimis Research Corp., Rockville, Maryland, USA). The 100 cells were then covered with gold ions in a JEOL JFC-110 ion sputterer (Tokyo, Japan) and analyzed in a JEOL 35-C scanning electron microscope.
Immunofluorescence assays
Immunofluorescence staining was performed as described previously [8]. Briefly, cells plated onto glass coverslips coated with laminin were fixed with 4% paraformaldehyde and permeated with 0.1% triton X100. Fixed cells were incubated overnight at 41C with both polyclonal antibody anti-b1-integrin (Santa Cruz Biotechnology, Santa Cruz, California, USA) and monoclonal antibody 5F3, raised against the last 31 amino acids of the Dp71f isoform [13]. Coverslips were incubated in PBS for 1h with both FITC-conjugated secondary anti-rabbit(ZYMED) and TRITC-conjugated secondary anti-mouse (ZYMED) to detect b1-integrin and Dp71, respectively. Coverslips were mounted with VectaShield (Vector Laboratories Inc., Burlingame, California, USA) and observed in an epifluorescence microscope coupled with a Leica confocal system TCS-SP2. From each image, eight optical Z-sections were scanned using the laser confocal microscope dual-channel imaging system.
Statistics
Statistical analysis was performed using the Mann-Whitney test with the v. 7.0 Stata program.
RESULTS
We have previously shown that the NGF-induced neuronal differentiation of antisense-Dp71 cells is drastically impaired [8]. However, it should be noted that those experiments were performed with cells grown on collagen. Therefore, to test whether such deficiency is substratum specific or not, the wild-type and antisense-Dp71 cells were plated on different substrata and their response to NGF after 6 days of treatment was evaluated. In all substrata tested, it was observed that wild-type cells displayed features that accompanied the neuronal differentiation, such as flattening, increase in cell body, and outgrowth of long and branched neurites (data not shown). In contrast, irrespective of the kind of substratum employed, the antisense-Dp71 cells exhibited only very short stubby processes, which failed to elongate even with longer NGF exposures (data not shown). Measurement of neurite outgrowth confirmed these observations, relative to wild-type cells, average reductions of 75%, 53%, 51% and 63% were observed in the neurite outgrowth score when the antisense-Dp71 cells were grown on poly-D-lysine, fibronectin, laminin and collagen, respectively (Fig. 1a).
Fig. 1. Neurite outgrowth and adhesion activity analyses in the wild-type and antisense-Dp71 cells grown on different substrata. Cells were seeded on dishes coated with poly-D-lysine, ¢bronectin, laminin or collagen.
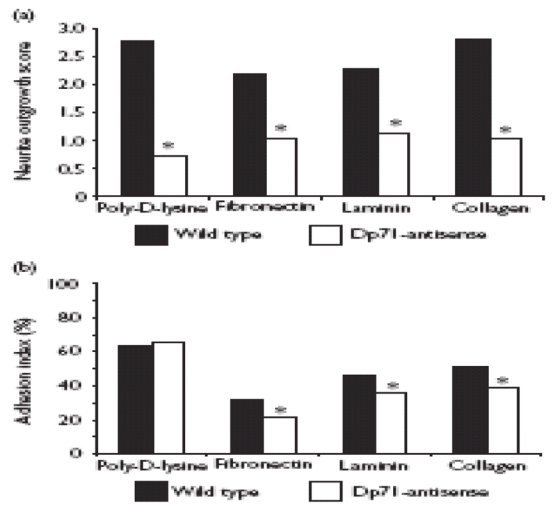
(a) Cells were stimulated with nerve growth factor for 6 days and the neurite outgrowth scores were calculated as described in Methods.
(b) The adhesion indexes of undifferentiated cells were obtained as described in Methods. Values are mean +/− SD of three independent experiments. Asterisks denote signifi¢ant differences (p<0.05).
Because Dp71 and several DAPC members have been related to cellular adhesion [9,10] and this process is intimately connected with neurite outgrowth in PC12 cells [11], it is likely that impairment in the neurite outgrowth of the antisense-Dp71 cells is due to a primary alteration in their adhesion activity. To test this hypothesis, we evaluated the capability of wild-type and antisenseDp71 cells to attach to different substrata. Figure 1b shows that poly-D-lysine is the best substratum for wild-type cell adhesion, followed by collagen, laminin and fibronectin. On the other hand, with the exception of poly-D-lysine, in the remaining substrata the antisense-Dp71 cells exhibited a statistically significant decrease in their adhesion activity, ranking from 23% to 35% (Fig. 1b).
To determine whether the impaired adhesion activity of the antisense-Dp71 cells correlates with any morphological change at ultrastructural level, these cells were examined at electron microscopic (EM) level. Under such analysis, the wild-type cells exhibited a flat, ovoid morphology with small filopodia in the periphery of the cell body and numerous microvilli on the cell surface (Fig. 2a). In contrast, the antisense-Dp71 cells displayed a round morphology with little or no peripheral filopodia (Fig. 2b). EM analysis of the NGF-treated antisense-Dp71 cells was reported elsewhere [8].
Fig 2.
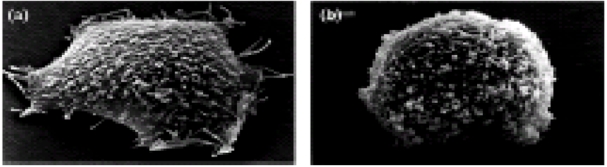
Morphology of (a) PC12 wild type and (b) anti-sense-Dp71 cells by scanning electron microscopy.
Because integrins mediate the adhesion of PC12 cells to different ECM components, the subcellular localization of Dp71 and b1-integrin in the wild-type and antisense-Dp71 cells was examined by immunofluorescence and confocal microscopy. We double-stained cells with rabbit anti-b1integrin (green channel) and mouse monoclonal anti-Dp71 (red channel) antibodies. In wild-type cells, the immunostaining of b1-integrin and Dp71 exhibited a punctuate cytoplasmic pattern that overlapped to a certain extent in the middle and basal portions of the cells (Fig. 3). Interestingly, in the antisense-Dp71 cells, the immunostaining of b1-integrin was significantly reduced in the basal cell area contacting the substrate while that of Dp71 was practically absent, which resulted in a pale colocalization between these two proteins (Fig. 3).
Fig. 3.
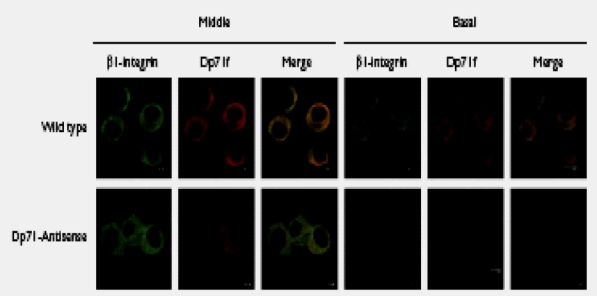
Confocal laser scanning images showing localization of b1-integrin and Dp71 in wild-type and antisense-Dp71 cells. Cells, cultured on laminincoated glass coverslips, were double stained with anti-b1-integrin (green channel) and anti-Dp71 (red channel) antibodies. Focus was adjusted to the middle or the basal portion of cells and merging of Dp71 and b1-integrin staining is shown in yellow.
DISCUSSION
Previous work from our laboratory has established the PC12 cell line as a model for studying the function of Dp71 in neuronal cells [14,15]. Following this, we recently reported that Dp71 is required for PC12 neurite outgrowth. We found that reduced protein levels of Dp71f and Dp71d, the predominant cytoplasmic and nuclear isoforms, respectively, caused an impairment in the ability of PC12 cells to extend neurites in response to NGF induction [8]. In the present study, we provide evidence suggesting that Dp71 deficiency also affects the adhesion of PC12 cells to ECM components; the antisense-Dp71 cells display a significant decrease in adhesion activity when grown on laminin, fibronectin and collagen, but not on poly-D-lysine. In addition, these cells exhibit an abnormal morphology, characterized by a rounded cell body devoid of filopodia, compared with wild-type cells. Since filopodia are cellular projections involved in the attachment of cell to substrata [16,17], it seems that the altered morphology of the antisense-Dp71 cells is related to their inability to attach to ECM components.
Adhesion of PC12 cells to ECM components takes place through different integrin heterodimers: a3b1 mediates adhesion to laminin, fibronectin and collagen while a1b1 leads cell adhesion to laminin and collagen [18,19]. On the other hand, PC12 adhesion to poly-D-lysine is nonspecific and takes place through electrostatic forces [20]. It should be noted that b1-integrin is the common subunit for PC12 adhesion to laminin, collagen and fibronectin. Therefore, our results suggest that Dp71 participates in some way in the b1-integrin-mediated cell adhesion of PC12 cells. The b1 integrin-containing adhesion sites of PC12 cells, named point contacts, are composed of vinculin, paxillin, talin and focal adhesion kinase (FAK +) [19,21].
Previous reports have provided biochemical evidence about the interaction between members of the DAP complex, including dystrophin, dystroglycans and sarcoglycans, and proteins involved in cell attachment, such as integrins, vinculin, talin, paxillin and FAK + [10,22,23]. Thus, dystrophin and DAPC seem to form an integral part of the focal adhesion complex in cultured cells. In this context, it could be proposed that Dp71 and DAPC members participate in the anchoring and stabilization of the b1-integrin-containing adhesion complex formed at the point contacts of PC12 cells. Alternatively, it is possible that Dp71 deficiency causes some instability at the PC12 cell membrane, which in turn could affect the assembly of b1integrin-containing adhesion complex. However, in support of our proposal, we demonstrated by immunofluorescence and confocal microscopy analyses that the subcellular distributions of Dp71f and b1-integrin overlap in the basal portion of the cell contacting the substratum, and the staining of both proteins is clearly diminished in such cell areas of the antisense-Dp71 cells. Since the antisense-Dp71 cells produce normal b1-integrin protein levels compared with wild-type cells (unpublished results), it seems that the diminished staining of b1-integrin corresponds to reduced amounts of the protein that are specifically engaged in the adhesion protein complex of the mutant cells. Furthermore, immunoprecipitation experiments using an anti-FAK antibody reveal that Dp71 copurifies with b1-integrin and FAK in the PC12 wild-type cells (unpublished results), which suggests that they are part of the same protein complex and probably interact with one another. In evidence of the role of Dp71 in cell adhesion, it has been shown that adhesion to collagen in response to thrombin was significantly decreased in platelets isolated from mdx3cv mouse, a Dp71 deficient animal model [9]. In addition, it has been reported that the isoform Dp71f colocalized with vinculin and actinin at focal contacts and lamellipodia of the astrocytoma cell line U373 MG [24]. Nevertheless, it is clear that further characterization of the adhesion point contacts components, such as paxillin, vinculin and talin, in the antisense-Dp71 cells is necessary to understand the role of Dp71 in PC12 cell adhesion.
The decreased adhesion activity of the antisense-Dp71 cells on laminin, fibronectin and collagen correlates with impairment in their ability to develop neurites in response to NGF. Since adhesion of growth cones to the substrate is essential for growth cone movement and directionality [11,25], it is likely that the deficiencies of antisense-Dp71 cells in b1-integrin-mediated cell adhesion and neurite outgrowth are functionality linked. Hence, an initial instability of the multiprotein adhesion complex in the antisense-Dp71 cells, due to the reduced levels of Dp71, could cause a further failure in the protrusive activity of the growth cone leading to neurite outgrowth inhibition. However, in spite of showing unaltered adhesion activity on poly-D-lysine, the antisense-Dp71 cells failed to extend neurites upon NGF treatment. These results might indicate that regardless of the ECM influence on neuritogenesis, the machinery and signaling pathway relevant to neurite extension are affected per se in the antisense-Dp71 cells.
CONCLUSION
Our findings suggest that Dp71 is required for the efficient adhesion of PC12 cells on b1-integrin-dependent substrata and that decreased adhesion activity of the antisense-Dp71 cells on b1-integrin-dependent substrata could determine their further failure to extend neurites upon NGF induction. Further biochemical evidence is necessary to clearly establish a role for Dp71 in the b1-integrin-mediated adhesion of PC12 cells.
Acknowledgments
This work was supported by CONACYT Mexico, Grant 43285M. We are grateful to Pablo Gomez Islas for cell culturing and Victor Rosales Garc|a for FACS counting.
References
- Ahn AH, Kunkel LM. The structural and functional diversity of dystrophin. Nat Genet. 1993;3:283–291. doi: 10.1038/ng0493-283. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Hugnot JP, Gilgenkrantz H, Vincent N, Chafey P, Morris GE, Monaco AP, et al. Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc Natl Acad Sci USA. 1992;89:7506–7510. doi: 10.1073/pnas.89.16.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramarcy NR, Vidal A, Froehner SC, Sealock R. Association of utrophin and multiple dystrophin short forms with the mammalian M(r) 58,000 dystrophin-associated protein (syntrophin) J Biol Chem. 1994;269:2870–2876. [PubMed] [Google Scholar]
- Greenberg DS, Schatz Y, Levy Z, Pizzo P, Yaffe D, Nudel U, et al. Reduced levels of dystrophin associated proteins in the brains of mice deficient for Dp71. Hum Mol Genet. 1996;5:1299–1303. doi: 10.1093/hmg/5.9.1299. [DOI] [PubMed] [Google Scholar]
- Jung D, Filliol D, Metz-Boutigue MH, Rendon A. Characterization and subcellular localization of the dystrophin-protein 71 (Dp71) from brain. Neuromuscul Disord. 1993;3:515–518. doi: 10.1016/0960-8966(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Moizard MP, Toutain A, Fournier D, Berret F, Raynaud M, Billard C, et al. Severe cognitive impairment in DMD: obvious clinical indication for Dp71 isoform point mutation screening. Eur J Hum Genet. 2000;8:552–556. doi: 10.1038/sj.ejhg.5200488. [DOI] [PubMed] [Google Scholar]
- Acosta R, Montanez C, Fuentes-Mera L, Gonzalez E, Gomez P, Quintero-Mora L, et al. Dystrophin Dp71 is required for neurite outgrowth in PC12 cells. Exp Cell Res. 2004;296:265–275. doi: 10.1016/j.yexcr.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Austin RC, Fox JE, Werstuck GH, Stafford AR, Bulman DE, Dally GY, et al. Identification of Dp71 isoforms in the platelet membrane cytoskeleton. Potential role in thrombin-mediated platelet adhesion. J Biol Chem. 2002;277:47106–47113. doi: 10.1074/jbc.M203289200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Pan Y, Hanada H, Iwata Y, Shigekawa M. Bidirectional signaling between sarcoglycans and the integrin adhesion system in cultured L6 myocytes. J Biol Chem. 1998;273:1583–1590. doi: 10.1074/jbc.273.3.1583. [DOI] [PubMed] [Google Scholar]
- Renaudin A, Lehmann M, Girault J, McKerracher L. Organization of point contacts in neuronal growth cones. J Neurosci Res. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Davidkova G, Zhang SP, Nichols RA, Weiss B. Reduced level of calmodulin in PC12 cells induced by stable expression of calmodulin antisense RNA inhibits cell proliferation and induces neurite outgrowth. Neuroscience. 1996;75:1003–1019. doi: 10.1016/0306-4522(96)00230-8. [DOI] [PubMed] [Google Scholar]
- Fabbrizio E, Nudel U, Hugon G, Robert A, Pons F, Mornet D. Characterization and localization of a 77 kDa protein related to the dystrophin gene family. Biochem J. 1994;299:359–365. doi: 10.1042/bj2990359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros B, Rendon A, Genty V, Aranda G, Marquez F, Mornet D, et al. Expression of dystrophin Dp71 during PC12 cell differentiation. Neurosci Lett. 1996;213:107–110. doi: 10.1016/0304-3940(96)12863-9. [DOI] [PubMed] [Google Scholar]
- Marquez FG, Cisneros B, Garcia F, Ceja V, Velazquez F, Depardon F, et al. Differential expression and subcellular distribution of dystrophin Dp71 isoforms during differentiation process. Neuroscience. 2003;118:957–966. doi: 10.1016/s0306-4522(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Damsky CH, Reichardt LF. Interactions of a neuronal cell line (PC12) with laminin, collagen IV, and fibronectin: identification of integrin-related glycoproteins involved in attachment and process outgrowth. J Cell Biol. 1987;105:2347–2358. doi: 10.1083/jcb.105.5.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loster K, Vossmeyer D, Hofmann W, Reutter W, Danker K. alpha1 Integrin cytoplasmic domain is involved in focal adhesion formation via association with intracellular proteins. Biochem J. 2001;356:233–240. doi: 10.1042/0264-6021:3560233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas RJ, Burmeister DW, Goldberg DJ. Rapid effects of laminin on the growth cone. Neuron. 1992;8:107–115. doi: 10.1016/0896-6273(92)90112-q. [DOI] [PubMed] [Google Scholar]
- Arregui CO, Carbonetto S, McKerracher L. Characterization of neural cell adhesion sites: point contacts are the sites of interaction between integrins and the cytoskeleton in PC12 cells. J Neurosci. 1994;14:6967–6977. doi: 10.1523/JNEUROSCI.14-11-06967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Hanada H, Iwata Y, Pan Y, Sigekawa M. Expression of a dystrophin-sarcoglycan complex in serum-deprived BC3H1 cells and involvement of alpha-sarcoglycan in substrate attachment. Biochem Biophys Res Commun. 1996;225:11–15. doi: 10.1006/bbrc.1996.1123. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Chiba A, Yamada H, Fukuta-Ohi H, Fujita S, Endo T, et al. A role of dystroglycan in schwannoma cell adhesion to laminin. J Biol Chem. 1997;272:13904–13910. doi: 10.1074/jbc.272.21.13904. [DOI] [PubMed] [Google Scholar]
- Garcia-Tovar CG, Luna J, Mena R, Soto-Zarate Cl, Cortes R, Perez A, et al. Dystrophin isoform Dp7l is present in lamellipodia and focal complexes in human astrocytoma cells U-373 MG. Acta Histochem. 2002;104:245–254. doi: 10.1078/0065-1281-00654. [DOI] [PubMed] [Google Scholar]
- Murnane AC, Brown K, Keith CH. Preferential initiation of PC12 neurites in directions of changing substrate adhesivity. J Neurosci Res. 2002;67:321–328. doi: 10.1002/jnr.10130. [DOI] [PubMed] [Google Scholar]


