SUMMARY
Quantitative differences in cadherin activity have been proposed to play important roles in patterning connections between pre- and post-synaptic neurons. However, no examples of such a function have yet been described, and the mechanisms that would allow such differences to direct growth cones to specific synaptic targets are unknown. In the Drosophila visual system, photoreceptors are genetically programmed to make a complex, stereotypic set of synaptic connections. Here we show that the atypical cadherin Flamingo functions as a short-range, homophilic signal, passing between specific R cell growth cones to influence their choice of post-synaptic partners. We find that individual growth cones are sensitive to differences in Flamingo activity through opposing interactions between neighboring cells, and require these interactions to be balanced in order to extend along the appropriate trajectory.
INTRODUCTION
The genetically programmed development of synaptic connectivity depends critically upon precisely regulated cell-surface contacts amongst afferent axons as well as between axons and their targets (reviewed in Yamagata et al., 2003; Takeichi, 2007). Members of the cadherin superfamily are hypothesized to direct individual axons to their appropriate post-synaptic partners (Fannon and Colman, 1996; Suzuki et al., 1997, Inoue and Sanes, 1997), yet the mechanisms by which they might play such roles in vivo are largely unknown. By analogy to studies of cadherin function in non-neuronal cells (Steinberg and Takeichi, 1994; Godt and Tepass, 1997; Hayashi and Carthew, 2004), one possibility is that different levels of cadherin adhesivity might sort axons toward specific targets. However, no such role has yet been uncovered in vivo, and no cellular mechanism for how developing axons might discriminate amongst differences in cadherin activity has been described. By systematically manipulating cadherin activity in adjoining pairs of afferent axons, we define an opponent strategy that allows neighboring growth cones to compare cadherin levels to determine outgrowth trajectory.
The Drosophila compound eye comprises 800 subunits, ommatidia, each containing 8 photoreceptors (R cells). Within each ommatidium, R cells detect light from different points in visual space, and each is genetically hard-wired to connect to a specific post-synaptic target (Figure 1A; reviewed in Clandinin and Zipursky, 2002). These events assemble a repeated array of axon fascicles, called cartridges, each of which comprises the terminals of all R cells that respond to light from the same part of visual space, clustered around the processes of their target neurons. Ablation studies, genetic analyses, and electron microscopic reconstruction experiments demonstrate that R cell axons require specific cell-surface interactions between afferent growth cones in order to reach their targets, and these interactions take place independent of neural activity (Meinertzhagen and Hanson, 1993; Clandinin and Zipursky, 2000; Hiesinger et al., 2006).
Figure 1. Flamingo is not required cell-autonomously in R cell axons.

(A). Schematic representation of R cells and their axons. In each ommatidium, in the retina, each R cell occupies an invariant relative position; this arrangement is preserved in the axon fascicle projecting into the brain, even as the bundle of axons twists 180° (curved line). In the brain, within the lamina plexus, each R cell axon (pink) then extends to a target (red), arranged in an invariant position relative to the fascicle. R cells axons from neighboring ommatidia (blue) choose an overlapping set of targets. The axons of R7 and R8 extend into the medulla, and are not shown. (B) In the retina each R cell sub-type is uniquely identifiable by its characteristic position and morphology (B, G, L, Q). Using the MARCM method, single R cells (green) are made homozygous for either a control chromosome (C-E, H-J, M-O, R-T) or a chromosome bearing a null allele in flamingo (F, K, P, U). During pupal development, R cell axons extend away from bundles of axons containing all R cells from a single ommatidium (D, I, N, S). Each R cell can be identified in the retina, and traced down into the brain, where it extends to a specific target located in an invariant position relative to the ommatial bundle. Arrowheads demark where each R cell axon starts its lateral extension across the lamina plexus; arrows demark the target cartridge.
The interactions amongst R cell growth cones that are necessary for target selection take place in a complex cellular milieu that is established in three steps (reviewed in Clandinin and Zipursky, 2002). First, axons from within each individual ommatidium extend to the lamina as part of a single fascicle, terminating between two layers of glial cells. After R cell axons have reached this plexus, lamina neuron processes, the post-synaptic targets of R cell axons, pass through the lamina into the second optic ganglion, the medulla. Second, each R cell growth cone defasciculates, forming a relatively flat process. At this stage, R cell growth cones from the same ommatidium maintain extensive contacts with one another, and begin to contact growth cones from neighboring ommatidia (Meinertzhagen and Hanson, 1993; Clandinin and Zipursky, 2000). Some unknown subset of these afferent-afferent interactions, taking place prior to growth cone extension, are required for R cell axons to choose appropriate targets (Clandinin and Zipursky, 2000). Finally, individual R cell axons extend away from the ommatidial bundle, making a directed projection along a specific trajectory toward the appropriate cartridge. Upon reaching this target, R cells initiate synapse assembly.
The molecular mechanisms that underlie connection specificity in this system are incompletely understood. Two cell adhesion molecules, the classical cadherin N-cadherin, and the atypical cadherin, Flamingo, play critical roles (Lee et al., 2001; Lee et al., 2003). N-cadherin mediates homophillic, stabilizing interactions between R cell axons and their target cartridges (Prakash et al., 2005). Flamingo, on the other hand, mediates interactions amongst R cell axons that are required for R cells to reach the appropriate target cartridge (Lee et al., 2003). This seven-pass transmembrane cadherin is evolutionarily conserved, mediates homophillic interactions in vitro, and regulates epithelial planar polarity, dendritic arborization and axon tract formation (Usui et al., 1999; Chae et al., 1999; Das et al., 2002; Lee et al., 2003; Senti et al., 2003; Gao et al., 2000; Grueber et al., 2002; Shima et al., 2004; Tissir et al., 2005; Kimura et al., 2006). However, its mechanism of action remains incompletely understood. Here we define how R cell subsets interact via Flamingo to influence target selection.
RESULTS AND DISCUSSION
Flamingo functions exclusively in R cells
As an entry point to examining the precise mechanism by which Flamingo influences R cell target selection, we first demonstrated that Flamingo exclusively mediates interactions amongst R1-R6 growth cones. In particular, genetic mosaic analyses in which Flamingo function is specifically removed from either all R cell axons, or their target neurons, demonstrate that Flamingo functions only within R cells, not their targets neurons, to control cartridge assembly (Lee et al., 2003; Senti et al., 2003; Figure S1A-C). This phenotype is distinct from, and more severe than that observed in other planar cell polarity mutants (Figure S1D and data not shown). Flamingo function is restricted to this specific aspect of R cell target choice, as detailed analysis of the flamingo mutant phenotype demonstrates that mutant R1-R6 axons fasciculate normally, stop appropriately in the lamina plexus, and ultimately form morphologically normal synapses, albeit with inappropriate partners (Lee et al., 2003, and data not shown).
Flamingo is not required cell-autonomously in R cell axons
To examine the mechanism by which Flamingo controls R cell connection patterns, we performed a series of single cell manipulations that alter Flamingo levels in individual growth cones. We began using mosaic analysis with a repressible cell marker (MARCM; Lee and Luo, 1999; Prakash et al., 2005), to generate single mutant R cells positively labeled with Green Fluorescent Protein (GFP) in an otherwise wild-type background (Figure 1). Clones were generated using the yeast FLP recombinase, under the control of a heat-shock promoter and, under appropriately mild heat shock conditions, it is possible to generate single cell clones in any R cell sub-type. As each R cell has a characteristic morphology and position within the ommatidium, each R1-R6 sub-type can be unambiguously identified independent of its axonal phenotype (Figure 1, B, C, G, H, L, M, Q, R). Since the projection of each R cell axon is both invariant, and uniquely specified by its sub-type identity, any variation would reflect a cell-autonomous function of flamingo. In this system, the axon of each R cell can be unambiguously traced from the retina into the brain, and defects that emerge in any step of the targeting process can be detected. Under these conditions, all R cells homozygous for a control chromosome extended out of the retina normally, associated with the appropriate ommatidial axon fascicle into the brain, and innervated the correct cartridge (n=33, Figure 1D, E, I, J, N, O, S, T). Remarkably, all isolated mutant R cells, homozygous for a null mutation in flamingo, were indistinguishable from control axons throughout their trajectory, and invariably chose appropriate post-synaptic targets (n=117, Figure 1F, K, P, U, and data not shown). Three lines of evidence demonstrate that we do achieve significant loss of Flamingo function in these single cell clones. First, as expected from previous studies, removal of Flamingo from single R3 and R4 cells caused ommatidial orientation defects with comparable expressivity to that seen in large clones using the same null alleles (33% misoriented, n=69 R3/4 clones versus 37% in large clones (Rawls and Wolff, 2003). To avoid possible confounding effects of ommatidial orientation on axonal projection, in all analyses, we excluded data from abnormally oriented ommatidia. Second, the dynamic expression of Flamingo in wild-type animals suggests that its axonal expression derives from synthesis after R cells complete their final cell division (and are thus potentially already homozygous for flamingo mutations (Lee et al., 2003). Indeed, in our studies, even single cell flamingo clones showed profound loss of expression (Figure S2). Third, as we describe below, removing Flamingo function from single cells does cause non-autonomous axon targeting defects. Thus, these experiments demonstrate that Flamingo is not required cell-autonomously in R cell axons.
Flamingo mediates interactions between neighboring R cell growth cones from within the same ommatidium
The fact that Flamingo does not function cell-autonomously in R cell axons raised the possibility that Flamingo might instead act non-autonomously, providing a signal between R cell growth cones necessary for targeting. We envisioned two extreme alternative models by which Flamingo could function in this context. In one view, Flamingo could act non-specifically, mediating interactions between any pair of R cell growth cones, regardless of their sub-type, or relative spatial position, within or between ommatidia, to control target selection. At the other extreme, Flamingo could function with absolute specificity, being required in only a particular R cell sub-type, sending a signal to specific R cell neighbor, either in the same ommatidium, or in one of the neighboring ommatidia. To distinguish between these two alternatives, we developed two variations of reverse MARCM (Lee et al., 2000), a strategy to positively label single identified wild-type cells while negatively labeling single, identified flamingo mutant cells. As flamingo function is not required cell autonomously in R cell axons (Figure 1), the targeting of these negatively marked, mutant cells is presumably normal. In both mosaic approaches, homozygous mutant and wild-type cells are generated at random in the retina, and identified by their morphologies and positions; in one approach, all wild-type cells are positively labeled with GFP using a pan-neuronal promoter; in the other, only wild-type R4 cells are positively labeled. In order to unambiguously label the mutant cells, we expressed Red Fluorescent Protein (RFP) under the control of the GMR promoter, expressed in all R cells, which allows unambiguous identification of mutant cells in the retina through their loss of RFP expression. In both reverse MARCM approaches, clones are induced by expressing the FLP recombinase under the control of the heat shock promoter, and due to the way in which precursor cells are recruited into adopting R cell fates, mutant cells and wild-type cells generated by the same recombination event are separated by a variable distance in the retina. We then reasoned, by direct analogy with previous mosaic analyses of other genes, that if Flamingo’s non-autonomous function was in any way specific, reflecting either sub-type identity, or relative position, either within an ommatidium, or between neighboring ommatidia, that the presence of a targeting defect in a wild-type cell should correlate with the presence of a flamingo mutant cell in some particular spatial, or R cell sub-type specific pattern.
Using these approaches, we find that Flamingo functions as a strictly non-autonomous signal between neighboring R cells. With both labeling methods, individual mutant R cells of all possible sub-types were generated at variable distances from labeled, wild-type neighbors of all sub-type combinations (Figures 2 and 3, Figure S3, and data not shown). We identified clones in which wild-type cells were separated from mutant neighbors by a variable distance (Figure 2A-E), lacked any mutant neighbors (Figure 2F-J), or directly abutted mutant cells (Figure 2K-O). Overall, we observed inappropriate targeting of wild-type R1, 4, 5, and 6 cells in the presence of flamingo mutant neighbors, demonstrating that Flamingo acts non-autonomously to influence the targeting of most, if not all, R cells. In order to define the range and specificity of these Flamingo interactions, we then mapped the relative positions of mutant and wild-type cells within each ommatidium such that mutant cells abutting the wild-type cell were designated 1°, cells that were separated by a single intervening cell were designated 2°, and the cell that was separated by two intervening cells was denoted 3° (Figure 3A, inset). That is, each ommatidium containing a single labeled wild-type cell had two 1° cells, two 2° cells, and one 3° cell. In this way, clones generated using either reverse MARCM method could be pooled and compared. By first examining only single cell clones (containing one mutant cell and at least one wild-type cell in the same ommatidium), we found a strong correlation between the frequency of mistargeting, and the relative distance between wild-type and mutant cells (Figure 3B). In clones in which the wild-type cells targeted normally, 1° and 2° cells were mutant with approximately equal frequency, while 3° cells were mutant about half as often. This distribution is as expected from the relative numbers of 1°, 2° and 3° cells in an ommatidium. By contrast, in cases where the wild-type axon mistargeted, the distribution was strongly skewed: 12/14 clones had a single 1° cell mutant, 2/14 had a single 2° cell mutant, and none had 3° mutants (p<0.01, χ2 Test) (Figure 3B). Consistent with these observations, all clones, including ones with multiple, labelled wild-type axons and multiple mutant cells, displayed the same positional effect (Figure 3C; p<.001, χ2 Test). In total, in clones where all wild-type axons targeted normally, most had no mutant cells in the same ommatidium (54%, n=362) and 1° mutant neighbors were about as frequent as 2° and 3° neighbors (26% versus 19%, n=362). However, in clones where a wild-type axon chose an incorrect target, 77% of clones included a 1° mutant cell (n=20/26), 11% included 2° or 3° mutant cells (but not a 1° cell, n=3/26), and 11% only had mutant neighbors in an adjacent ommatidium (n=3/26). Our single and multiple mutant cell analyses (Figure 3B, C) demonstrate that the frequency of targeting defects correlates with the relative distance between mutant and wild-type cells. In addition, when there was at least one mutant cell in the same ommatidium as the wild-type cell, there was no correlation between the number mutant cells found and the behavior of the wild-type axon. In particular, we found approximately 1.5 mutant cells/ommatidium (n=171) in cases where the wild-type axon targeted normally, and 1.7 mutant cells/ommatidium, (n=23) when the axon mistargeted. Moreover, our analyses also demonstrate that the spatial relationship between wild-type and mutant cells, not the specific sub-type identity of the mutant cell, is what influences axonal behavior. That is, for each axon that mistargeted in the presence of a 1° mutant, we observed that the mutant could fall on either side of the wild-type cell; for R4, for example, mistargeting was associated with mutant R3 or mutant R5 cells, with R5 predominating. Thus, in contrast to the role for Flamingo in retinal fate patterning, where Flamingo acts strictly in R3 and R4, in the brain, its function extends to all R1-6 cell sub-types. Note that the rare cases in which a wild-type cell (containing 2 wild-type copies of the flamingo locus) mistargeted, yet had only 2° or 3° mutant neighbors, could reflect either failed interactions with the relatively remote homozygous mutant cell, or by abnormal interactions with its 1° neighbors (which contain only 1 normal copy, and hence a lower relative level, of flamingo). In aggregate, 17% of wild-type axons adjacent to a mutant 1° cell chose inappropriate targets (20/115 cases), while 4% (n=3/73) of axons with only 2° or 3° mutant neighbors, and only 1.5% (n=3/200) of axons with mutants in adjacent ommatidia targeted inappropriately (Figure 3D). Thus, as the relative distance between wild-type and mutant cells increases, the expressivity of the targeting phenotype decreases. These mistargeting defects were of two types: either the wild-type axon failed to extend away from the ommatidial fascicle, and innervated the nearest cartridge (n=14/26), or it extended toward another inappropriate target (n=12/26). In many cases, these mis-targeting wild-type axons innervated two targets. Taken together, these studies demonstrate that Flamingo acts locally, as a non-autonomous signal between immediate neighbors, sorting most, if not all, R cells toward appropriate postsynaptic targets. These studies thus provide the first evidence for a strictly non-autonomous role for a cadherin in patterning neuronal connections. Moreover, as we detected no strong correlation between axonal targeting and the presence of a flamingo-mutant cell in a neighboring ommatidium, flamingo-mediated interactions likely take place before R cell growth cones reach their target cartridge (where they might interact with R cell axons from the neighboring ommatidia that contribute to the cartridge). These interactions could, in principle, take place either anywhere along the length of the R cell axon, but because ommatidial axon bundles in flamingo mutants display no defects in fasciculation above the lamina (Lee et al., 2003), we favor the notion that the interactions we describe take place amongst R cell growth cones within the lamina plexus, prior to extension.
Figure 2. Flamingo acts non-autonomously to control the targeting of neighboring photoreceptors.
(A-C, F-H, K-M) Retina. Homozygous wild type cells (green) abutting flamingo null mutant cells (black) are generated at random, and are uniquely 23 identified by their morphology (red) (B, G, L). Retina. (C, H, M) Flamingo null mutant cells (chevrons) arise within ommatidia containing wild-type neighbors. Mutant cells can be identified by the absence of GMR-RFP expression (white), which is particularly noticeable in the rhabdome. (D, I, N) Schematic images of the labeled R cell axons (green), homozygous mutant axons (black) and heterozygous axons (gray) extending to their targets (red). (E, J, O) Confocal images of labeled R cell projections (green) extending to their targets (red). Normally extending axons (arrows) extend to specific targets; mistargeting axons extend two growth cones, innervating inappropriate targets (arrowheads). Inset panels depict single axons at high magnification. In (E), neither growth cone innervated the appropriate target (denoted * in D, E). Scale bar 10μm.
Figure 3. Flamingo mediated interactions between immediately neighboring R cells are critical for target selection.
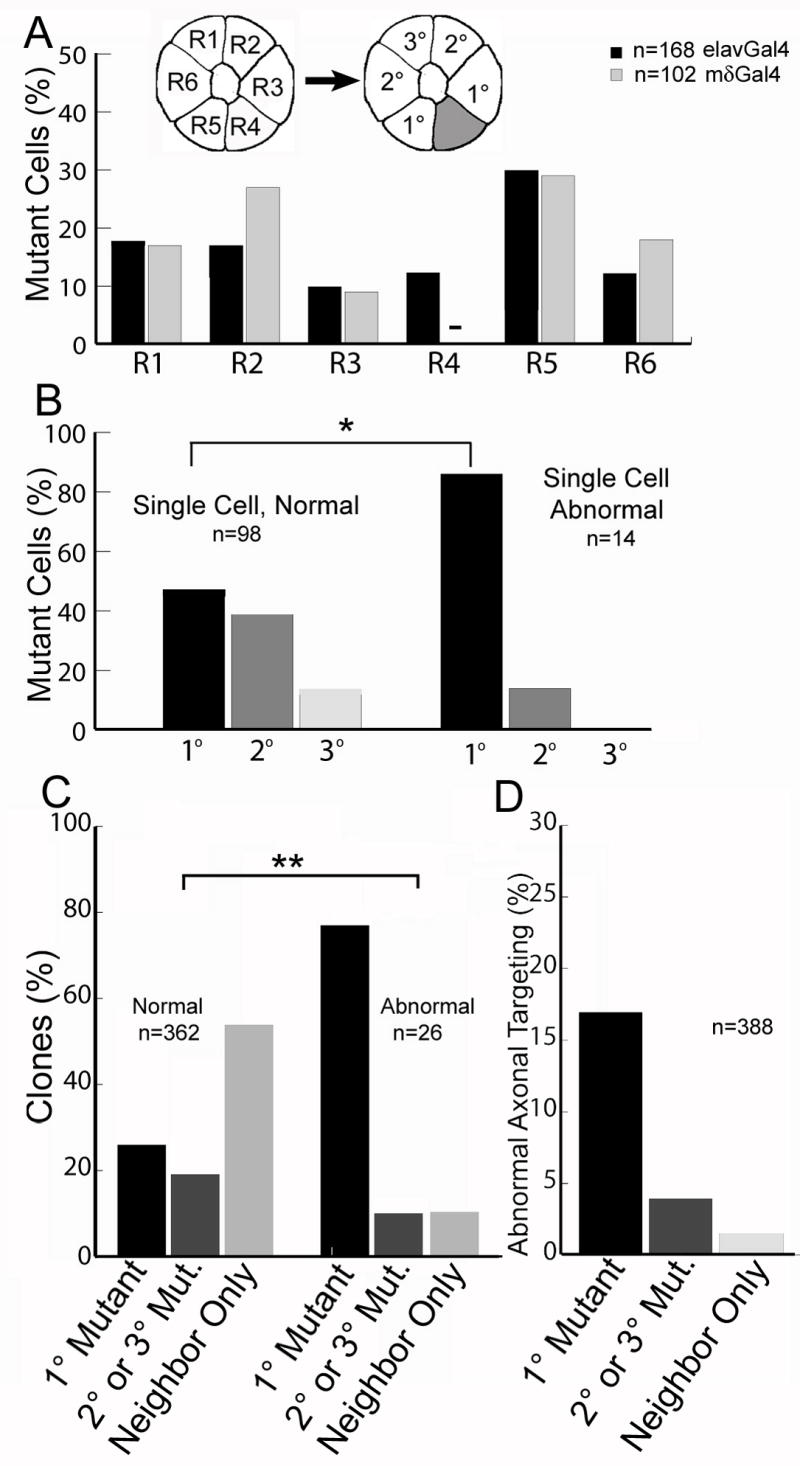
(A) The distribution of flamingo mutant R cells in two variations of reverse marcm. Flamingo mutant R cells, elavGal4 (black bars), mδGal4 (grey bars, n=102). In mδGal4 clones, all labeled wild-type axons are R4, so none are ever mutant (-). Inset panel designates the neighbor relationships between R cells relative to a single wild type cell (grey). (B) The distribution of projection defects in ommatidia containing only 1 mutant cell, and 1-3 wild-type cells, pooled between elavGal4 and mδGal4 datasets. The data is divided into those cases where the wild-type axons targeted normally (left bars), and abnormally (right bars). The fraction of clones is plotted as a function of the separation between the mutant cell and the wild-type cell (* p<0.01, χ2 test). (C) Plot of the fraction of clones of each type, dividing the data into two groups. Left bars: normal targeting. Right bars: abnormal targeting. These distributions are different (p<.001; χ2 test). “Neighbor Only” denotes clones in which the only mutant cells were in the neighboring ommatidium (185 cases), or clones in which there were no mutant neighbors at all (12 cases). (D) The frequency of abnormal axon targeting as a function of the neighbor relationship between wild-type and mutant cells.
Flamingo-mediated interactions amongst R cell axons are homophillic
Flamingo has previously been shown to mediate homophillic interactions in vitro (Usui et al., 1999), yet whether Flamingo mediates homophilic interactions or functions as a heterophilic signal, through some as-yet unidentified receptor, has not previously been demonstrated in vivo. To address this issue, we reasoned that if Flamingo was to function in a heterophillic fashion, the targeting phenotypes we observe when a mutant cell abuts a wild-type cell in a two cell pair should be preserved when both cells are mutant for flamingo (since the phenotype of the cell responding to the flamingo signal would depend on an unknown receptor, not on Flamingo; Figure S4). That is, if Flamingo functions in heterophilic fashion, a neighboring two cell clone generated by forward MARCM should display the same level of targeting defects as a reverse MARCM clone in which a wild-type cell is adjacent to its “twinspot” mutant cell. On the other hand, if Flamingo mediates homophillic interactions strictly non-autonomously, the two neighboring mutant cells should target normally, as Flamingo has no cell-autonomous effect on targeting. That is, in this situation, the two mutant cells should be “blind” to one another (since neither can produce, or respond, to a Flamingo signal), and should behave as if they were two single mutant cells produced in isolation from one another (which target normally, see Figure 1). We therefore examined the behavior of adjacent pairs of flamingo mutant cells using MARCM, and found mistargeting in only 1/40 pairs (data not shown). By comparison, using reverse MARCM, in two cell clones comprising pairs with one mutant cell abutting one wild-type neighbor, we observed mistargeting in 9/39 cases (p<0.01, Fisher’s Exact Test). Thus, the simplest explanation for the strictly non-autonomous role of Flamingo in mediating afferent-afferent interactions between R cell growth cones is one that reflects homophilic function.
Flamingo over-expression in a single R cell type is sufficient to cause non-autonomous targeting errors
These loss-of-function studies raised the possibility that appropriate R cell axon targeting might rely on relative differences in Flamingo protein expression between R cell growth cones. Consistent with this view, Flamingo protein was unequally distributed across R cell growth cone boundaries at the developmental stage at which R cell axons extend to their target (Lee et al., 2003). To test this notion, we reasoned that over-expression of Flamingo in a single R cell growth cone should alter the targeting of neighboring cells. The most specific driver available, mδGal4, is expressed in only R4 cells at this stage, as well as in most lamina target neurons (Figure 4A), so we compared the phenotypes associated with over-expression of a wild-type Flamingo transgene using mδgal4 to those seen using the strong, well-characterized lamina-neuron specific driver gcmGal4 (Figure 4B). Strikingly, in animals over-expressing wild-type Flamingo protein using mδGal4, but not gcmGal4, many cartridges contain abnormal numbers of R cell axons, indicative of widespread targeting defects, in both pupal and adult brains (Figure 4C, D; data not shown). This observation suggests that phenotypes associated with Flamingo over-expression are specific to altered interactions between R4 and other R cell growth cones. Moreover, this phenotype is strongly dose sensitive, as reducing either the temperature of cultivation (lowering the activity of Gal4), or reducing the copy number, reduces the strength of the phenotype, and the fraction of brains in which it is observed (Figure 4E-G, and data not shown). Finally, over-expression of a truncated form of Flamingo that lacks the intracellular domain is sufficient to generate the same phenotype, consistent with the notion that it is the relative levels of the extracellular, adhesive portion of the molecule that directs R cell target choice (Figure 4H).
Figure 4. Overexpression of Flamingo in R4 is sufficient to cause mistargeting of neighboring axons.

(A) The expression pattern of mδGal4 (green) and Flamingo protein (red) in the lamina plexus. (B) The GcmGal4 expression pattern (green), and Flamingo (red). (C) The pattern of cartridges (red) in the adult lamina of animals overexpressing flamingo using mδGal4. Single cartridges are inset. (D) The pattern of cartridges (red) in the adult lamina of animals overexpressing Flamingo using GcmGal4. Scale bar, 20μm. (E-H). The pattern of cartridges in the adult lamina overexpressing either 2 copies (X2) or 1 copy (X1) of either full-length Flamingo (E-G) or a truncated form of Flamingo lacking the intracellular domain (ΔC, H). Single cartridges are inset. (I-L) Dye injection into single ommatidia (red). Panels contain all of the axons from one ommatidium; insets display the corresponding retinal image.(I)Control. (J-H)Flamingo overexpression in R4 using mδGal4. Arrows indicate R4 axons, arrowheads denote R cell growth cones that have targeted inappropriately. Scale bar, 10 μm.
To examine this phenotype with single cell resolution, we injected fluorescent dye into single ommatidia in which full-length Flamingo was over-expressed in R4, and visualized the pattern of R1-R6 connections (Figure 4I-L). In 8/9 injected ommatidia, defects in R cell targeting were observed. In 6/8 cases, the apparent R4 axon targeted normally, while neighboring R cells followed inappropriate trajectories; in the remaining 2 cases, the majority of axons chose inappropriate targets, and a presumptive R4 axon could not be discerned. In all 6 cases in which R4 targeted normally, the targeting phenotypes seen in surrounding R cell axons are not consistent with a non-specific association between the mistargeting axon and R4, which one might have expected if a non-specific “stickiness” had been caused by over-expression. These results demonstrate that altering the relative levels of Flamingo on R cell growth cones disrupts the short-range signaling events necessary for R cell growth cones to reach appropriate targets. As R cell growth cones extend toward their targets, they maintain close contact with lamina neurons in the target field. However, over-expression of Flamingo in the target neurons that contact R cell axons has no effect on R cell target choice. Thus, this result demonstrates that the effects of Flamingo over-expression on R cell target choice specifically reflect manipulations of Flamingo-mediated signaling between R cell growth cones. This observation also implies that Flamingo mediated interactions can only take place in the context of R cell growth cones.
MODEL
We have shown that the atypical cadherin Flamingo functions as a short-range, homophilic signal, passing between neighboring R cell processes to influence their choice of post-synaptic partners. In contrast to Flamingo’s requirement in dendritic tiling and layer-specific targeting (Lee et al., 2003; Senti et al., 2003; Gao et al., 2000; Grueber et al., 2002; Kimura et al., 2006), Flamingo acts in a strictly non cell-autonomous fashion to control R1-6 cell connectivity (Figure S5). The observation that targeting phenotypes in wild-type cells next to mutant neighbors are much more severe than those seen in two adjacent mutant cells demonstrates that growth cone behavior is sensitive to relative differences in Flamingo activity between neighboring cells, not the absolute level of Flamingo activity in any single cell. How could a homophillic molecule act strictly non-autonomously? We propose that, in wild-type animals, each R cell growth cone compares its level of Flamingo with that of its two neighbors, and either increases, or decreases, its contacts with them in order to precisely balance the Flamingo-mediated interactions on both sides. That is, interactions either “pulling” (attractive) or “pushing” (repulsive) with one neighbor are physically opposed by interactions with the neighbor on the opposite side of the growth cone. A simple means of balancing these opposing forces would be for a growth cone to alter the extent of its contacts with its neighbors, either by adjusting its morphology, or by changing its relative position. As this comparison process must take place prior to axon extension, yet affects target selection, it likely determines axon trajectory, influencing which potential post-synaptic targets will ultimately be contacted. When one neighbor lacks Flamingo, this balance becomes disrupted, causing the wild-type growth cone abutting the mutant cell to interact excessively with its remaining wild-type neighbor, via Flamingo, and mis-target. That is, we propose that the amount of Flamingo on each surface of each R cell is “titrated” by homophilic interactions with its neighbors; when interactions with one neighbor are lost, the Flamingo molecules that would normally have been engaged with the mutant neighbor now shift to the other side of the neurite, and engage Flamingo molecules on the processes of the remaining wild-type neighbor. This increases the strength of the homophillic interaction with that neighbor to an abnormally high level. The single mutant cell does not experience this unbalance, as it loses Flamingo-mediated interactions symmetrically, with neighbors on both sides. It thus innervates the appropriate target through the action of Flamingo-independent targeting signals. Since removing Flamingo from all R cells (in large clones) causes severe targeting errors, these Flamingo-independent signals must be only partially redundant with Flamingo, as they can only act effectively when very few cells are mutant. Our model is consistent with electron microscopic reconstructions showing that the relative positions of R cell axons are invariably maintained from the eye to the brain, and that contacts between immediate neighbors within an ommatidial group are the major component of each R cell growth cone’s surface interaction prior to target selection (Meinertzhagen and Hanson, 1993).
Flamingo’s molecular structure defines it as an unusual member of the cadherin superfamily, raising the possibility that the mechanism we propose may not be general to all cadherins (Usui et al., 1999). Indeed, it is unclear whether Flamingo acts as a purely adhesive factor, or through some as-yet unknown signaling cascade (Das et al., 2002; Shima et al., 2006). However, our mosaic analysis argues strongly that Flamingo acts locally, within the growth cone (or axon), and does not send a long-range signal (to the nucleus, for example) that indirectly alters target selection. The critical observation is that the correct spatial pattern of Flamingo activity around a given R cell neurite, balanced between its immediate neighbors, but not the absolute amount of activity (which can be lowered to zero without cell-autonomous effect), is critical for target selection. As transmitting a signal outside the growth cone could, at most, preserve information about the strength of the Flamingo-mediated interactions, not information about whether these interactions occurred on one, or both, sides of the neurite, a long-range signaling model is inconsistent with our data. Thus, as Flamingo’s role in R cell target selection reflects a direct, local effect on growth cone trajectory, this activity does not necessarily demand an unusual signaling mechanism unavailable to other cadherins in other contexts.
The balanced neighbor-neighbor interaction system we propose represents a cellular form of opponency that provides a means of detecting small differences in cadherin activity between cells. It serves an ideal substrate for afferent-afferent interactions in R cells, as it allows growth cones to directly read-out the differences in Flamingo expression that we see in wild-type animals, and use these differences to determine their relative orientations. More broadly, this opponent model provides a sensitive, general mechanism for measuring relative differences in the expression of any homophilic adhesion molecule, and translating these differences into changes in axon trajectory. Thus, our studies provide the first evidence for a novel mechanism organizing the precise pattern of synaptic connections in the developing brain.
MATERIALS AND METHODS
Fly Stocks
The following stocks were used: eyFLP, ey3.5FLP,the FRT42D ELF system, FRT42D fmi e59/CyO KrGFP, FRT42D fmi e45 tubP-Gal80/CyO UbqGFP, UAS fmi, FRT42D tubPGal80, mδGal4, hsFLP22, elavGal4 UASmCD8GFP hsFLP, GMRRFP, gcmGal4. For MARCM experiments, 3rd instar larvae, between 3-4 days AEL, were incubated at 37°C for 27 minutes, and pupae with appropriate clones were selected for analysis at 42hrs after puparium formation. For reverse MARCM, 3rd instar larvae between 3-4 days AEL were incubated at 37°C for 22 minutes.
Immunohistochemistry and Dye Injection
Pupal and adult brains were stained as previously described (Prakash et al., 2005; Lee et al., 2001). The following antibodies were used: rabbit anti GFP (Promega), mAb24B10 (Developmental Studies Hybridoma Bank (DSHB)), mouse anti flamingo (DSHB), mouse anti csp2a (DSHB), goat anti rabbit AlexaFluor 488 (Invitrogen), goat anti rabbit AlexaFluor 488 IgG2a (Invitrogen), goat anti mouse Cy5 (Jackson Immunoresearch), goat anti mouse AlexaFluor 594 (Invitrogen). Labeling of R cell projections using fluorescent diI was as described (Clandinin and Zipursky, 2000).
Imaging
All images were acquired on a Leica TCS SP2 AOBS confocal microscope, using either a 40X NA 1.25 lens, or a 100X N.A. 1.4 lens, and were processed using Huygens Pro (SVI), rendered using Imaris (Bitplane) and mounted using Photoshop (Adobe).
Supplementary Material
Acknowledgments
The authors thank Jeff Axelrod, T. Uemura, I. Salecker, L. Luo, M. Krasnow, and P. Garrity for reagents, L. Luo, K. Shen, and members of the Clandinin lab for helpful comments on the manuscript. This work was supported by a Stanford Graduate Fellowship (PLC) and by NIH R01 EY015231 (TRC). TRC was a recipient of a career development award from the Burroughs-Wellcome Foundation, and a Searle Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barth M, Hirsch HV, Meinertzhagen IA, Heisenberg M. Experience-dependent developmental plasticity in the optic lobe of Drosophila melanogaster. J Neurosci. 1997;17:1493–1504. doi: 10.1523/JNEUROSCI.17-04-01493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Das G, Reynolds-Kenneally J, Mlodzik M. The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev Cell. 2002;2:655–666. doi: 10.1016/s1534-5807(02)00147-8. [DOI] [PubMed] [Google Scholar]
- Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Gao FB, Kohwi M, Brenman JE, Jan LY, Jan YN. Control of dendritic field formation in Drosophila: the roles of flamingo and competition between homologous neurons. Neuron. 2000;28:91–101. doi: 10.1016/s0896-6273(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 1998;395:387–391. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, Meinertzhagen IA, Bellen HJ. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Kimura H, Usui T, Tsubouchi A, Uemura T. Potential dual molecular interaction of the Drosophila 7-pass transmembrane cadherin Flamingo in dendritic morphogenesis. J Cell Sci. 2006;119:1118–1129. doi: 10.1242/jcs.02832. [DOI] [PubMed] [Google Scholar]
- Lee RC, Clandinin TR, Lee CH, Chen PL, Meinertzhagen IA, Zipursky SL. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. 2003;6:557–563. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, H TE. In: The Development of Drosophila Melanogaster. Bate M, Martinez-Arias A, editors. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. pp. 1363–1491. [Google Scholar]
- Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Rawls AS, Wolff T. Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development. 2003;130:1877–1887. doi: 10.1242/dev.00411. [DOI] [PubMed] [Google Scholar]
- Senti KA, Usui T, Boucke K, Greber U, Uemura T, Dickson BJ. Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Curr Biol. 2003;13:828–832. doi: 10.1016/s0960-9822(03)00291-4. [DOI] [PubMed] [Google Scholar]
- Shima Y, Kengaku M, Hirano T, Takeichi M, Uemura T. Regulation of dendritic maintenance and growth by a mammalian 7-pass transmembrane cadherin. Dev Cell. 2004;7:205–216. doi: 10.1016/j.devcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci. 91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Tissir F, Bar I, Jossin Y, De Backer O, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nat Neurosci. 2005;8:451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



