Summary
The Notch proteins play a vital role in cell fate decisions in both invertebrate and vertebrate development. Careful analysis of this role has led to a model of signalling downstream of these receptors, via the CSL (CBF1, Suppressor of Hairless, Lag-1) family of transcription factors. However there have been suggestions that Notch can signal through other pathways. In the current paper, Ramain et al. (1) provide compelling evidence for Notch signalling through a CSL-independent pathway and they demonstrate that the cytoplasmic protein, Deltex, is required for this signal. In addition they show that Wnt signalling may regulate this Deltex dependent signal.
Keywords: Notch, Deltex, Wnt, cell signalling, cross-talk
Introduction
Notch genes encode large transmembrane proteins that act as receptors for the DSL (Delta, Serrate and Lag-2) family of ligands (2). These receptors are highly conserved, and play a crucial role in cell fate decisions during the development of organisms as diverse as sea urchins and humans (3). In addition, aberrant Notch signalling has been linked to several human diseases including a number of cancers, Alagille's syndrome and the neural degenerative disease, CADASIL, (4).
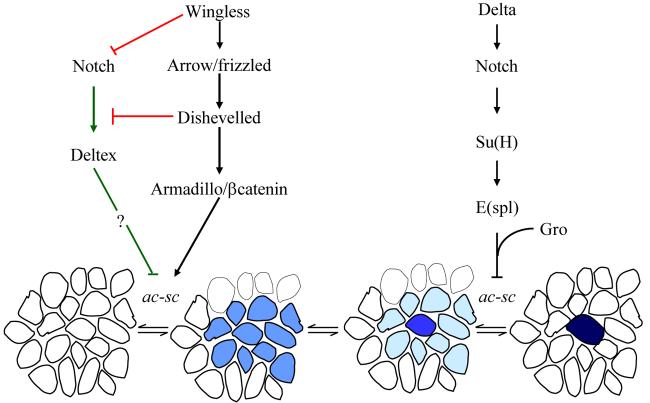
The best understood role of the Notch receptors in cell fate decisions is in the process of “lateral inhibition” which was first described during peripheral nervous system development in Drosophila (see figure 1) (5). The Drosophila thorax carries two types of sensory bristles, macrochaetae and microchaetae. Bristle development is initiated by prepattern genes and signalling through the Wingless pathway, which leads to the expression of proneural genes of the achaete-scute complex in small groups of cells (6-8). All the cells within these proneural clusters have the potential to develop into sense organ precursors (SOPs). However only one or two cells maintain achaete-scute expression and differentiate into SOPs, and in doing so emit an inhibitory signal that extinguishes proneural gene expression in their neighbours. This process is known as lateral inhibition. The selected SOPs will divide three times to produce the five cells of the sensory bristles including the external socket and bristle cells, and innervating neurone (9).
Figure 1. Summary of SOP development in wild type and Notch mutant backgrounds.
In wild type flies, the combined action of the prepattern genes and signalling through the Wingless pathway leads to the expression of proneural genes of the achaete-scute complex in small groups of cells. All the cells within these proneural clusters have the potential to develop into SOPs. However lateral inhibition signalling restricts achaete-scute expression to one or two cells. These cells will divide three times to produce the socket, bristle, supporting, glial and neural cells of the sensory bristles. In the NMcd and NAx mutants there is increased Notch signalling via Deltex which represses proneural gene expression. This signalling prevents proneural cluster specification in NMcd flies and consequently no SOPs develop. In NAx mutants the increase in signalling via Deltex is not as great and proneural clusters of reduced size develop. The process of lateral inihibition then restricts proneural gene expression to one cell. Both signalling via Deltex and lateral inhibition are abolished in Nnull clones leading to robust achaete-scute expression and the development of multiple SOPs.
Careful analysis of this lateral inhibition signal, together with experiments in other systems, has provided a detailed model for canonical DSL signalling (2) and a sensitive assay for Notch function. The signal is initiated by the interaction of the DSL ligands on the differentiating SOPs with the extracellular domain of the Notch proteins on neighbouring cells (see figure 1). This leads to two sequential proteolytic cleavages of the Notch protein, releasing the intracellular domain. This fragment of Notch enters the nucleus where it interacts with members of the CSL (CBF1, Suppressor of Hairless, Lag-1) family of transcription factors, converting the CSL proteins from transcriptional repressors into activators. During bristle development in Drosophila the association of the Notch intracellular domain with the Drosophila CSL protein, Suppressor of Hairless (Su(H)), leads to the expression of the bHLH transcription factors of the Enhancer of split Complex (E(spl)-C) (10). In turn the E(spl) proteins associate with the transcriptional co-repressor, Groucho (Gro), to inhibit achaete-scute expression.
Identification of a new class of Notch alleles
In the current paper, Ramain et al. (1) have isolated six new alleles (NMcd1, NMcd2, NMcd5, NMcd7, NMcd8 and NMcd9) and identified one existing allele (NMcd3) of Notch in a genetic screen for mutations that specifically reduce the number of thoracic microchatae (see figure 1). These alleles appear to affect a Notch function as the NMcd phenotype changes when the copy number of the wild type Notch allele is altered. Furthermore, as Notch signalling inhibits bristle formation during normal development (11), the phenotype of the NMcd alleles suggests that they are gain of function mutations.
The phenotype of the NMcd alleles is reminiscent of two other classes of Notch alleles, the l(1)NB-like and Abruptex (NAx) alleles (12). Like NMcd mutants, the number of microchaetae are reduced in flies of both these classes. The l(1)NB-like class are easily distinguishable from the NMcd alleles genetically as the phenotype of the l(1)NB-like mutants behaves differently when the copy number of the wild type Notch allele is altered (12). In contrast, the NAx alleles exhibit similar genetic behaviour to the NMcd alleles when wild type Notch function is increased or decreased (12). However NAx and NMcd alleles are distinguishable phenotypically. In the NAx mutants, macrochaetae are lost as well as microchaetae and they have broader wings, with shortened veins, than wild type flies. These phenotypes are not observed in NMcd/+ animals and suggest that increased canonical DSL signalling is occurring in NAx/+ flies.
Notch gain of function is independent of Lateral Inhibition
If the loss of microchaetae observed in the NMcd mutants is caused by increased signalling during lateral inhibition (see figure 1), the phenotype should be rescued when lateral inhibition is abolished. To test this possibility the authors have generated clones of NMcd cells that lack components required for lateral inhibition signalling (5). In all cases microchaetae fail to develop, indicating that the NMcd phenotype is not due to excessive signalling during lateral inhibition (see figure 1). Furthermore, as NMcd clones that lack Delta and Serrate function (the two known Drosophila DSL ligands) are indistinguishable from NMcd clones, the NMcd phenotype must be due to signalling of an unknown Notch ligand or an intrinsic activity of the Notch protein. Together these data indicate that the NMcd phenotype is due to increased signalling through a distinct intracellular pathway.
Proneural clusters are not defined in NMcd mutants
To analyse further the cause of NMcd phenotype the authors examined the expression of several marker genes that allow microchaetae differentiation to be monitored. Using the neural-specific antibody 22C10 (13), they demonstrated that the neurones that innervate the sensory bristles are absent. Next they showed that SOPs fail to differentiate as expression of the SOP marker gene, neuralised, is absent (14). Further, they found that the proneural gene Achaete is not expressed in the NMcd mutants, indicating that the proneural clusters are not defined in the first place (6). Consequently it appears that the aberrant Notch signalling in the NMcd mutants is preventing the establishment of the proneural clusters.
A similar failure to establish normal proneural clusters is observed in the NAx mutants, (15) but in these flies the clusters are reduced in size rather than absent. This difference can explain the different phenotypes observed in clones of NMcd and NAx alleles where lateral inhibition signalling is abolished (see figure 1) (1, 16). In the NAx clones, multiple SOPs arise from the small proneural clusters when lateral inhibition is abolished leading to a tuft of bristles on the thorax. In contrast, in the NMcd animals no SOPs can develop even in the absence of lateral inhibition as the proneural clusters are absent.
The NMcd phenotype requires both Deltex and Shaggy
Deltex was originally implicated in Notch signalling because the phenotypes when the gene is mutated (17) or when the protein is over expressed (1, 18) mimic the phenotypes observed when Notch signalling is disrupted or activated respectively. In addition the Deltex protein has been shown to interact with the Notch intracellular domain suggesting that it functions downstream of the receptor (19). Ramain et al. (1) have shown that regularly spaced microchaetae develop in NMcd,deltex double mutant clones, indicating that Deltex function is required for the NMcd phenotype. As the bristles are regularly spaced lateral inhibition signalling must be occurring normally. The authors have confirmed this by demonstrating that the NMcd proteins are cleaved to release the intracellular fragment which is indicative of DSL signalling (20, 21). Interestingly, deltex was originally isolated in a genetic screen for suppressers of a lethal combination NAx alleles (17) suggesting that the NAx phenotype is partly dependent upon signalling via Deltex as well.
Shaggy (Drosophila GSK-3β) is a central component of the Wingless signalling pathway which negatively regulates signalling through the pathway (22). However shaggy has also been shown to be epistatically downstream of Notch as shaggy mutations will rescue the NAx phenotype (23). Although this suggests that Shaggy may also be a component of a signalling pathway downstream of Notch, this result has generally been interpreted in the light of the fact that Notch and Wingless signalling have opposing effects on bristle development (see figure 1) (7, 11). Wingless signalling is required for the expression of the proneural gene, achaete. Therefore it has been suggested that unregulated Wingless signalling in the absence of Shaggy function will lead to Achaete expression and bristle development even when excessive Notch signalling is occurring (15, 24). It also appears that an unregulated Wingless signal can rescue the NMcd phenotype as microchaetae develop in double mutant clones for NMcd and shaggy.
Regulation of signalling by the NMcd proteins
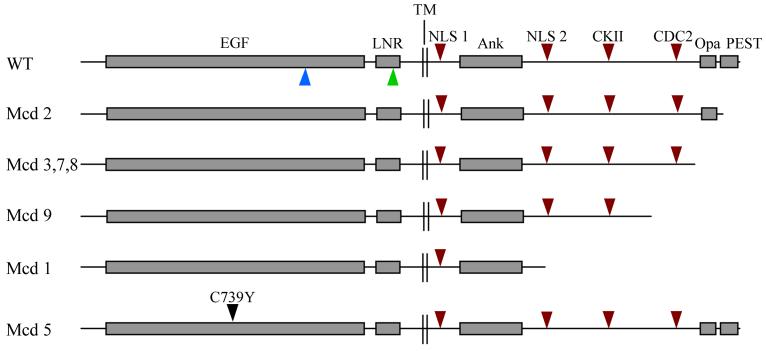
The authors have characterised all seven alleles and found that all of them contain a mutation that will prematurely terminate the Notch protein C-terminal to the cdc10/ankyrin repeats, with the exception of NMcd5 which is a mutation within EGF-like repeat 18 (see figure 2). In addition they noted that the severity of the phenotype is correlated with extent of the deletion. This region of Notch has previously been shown to interact with the Dishevelled protein, another intracellular component of the Wingless signalling pathway (25). The authors have confirmed in a two-hybrid analysis that the C-terminally deleted NMcd proteins are unable to interact with Dishevelled.
Figure 2. A schematic representation of the Notch protein.
The Notch protein contains 36 tandemly repeated epidermal growth factor (EGF) -like and three LNG (Lin-12, Notch, Glp-1) repeats in its extracellular domain. The intracellular domain contains a juxtamembrane RAM23 domain, six cdc10/ankyrin repeats, two nuclear localisation sequences (NLS), caesin kinase II (CKII) and CDC2 phosphorylation sites, a poly glutamine (OPA) repeat and a PEST sequence. The seven different NMcd proteins are shown below the wild type protein and position of the NAx and l(1)NB mutations is indicated by blue and green arrowheads respectively.
The rescue of the NMcd phenotype by removing Shaggy function suggests that a Wingless signal can inhibit the Notch signal that is activated by the NMcd mutations. Also Wingless signalling may inhibit Notch signalling via Deltex during normal development, as Wingless signalling is required for Achaete expression. Data from the careful analysis of two Wingless target genes support this possibility (26, 27). The expression of S59 and Ultrabithorax in the somatic and visceral mesoderm respectively is dependent upon Wingless signalling, and expression of the two genes is lost or reduced in wingless mutant embryos (28, 29). However both genes are robustly expressed in the absence of a Wingless signal if Notch function is also removed (26, 27). In contrast removal of Su(H) function in a wingless mutant does not rescue the expression of either gene. This suggests that the expression of both genes is inhibited by Notch prior to the receipt of a Wingless signal and that a pathway that is distinct from the canonical Notch pathway is mediating the repression. It also suggests that the first step in Wingless signalling is to break this repression.
One way for a Wingless signal to regulate Notch signalling is through the interaction between Notch and Dishevelled (25). As the region of Notch required for this interaction is deleted in the NMcd proteins, this regulation would be abolished leading to unregulated Notch signalling via Deltex. The authors tested this possibility by over expressing Dishevelled in wild type and NMcd flies. In wild type flies, they found a mild but significant increase in the number of microchaetae, whereas microchaetae numbers are unaltered in the NMcd flies. This suggests that Dishevelled is able to regulate Notch signalling via Deltex through its interaction with the C-terminus of the Notch protein.
On the other hand, the clustering of the NMcd5 and NAx mutations to a defined group of EGF-like repeats suggest that Notch signalling activated by the NMcd mutations could also be regulated by an extracellular ligand. For example the NMcd5 and NAx mutations may be increasing the affinity of Notch for an unknown ligand that activates signalling via Deltex. On the other hand the mutations could be preventing the interaction of Notch with a ligand that inhibits Deltex dependent signalling. One interesting candidate for the inhibitory ligand is Wingless which has been shown by biopanning, immunoprecipitation and co-localisation studies to interact with the EGF-like repeats of Notch that are mutated in the NMcd5 and NAx alleles (30).
Conclusions
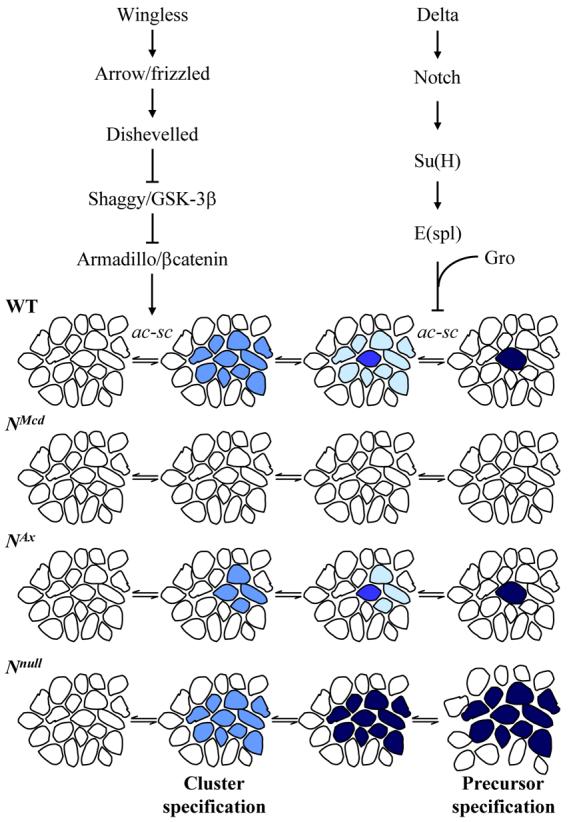
Altogether these results, along with published data, suggest the following model for the development of the thorax microchaetae (see figure 3). The definition of the proneural clusters is initiated in the pupal wing disc by prepattern genes such as pannier, ushaped, Bar and elements of the Iroquois complex (6, 8). However Notch signalling via Deltex initially inhibits expression of the proneural genes in these clusters. Wingless signalling alleviates this repression through either the interaction of Dishevelled, Wingless or both with the Notch protein. This regulation of Notch signalling along with signalling through the canonical Wingless signalling pathway (7) leads to Achaete/Scute expression. Then lateral inhibition signalling via the canonical DSL pathway restricts Achaete/Scute expression to the one or two cells that will differentiate into SOPs (11). In this model, NMcd mutations disrupt the regulation Notch signalling via Deltex by Wingless or Dishevelled. Consequently the initial repression of Achaete/Scute expression is not broken and the proneural clusters fail to develop. In contrast, the reduced cluster size in the NAx mutants suggest that the ability of Wingless signalling to regulate Notch signalling via Deltex is reduced rather than abolished. Regulation of Notch signalling via Deltex by Wingless signalling could also explain the differences in phenotypes sometimes observed when Wingless signalling is activated by expressing Wingless and an activated Armadillo protein (see figure 3) (31, 32).
Figure 3. A model for regulation of Notch signalling via Deltex by Wingless signalling.
The achaete-scute expression is initiated by the combined action of the prepattern genes. However Notch signalling via Deltex represses proneural gene expression. Wingless signalling alleviates this repression locally through the interactions of Dishevelled, Wingless or both with the Notch protein. Inhibition of signalling via Deltex and signalling through the canonical Wingless pathway leads to proneural cluster development from which one or two SOPs will arise.
Notch signalling via Deltex may have a more general role in repressing the expression of Wingless target genes. As described above Notch is required to repress S59 and Ultrabithorax expression in the somatic and visceral mesoderm respectively prior to a Wingless signal (26, 27). Also ectopic and premature engrailed expression is observed in Notch mutant embryos (K. Brennan and A. Martinez Arias unpublished results).
Further evidence for Notch signalling via other intracellular pathways has come from experiments using the murine myoblast cell line C2C12 (33, 34). In these experiments the differentiation of C2C12 cells into myotubes is prevented by ectopically expressing a form of the Notch intracellular domain that cannot interact with CBF1, the mammalian CSL family protein. This suggests that activating a CBF1-independent pathway inhibits differentiation. The differentiation of C2C12 cells is also blocked by Deltex over expression, implicating Deltex in this pathway (35). Similar results have been obtained from related experiments examining the inhibition of the bHLH transcription factors E47 and Mash1 by Notch signalling (36, 37). Finally how Notch signalling via Deltex regulates differentiation remains unclear and further experiments will be necessary to resolve the molecular mechanism.
Acknowledgements
We are grateful for the comments of Maggy Fostier, Martin Baron and Alfonso Martinez Arias on the manuscript, and the support of the Wellcome Trust.
Abbreviations
- CSL
CBF1, Suppressor of Hairless, Lag-1
- DSL
Delta, Serrate, Lag-2
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- SOP
sense organ precursor
- Su(H)
Suppressor of Hairless
- bHLH
basic helix-loop-helix
- E(spl)
Enhancer of split
- Gro
Groucho
- Mcd
microchaete defective
- Ax
Abruptex
- EGF
epidermal growth factor
- LNG
Lin-12, Notch, Glp-1
References
- 1.Ramain P, Khechumian K, Seugnet L, Arbogast N, Ackermann C, Heitzler P. Novel Notch alleles reveal a Deltex-dependent pathway repressing neural fate. Curr. Biol. 2001;11:1729–1738. doi: 10.1016/s0960-9822(01)00562-0. [DOI] [PubMed] [Google Scholar]
- 2.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev. Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Joutel A, Tournier-Lasserve E. Notch signalling pathway and human diseases. Semin Cell Dev Biol. 1998;9:619–615. doi: 10.1006/scdb.1998.0261. [DOI] [PubMed] [Google Scholar]
- 5.Artavanis Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 6.Simpson P. A prepattern for sensory organs. Drosophila development. Curr. Biol. 1996;6:948–950. doi: 10.1016/s0960-9822(02)00635-8. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Garcia MJ, Ramain P, Simpson P, Modolell J. Different contributions of pannier and wingless to the patterning of the dorsal mesothorax of Drosophila. Development. 1999;126:3523–3532. doi: 10.1242/dev.126.16.3523. [DOI] [PubMed] [Google Scholar]
- 8.Sato M, Kojima T, Michiue T, Saigo K. Bar homeobox genes are latitudinal prepattern genes in the developing Drosophila notum whose expression is regulated by the concerted functions of decapentaplegic and wingless. Development. 1999;126:1457–1466. doi: 10.1242/dev.126.7.1457. [DOI] [PubMed] [Google Scholar]
- 9.Gho M, Bellaïche Y, Schweisguth F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- 10.Bray SJ. Expression and function of Enhancer of split bHLH proteins during Drosophila neurogenesis. Perspect. Dev. Neurobiol. 1997;4:313–323. [PubMed] [Google Scholar]
- 11.Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- 12.Brennan K, Tateson R, Lewis K, Martinez Arias A. A functional analysis of Notch mutations in Drosophila. Genetics. 1997;147:177–188. doi: 10.1093/genetics/147.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 14.Bouiliane GL, De la Concha JA, Campos Ortega JA, Jan LY, Jan YN. The Drosophila gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. The EMBO J. 1991;10:2975–2984. doi: 10.1002/j.1460-2075.1991.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan K, Tateson R, Lieber T, Couso JP, Zecchini V, Martinez Arias A. The Abruptex mutations of Notch disrupt the establishment of proneural clusters in Drosophila. Dev. Biol. 1999;216:230–242. doi: 10.1006/dbio.1999.9501. [DOI] [PubMed] [Google Scholar]
- 16.Heitzler P, Simpson P. Altered epidermal growth factor-like sequences provide evidence for a role of Notch as a receptor in cell fate decisions. Development. 1993;117:1113–1123. doi: 10.1242/dev.117.3.1113. [DOI] [PubMed] [Google Scholar]
- 17.Xu T, Artavanis Tsakonas S. Dx, a locus interacting with the neurogenic genes, Notch, Delta and mastermind in Drosophila melanogaster. Genetics. 1990;126:665–677. doi: 10.1093/genetics/126.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis Tsakonas S. Deltex acts as a positive regulator of notch signaling through interactions with the notch ankyrin repeats. Development. 1995;121:2633–2644. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- 19.Diederich RJ, Matsuno K, Hing H, Artavanis Tsakonas S. Cytosolic interactions between Deltex and Notch ankyrin repeats implicates Deltex in the Notch signalling pathway. Development. 1994;120:473–481. doi: 10.1242/dev.120.3.473. [DOI] [PubMed] [Google Scholar]
- 20.Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal-transduction by activated mNotch: Importance of proteolytic processing and its regulation by the extracellular domain. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeter E, Kisslinger J, Kopan R. Notch-1 signalling requires ligand induced proteolytic release of the intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 22.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 23.Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P. Drosophila Shaggy kinase and rat glycogen-synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993;362:557–560. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- 24.Couso JP, Martinez Arias A. Notch is required for wingless signaling in the epidermis of Drosophila. Cell. 1994;79:259–272. doi: 10.1016/0092-8674(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 25.Axelrod JD, Matsuno K, Artavanis Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by Dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- 26.Brennan K, Baylies M, Martinez Arias A. Repression by Notch is required before Wingless signalling during muscle progenitor cell development in Drosophila. Curr. Biol. 1999;9:707–710. doi: 10.1016/s0960-9822(99)80313-3. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence N, Langdon T, Brennan K, Martinez Arias A. Notch signaling targets the Wingless responsiveness of a Ubx visceral mesoderm enhancer in Drosophila. Curr. Biol. 2001;11:375–385. doi: 10.1016/s0960-9822(01)00120-8. [DOI] [PubMed] [Google Scholar]
- 28.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signalling inputs from Wingless and Decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 29.Baylies MK, Martinez Arias A, Bate M. wingless is required for the formatiom of a subset of muscle founder cells during Drosophila embryogenesis. Development. 1995;121:3829–3837. doi: 10.1242/dev.121.11.3829. [DOI] [PubMed] [Google Scholar]
- 30.Wesley CS. Notch and Wingless regulate expression of cuticle patterning genes. Mol. Cell. Biol. 1999 doi: 10.1128/mcb.19.8.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan K, Klein T, Wilder E, Martinez Arias A. Wingless modulates the effects of dominant negative Notch molecules in the developing wing of Drosophila. Dev. Biol. 1999;216:210–229. doi: 10.1006/dbio.1999.9502. [DOI] [PubMed] [Google Scholar]
- 32.Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- 33.Shawber C, Nofziger D, Hsieh JJ-D, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signalling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–3773. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- 34.Nofziger D, Miyamoto A, Lyons KM, Weinmaster G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development. 1999;126:1689–1702. doi: 10.1242/dev.126.8.1689. [DOI] [PubMed] [Google Scholar]
- 35.Kishi N, Tang Z, Maeda Y, Hirai A, Mo R, Ito M, Suzuki S, Nakao K, Kinoshita T, Kadesch T, et al. Murine homologs of deltex define a novel gene family involved in vertebrate Notch signaling and neurogenesis. Int. J. Devl. Neuroscience. 2001;19:21–35. doi: 10.1016/s0736-5748(00)00071-x. [DOI] [PubMed] [Google Scholar]
- 36.Ordentlich P, Lin A, Shen CP, Blaumueller C, Matsuno K, Artavanis-Tsakonas S, Kadesch T. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol. Cell. Biol. 1998;18:2230–2239. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto N, Yamamoto S-i, Inagaki F, Kawaichi M, Fukamizu A, Kishi N, Matsuno K, Nakamura K, Weinmaster G, Okano H, et al. Role of Deltex-1 as a Transcription Regulator Downstream of the Notch Receptor. J. Biol. Chem. 2001;276:45031–45040. doi: 10.1074/jbc.M105245200. [DOI] [PubMed] [Google Scholar]