Abstract
Previously, we identified 14-3-3 β and ζ isoforms and proteolytic fragments of α-spectrin as proteins released from degenerating neurons that also rise markedly in cerebrospinal fluid (CSF) following experimental brain injury or ischemia in rodents, but these proteins have not been studied before as potential biomarkers for ischemic central nervous system injury in humans. Here we describe longitudinal analysis of these proteins along with the neuron-enriched hypophosphorylated neurofilament H (pNFH) and the deubiquitinating enzyme UCH-L1 in lumbar CSF samples from 19 surgical cases of aortic aneurysm repair, 7 involving cardiopulmonary bypass with deep hypothermic circulatory arrest (DHCA). CSF levels of the proteins were near the lower limit of detection by Western blot or enzyme-linked fluorescence immunoassay at the onset of surgical procedures, but increased substantially in a subset of cases, typically within 12–24 hours. All cases involving DHCA were characterized by >3-fold elevations in CSF levels of the two 14-3-3 isoforms, UCH-L1, and pNFH. Six of 7 also exhibited marked increases in α-spectrin fragments generated by calpain, a protease known to trigger necrotic neurodegeneration. Among cases involving aortic cross-clamping but not DHCA, the proteins rose in CSF preferentially in the subset experiencing acute neurological complications. Our results suggest the neuron-enriched 14-3-3β, 14-3-3ζ, pNFH, UCH-L1, and calpain-cleaved α-spectrin may serve as a panel of biomarkers with clinical potential for the detection and management of ischemic central nervous system injury, including for mild damage associated with surgically-induced circulation arrest.
Keywords: ischemia, acute CNS damage, surrogate marker, calpain, circulation arrest
1. Introduction
Acute brain injuries resulting from cardiac arrest, stroke, or head trauma sometimes result in lasting neurologic and cognitive problems even when they are of mild severity. Currently, mild acute brain injuries are difficult to diagnose, and there are neither prognostic methods for identifying patients at risk for developing sustained abnormalities, nor proven therapeutic strategies for improving long-term functional outcome. To help circumvent these problems, considerable effort is being devoted to the establishment and validation of biochemical surrogate markers for acute brain damage. Conceptually, proteins that are expressed primarily in the brain could appear outside the central nervous system preferentially under neurodegenerative conditions accompanied by disruption of the barriers separating the brain interstitial fluid from cerebrospinal fluid (CSF) and blood. Supporting this concept, there are numerous reports that cardiac arrest, stroke, and traumatic brain injury lead to at least transient breakdown of the blood-brain barrier (del Zoppo and Hallenbeck, 2000; Ballabh et al., 2004), and certain proteins enriched in the nervous system are detectable in human CSF and serum following acute brain injuries. Among the most widely studied candidate markers for brain damage are the astroglial protein S100β and the neuronal proteins neuron-specific enolase and a proteolytic fragment of tau of indeterminate origin. In many cases, CSF and serum alterations in these brain-enriched proteins are related to prognosis (Fassbender et al., 1997; McKeating et al., 1998; Wunderlich et al., 1999; Mussack et al., 2002; Singhal et al., 2002; Zemlan et al., 2002), and could potentially facilitate assessment of experimental neuroprotectant treatment strategies (Tanaka et al., 2007). Unfortunately, owing to one or more limitations in sensitivity and specificity, none of these has emerged as a widely used diagnostic or prognostic clinical tool or a validated surrogate endpoint measure for irreversible brain damage. For example, although serum levels of S100β change in relation to short-term mortality and morbidity, as well as long-term neurologic outcome, the protein also markedly increases in serum during surgical procedures unrelated to acute brain injuries (Anderson et al., 2001; Routsi et al., 2006) as well as marathon runners (Hasselblatt et al., 2004), from which it is derived from adipose and other extracranial sources (Kleine et al., 2003). Furthermore, acute alterations in serum S100β are not consistently predictive of brain dysfunction resulting from mild brain injury (reviewed by Begaz et al., 2006). In an analysis of 4 candidate biomarkers in over 300 stroke patients, an efficacious tissue plasminogen activator treatment did not alter any of the stroke-induced serum marker increases (Jauch et al., 2006), suggesting none of these markers could be used as pharmacodynamic measures of brain damage. Consequently, the need remains for new, highly sensitive biomarkers which may be combined with neurologic and neuroradiologic methods to improve the diagnosis, prognosis and experimental therapeutic evaluation of acute brain injuries.
Toward this objective, we devised an alternative neurobiological approach for identifying novel candidate biomarkers for acute brain damage. Using proteomics methods and an antibody library, we identified the most abundant proteins released by degenerating cultured rat cortical neurons (Siman et al., 2004). The validity of this approach was established by immunodetection of released proteins in CSF following experimental traumatic brain injury or transient global forebrain ischemia (Siman et al., 2004; 2005). Several of these are expressed predominantly or exclusively in neurons, including the β and ζ isoforms of 14-3-3 (Aitken et al., 2003), a hypophosphorylated form of the neurofilament triplet polypeptide NFH (pNFH; Lasek et al., 1985; Jung et al., 2000), and the deubiquitinating enzyme UCH-L1 (Doran et al., 1983). Others are proteolytic fragments of abundant neuronal proteins, including an α-spectrin breakdown product generated by calpain, a family of calcium-dependent proteases associated with neuronal necrosis (Siman et al., 1989; Roberts-Lewis et al., 1993; Zhang et al., 2002). None of these neuron-enriched proteins has been evaluated before as candidate biomarker for the brain and spinal cord damage that can result from surgically-induced deep hypothermic circulation arrest (DHCA; Roche et al., 1996; Watanabe et al., 2004; Selnes and McKhann, 2005). Here we describe the study of changes in these candidate biomarkers in serial CSF samples derived from cases of aortic aneurysm surgical repair, including a subset subjected to cardiopulmonary bypass and DHCA. We addressed whether CSF elevations in the panel of neuron-enriched proteins may be indicators of circulation arrest-induced injury to the central nervous system (CNS), and evaluated the relation between biomarker changes and CNS injury for the subset of cases not subjected to cardiopulmonary bypass and DHCA, but experiencing acute neurological complications.
2. Results
Thoracoabdominal surgery provides an opportunity to evaluate candidate biomarkers for ischemic central nervous system injury in a longitudinal fashion and with pre-insult baseline measures. The diversity of surgical repairs allows patients undergoing deep hypothermic arrest on cardiopulmonary bypass to be compared with patients who undergo the procedure with partial left heart (LA/FA) bypass or transthoracic endovascular repair (TEVAR). In addition, surgical repair of the descending aorta has a high risk of both transient and permanent neurological complications, thereby permitting investigation of the relationship between candidate biomarker alterations and neurologically-confirmed CNS injury.
Toward these ends, we sampled lumbar CSF from nineteen patients undergoing aortic aneurysm surgical repair with aortic artery cross-clamping, 7 of whom underwent their repairs with cardiopulmonary bypass and DHCA, 10 utilizing LA/FA bypass, and 1 undergoing TEVAR. Clinical data on this patient population are summarized in Table 1. Ten males and 9 females ranging from 31 to 99 years of age were evaluated, 14 of whom were Caucasian, 4 African American, and 1 Hispanic. A baseline CSF sample was taken prior to surgical incision. For the 7 cases involving DHCA with circulation arrest, the durations of DHCA ranged from 7 to 64 minutes and cardiopulmonary bypass times ranged from 159–260 minutes. In the 10 patients undergoing LA/FA bypass, the bypass times ranged from 54–164 minutes and aortic cross-clamp times ranged from 43–136 minutes. Of the 19 patients studied here, 13 were discharged with normal scores on the NIH stroke and ASIA scales. One patient (case 8) suffered a delayed episode of paraparesis on post-operative day 4 that resolved to baseline with treatment. Of the 6 patients who suffered persistent neurologic injuries, two (cases 1 and 14) suffered severe irreversible injuries detected intraoperatively by SSEPS and on arousal fromanesthesia. Both of these patients expired. One additional patient (case 45) suffered moderate intraoperative paraparesis, detected by SSEPS, and a moderate persistent paraparesis was still detected on arousal from anesthesia. Three additional patients suffered episodes of persistent delayed post-operative paraparesis, and expired during their hospitalization: one was mild and occurred on post-operative day 2 (case 49), while the other two experienced moderate paraparesis starting either on day 4 (case 38) or day 11 (case 39).
Table 1. Clinical data on thoracoabdominal surgical cases.
Clinical data summary of aortic surgical cases. Cases involving deep hypothermic circulatory arrest are denoted by the indicated durations of circulation arrest. All other cases, except for #31, were treated with partial left heart (left atrium/femoral artery) bypass. Case 31 received a transthoracic endovascular repair.
| # | Age | Gender | Stroke | Prior Aortic | Cross-Clamp (min) | Bypass (min) | Circ Arrest (min) |
|---|---|---|---|---|---|---|---|
| 1 | 74 | M | No | Yes | 87 | 224 | 64 |
| 3 | 80 | F | No | No | 47 | 109 | NA |
| 5 | 31 | F | No | Yes | 136 | 233 | 7 |
| 6 | 68 | F | No | No | 85 | 91 | NA |
| 8 | 75 | F | No | Yes | 69 | 115 | NA |
| 9 | 68 | F | No | No | 96 | 105 | NA |
| 11 | 43 | M | No | No | 43 | 54 | NA |
| 12 | 71 | F | No | No | 44 | 55 | NA |
| 13 | 68 | F | No | Yes | 61 | 159 | 36 |
| 14 | 99 | M | No | No | 68 | 111 | NA |
| 15 | 64 | M | No | No | 71 | 182 | 38 |
| 16 | 69 | M | No | Yes | 63 | 175 | 36 |
| 18 | 54 | M | Yes | Yes | 66 | 176 | 39 |
| 31 | 77 | M | No | Yes | NA | NA | NA |
| 37 | 65 | M | No | No | 56 | 75 | NA |
| 38 | 75 | M | No | No | 64 | 260 | 24 |
| 39 | 70 | F | Yes | No | 59 | 111 | NA |
| 45 | 79 | M | No | Yes | 104 | 164 | NA |
| 49 | 76 | F | No | No | 89 | 109 | NA |
NA – not applicable
Multiple neuron-enriched proteins are elevated in CSF following surgically induced circulation arrest
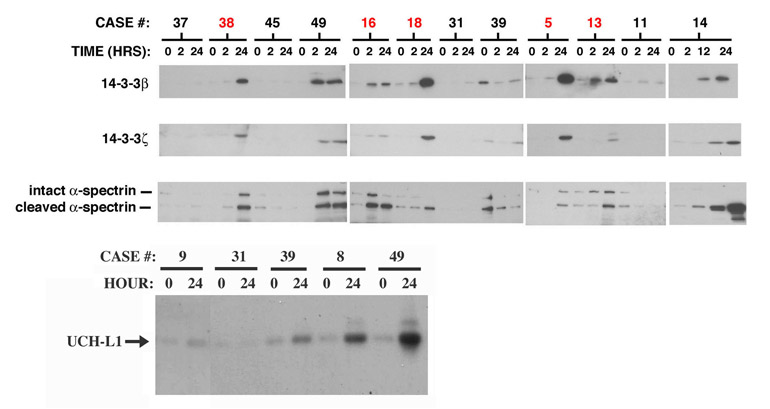
In searching for novel markers for acute brain injuries, we have identified the β and ζ isoforms of 14-3-3 as abundant neuron-enriched proteins that are released from degenerating cultured neocortical neurons, and rise markedly in CSF of rats following transient global forebrain ischemia or traumatic brain injury (Siman et al., 2004; 2005). Additional candidate biomarkers for brain injury identified by this neurobiological approach include proteolytic fragments of tau and α-spectrin derived by the calcium-dependent protease calpain, and also the neuron-enriched ubiquitin hydrolase UCH-L1. These proteins have not been evaluated as possible markers for acute ischemic CNS injury in humans. We analyzed these candidate biomarkers by quantitative Western blotting in the CSF samples described above. Results for 14-3-3β, 14-3-3ζ, calpain-cleaved , α-spectrin and UCH-L1 are illustrated in Figure 1. For cases involving circulation arrest, CSF levels of 14-3-3β (~29 kDa) increased by more than 3-fold by 24 hours, compared with baseline levels (Figure 1 top). In contrast, only a subset of the cases not including circulation arrest exhibited elevations in CSF 14-3-3β. There was no direct relationship between the absolute CSF level of 14-3-3β and the duration of either the aortic cross clamping, cardiopulmonsary bypass, or circulation arrest.
Figure 1.
Time-dependent increases in CSF 14-3-3β, 14-3-3ζ, cleaved α-spectrin, and UCH-L1 following aortic aneurysm surgical repair. Western blot analysis of unfractionated lumbar CSF samples taken at the indicated times following aortic cross-clamping. (Top) The cases shown in red included cardiopulmonary bypass with DHCA. The levels of 14-3-3β, ζ and cleaved α-spectrin were near or below the lower limit of detection prior to the onset of the surgical procedures. Note that for a subset of surgical cases, there are coordinate increases in the levels of the three biomarkers between 2–24 hours after the beginning of surgery. (Bottom) Western analysis of CSF levels of the neuron-enriched deubiquinating enzyme UCH-L1. Shown are 5 representative cases, two of which exhibit a time-dependent increase in the ~25 kDa UCH-L1 polypeptide of >3-fold.
In addition to 14-3-3β, 14-3-3ζ (~30 kDa) and a proteolytic cleavage product of α-spectrin (~150 kDa) were near or below the limit of detection of immunoblotting for 17 of the 19 cases prior to surgery. For the subset of cases exhibiting a marked rise in CSF 14-3-3β, there were large and parallel increases in intact14-3-3ζ and a proteolytic fragment of α-spectrin (Figure 1 top). As shown in the bottom panel of Figure 1, levels of the ~25 kDa UCH-L1 also increased in CSF a subset of cases. In some instances, elevations in cleaved α-spectrin were accompanied by increases in the intact ~250 kDa α-spectrin polypeptide. Despite the coincident increases in CSF levels of the four proteins by 24 hours after cardiopulmonary bypass with DHCA, the absolute magnitude of the marker changes were not always related. Cases 16 and 18 involving DHCA are illustrative. Whereas levels of all four neuron-enriched proteins were elevated 24 hours after circulation arrest, the β and ζ 14-3-3 isoforms were higher in case 18, while intact and cleaved α-spectrin were more abundant in case 16 and UCH-L1 was comparable between the two. Nevertheless, for the 9 cases that exhibited >3-fold elevations in CSF 14-3-3β, the CSF levels of 14-3-3ζ, UCH-L1 and cleaved α-spectrin also consistently increased well above baseline levels. Calpain-cleaved tau, on the other hand, was below the limit of detection by Western blotting for all cases, and will not be considered further herein.
A hypophosphorylated form of the high molecular weight neurofilament subunit, pNFH, is another neuron-enriched protein that reportedly increases in CSF and serum in association with subarachnoid hemorrhage and acute spinal trauma (Petzold et al., 2003, 2005; Petzold, 2005; Shaw et al., 2005). We have found that pNFH levels rise in the CSF and serum following experimental brain trauma in the rat (Siman et al., manuscript in preparation). Consequently, we evaluated CSF levels of pNFH in the same set of aortic surgical cases, some of which exhibit alterations in the two 14-3-3 isoforms, UCH-L1 and cleaved α-spectrin. A highly-sensitive fluorescence-based sandwich immunoassay quantified pNFH, and was standardized using varying amounts of mouse spinal cord extract rich in pNFH. As summarized in Figure 2, a subset of the surgical cases showed marked CSF elevations in pNFH when compared with their baseline values. Of those cases involving cardiopulmonary bypass with DHCA, pNFH levels invariably increased post-surgery by at least 3-fold. Among the remaining cases, a subset demonstrated elevations in pNFH; strikingly, these were the same patients exhibiting marked increases in the two 14-3-3 isoforms, UCH-L1 and the α-spectrin fragment. On the other hand, cases that did not show measurable alterations in CSF levels of the 4 neuron-enriched proteins also did not exhibit time-dependent changes in pNFH.
Figure 2.
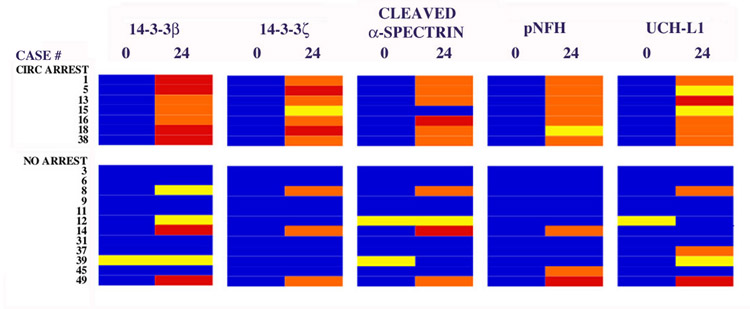
Coordinate increases in multiple CSF markers occur preferentially in cases cardiopulmonary bypass with DHCA. CSF levels of 14-3-3β, 14-3-3ζ, cleaved α-spectrin, a hypophosphorylated form of neurofilament H (pNFH), and UCH-L1 were quantified prior to and 24 hoursafter reestablishment of cardiopulmonary bypass following DHCA, and divided into 4 quadrants as described in Materials and Methods: low, moderate, high and very high levels. The surgical cases are divided into groups that either were subjected to or lacked DHCA. Note that all cases of DHCA are accompanied by time-dependent CSF increases in at least 4 of the proteins. In addition, 3 of 12 cases not involving cardiopulmonary bypass with DHCA also show time-dependent CSF increases in at least 4 of the proteins. Strikingly, all 3 of these cases suffered acute neurological complications indicative of CNS injury.
The collective changes in the five biomarkers for the 19 cases under study herein are summarized in Figure 2. These data demonstrate a remarkable divergence in CSF marker changes as a function of the presence or absence of circulation arrest. Thus, all 7 cases of cardiopulmonary bypass with DHCA are accompanied within 24 hours by consistent and coordinate rises in the CSF levels of at least four different neuron-enriched protein biomarkers for acute ischemic CNS injury. In sharp contrast, only 3 of the 12 cases not involving cardiopulmonary bypass with DHCA also demonstrate coordinate increases in multiple protein markers for neurodegeneration, a finding that will be detailed further below.
Time course for neuronal protein changes in CSF after reperfusion
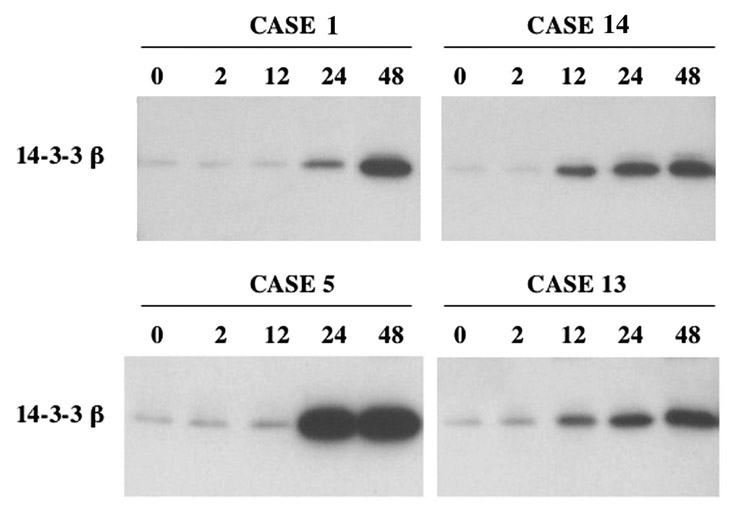
A temporal analysis was performed of CSF 14-3-3β changes following thoracoabdominal surgery with aortic cross-clamping (Figure 3). Cases 1,5, and 13 are representative of the 7 cases of cardiopulmonary bypass with DHCA, while case 14 is an example of increased CSF marker levels even in the absence of surgically-induced DHCA. All of these cases showed >3-fold increases in 14-3-3β above baseline levels at 24 and 48 hours after the reinstitution of cardiopulmonary bypass following DHCA or aortic crossclamp depending of the surgical subtype. For cases 1 and 5, significant increases were not apparent until between 12–24 hours, whereas 14-3-3β levels were elevated initially between 2–12 hours for cases 13 and 14. In all cases, marker levels were elevated >5-fold above baseline for at least 48 hours.
Figure 3.
Time course for surgically-induced increase in CSF 14-3-3β. Western blot analysis of 14-3-3β is shown for 4 representative cases. In all cases, 14-3-3β was elevated markedly within 24 hours, but not at 2 hours. In two instances, the major rise occurred between 2–12 hours, whereas in the other cases 14-3-3β increased predominantly between 12–24 hours.
The time-dependent alterations in CSF pNFH and UCH-L1 were essentially identical. Compared with measures taken at baseline and 1 hour after recirculation or crossclamp, pNFH and UCH-L1 increased uniformly beginning at 12 hours and remained elevated for at least the next 36 hours (data not shown).
Surgically-induced circulation arrest leads to activation of calpain, a protease associated with necrotic neurodegeneration
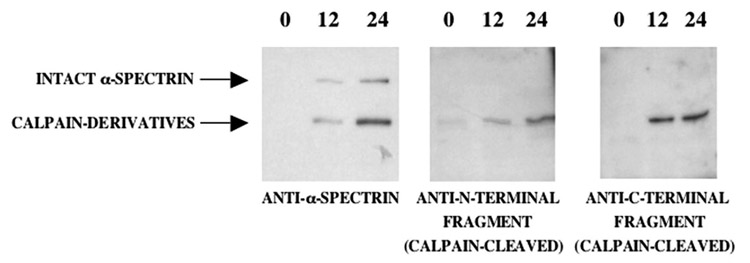
The ~150 kDa proteolytic fragment of α-spectrin detected in CSF following surgically-induced DHCA using an antibody directed toward the α-spectrin holoprotein (Figure 1) is similar in size to a derivative generated by the calpain family of calcium-dependent proteases. This calpain derivative of α-spectrin is markedly increased in the brain in multiple experimental models of necrotic neurodegeneration, including following transient global forebrain ischemia (Siman et al., 1988; 1989; 1994; Saatman et al., 1996; Zhang et al., 2002). To investigate directly whether calpain is activated by DHCA in human patients, we examined whether calpain derivatives of α-spectrin increase in human CSF using Ab38 and Ab41, cleavage site-specific antibodies that react specifically with the ~ 150 and ~145kDa NH2- and COOH-terminal fragments of α-spectrin degenerated by calpain cleavage, respectively (Roberts-Lewis et al., 1994; Siman et al., 2001). As exemplified by case 5, CSF levels of the NH2- and COOH-terminal calpain derivatives of α-spectrin increase at 12–24 hours after circulation arrest and reperfusion (Figure 4). This provides confirmatory immunochemical evidence that DHCA in humans triggers CNS calpain activation.
Figure 4.
Evidence for activation of calpain, a protease associated with necrotic neurodegeneration, following repairs employing cardiopulmonary bypass with DHCA. The intact α-spectrin and its proteolytic fragments detectable in CSF following DHCA were characterized for case 5 by Western blot analysis using either an antibody reactive with all forms of α-spectrin (>250 kDa), or cleavage site-specific antibodies reactive with the NH2-or COOH-terminal fragments (~150 kDa each) generated by calpain proteolysis. Note that there is a large time-dependent increase in CSF levels of the two α-spectrin fragments derived from calpain cleavage.
Marker elevations in CSF are associated with surgical and post-surgical neurological complications
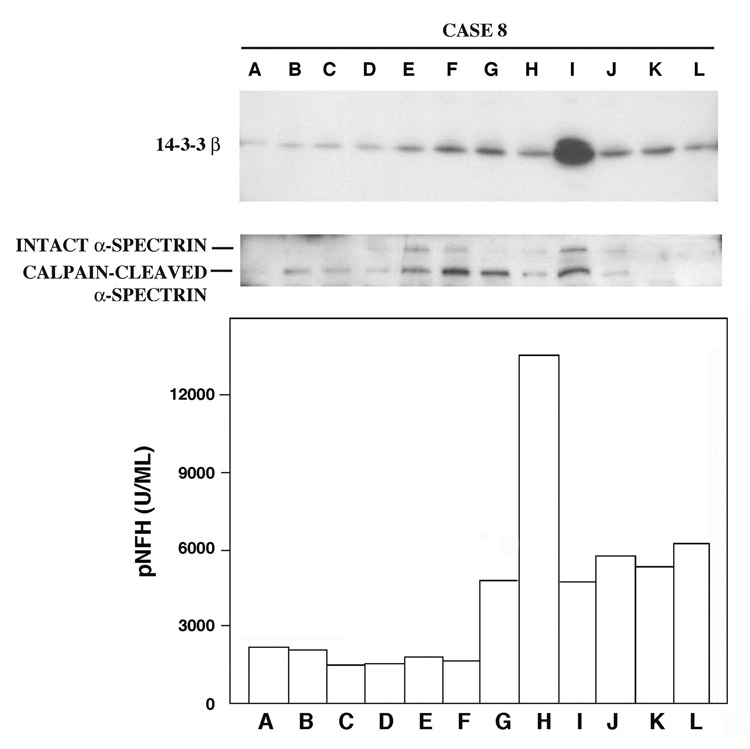
Among the 12 cases of aortic surgical repair that did not involve cardiopulmonary bypass with DHCA, only 3 showed consistent CSF elevations of at least 4 of the marker proteins measurable at 24 hours. Strikingly, marker elevations corresponded to the incidence of neurological complications indicative of acute CNS injury. For example, case 8 suffered a transient spinal ischemia during the hospitalized recovery on post-operative day 4. This event enabled the kinetic analysis of CSF levels of 14-3-3 isoforms, calpain-cleaved α-spectrin, and pNFH prior to and shortly after ischemia onset, as well as the duration of increased biomarker levels upon cessation of the ischemic episode. As shown in Figure 5, levels of the neurodegeneration markers rose markedly in association with the onset of neurologic symptoms, which began at 96 hours (time H). Thus, CSF levels of 14-3-3β, calpain-cleaved α-spectrin (Figure 5), and 14-3-3ζ (data not shown) rose modestly at 24 hours, prior to the onset of overt neurological symptoms, and increased still further in association with the onset of paraparesis. The CSF pNFH content also increased, but only in temporal association with the spinal ischemic event. All four biomarkers remained elevated above pre-ischemic baselines but declined sharply within hours of reaching peak values, coincident with the recovery of neurologic function.
Figure 5.
Rapid elevations in 14-3-3β and pNFH in CSF following transient spinal cord ischemia. Western blot analyses of 14-3-3β and calpain-cleaved α-spectrin are illustrated, along with ELIFA quantitation of pNFH. Case 8 suffered a transient spinal cord ischemic event during post-surgical hospital recovery, with symptoms first noted at 96 hours after the aortic artery cross-clamping and having recovered to baseline at 102 hours. The neurological complication was accompanied by rapid and transient increases in the 3 neurodegeneration markers. Time points A–L represent 0, 1, 2, 12, 24, 36, 48, 96, 102, 110, 117 and 135 hours.
There were six additional cases that developed neurological complications within 4 days of the surgical procedure, and in each case at least one of the biomarkers under study herein rose in CSF prior to or coincident with the neurological signs. Intraoperative strokes and paraplegia occurred in cases 1 (DHCA) and 14 (no DHCA), and both were accompanied by large and coordinate CSF elevations in all 5 protein markers. Cases 38 (DHCA) and 39 (no DHCA) developed delayed paraplegia, with the former manifested 4 days post-surgery and the latter not developing until day 11. Only the former case exhibited large and consistent biomarker increases measurable at 24 hours. Case 45 suffered moderate intraoperative paraparesis that persisted upon arousal from the anesthesia. Among the five biomarkers, only pNFH levels were elevated in CSF in this patient. Finally, case 49 (no DHCA) developed delayed paraparesis at 2 days, preceded at 24 hours by large and coordinate increases in all 5 protein biomarkers in CSF.
3. Discussion
We report here that a panel of neuron-enriched proteins is markedly elevated in cerebrospinal fluid following aortic aneurysm surgical repair involving cardiopulmonary bypass and deep hypothermic circulation arrest. The CSF levels of 14-3-3β, 14-3-3ζ, a hypophosphorylated form of the high molecular weight neurofilament subunit (pNFH), the deubiquitinating enzyme UCH-L1, and a calpain-derived proteolytic fragment of the microfilament-associated protein α-spectrin all show large increases in a delayed and generally coordinated fashion. Thus, all 7 cases involving cardiopulmonary bypass with DHCA show CSF increases in at least 4 of these neuronal proteins of >3-fold beginning between 2–12 hours after the onset of aortic cross-clamping, peaking in most cases at 24–48 hours. In light of considerable evidence that DHCA during aortic surgeries leads in some cases to neurologic dysfunction and lasting functional deficits (Roche et al., 1996; Ergin et al., 1999; Arrowsmith et al., 2000; Selnes and McKhann, 2005), these post-surgical CSF protein alterations might be surrogate indices for acute ischemic damage to the brain or spinal cord. Consistent with this concept, surgical cases involving LA/FA with shorter bypass durations and no cardiopulmonary bypass, but exhibiting overt neurological complications, also are associated with CSF elevations in the 5 proteins either shortly preceding or at the onset of neurologic symptoms. On the other hand, patients undergoing thoracoabdominal aortic aneurysm surgical repairs involving LA/FA circulation management did not have significant elevations in these protein markers for neurodegeneration when neurologic injury was not detected. Collectively, these findings support the possibility that 14-3-3β, 14-3-3ζ, UCH-L1, calpain-cleaved α-spectrin, and pNFH might serve as a panel of novel biomarkers with potential clinical utilities in the detection and management of acute ischemic brain and spinal cord injuries.
Conceivably, the leakage of neuronal proteins into CSF following surgically-induced transient episodes of CNS ischemia may result from reversible permeation of neurons during conditions of stress, rather than as a response to irreversible neuronal degeneration. This possibility is unlikely, however, based on prior studies in experimental animals and findings reported herein. The discovery of 14-3-3β and 14-3-3ζ as novel biomarker candidates for brain injury was based on a neurobiological approach for identifying the most abundant neuron-enriched proteins released during neurodegeneration (Siman et al., 2004). In cultured neurons, 14-3-3 isoform release occurs only in association with neuronal death, and is reduced by a neuroprotective intervention (Siman et al., 2004; Lawrence et al., 2005). In a rat model of transient global forebrain ischemia designed to mimic cardiac arrest-induced brain damage, CSF levels of the two 14-3-3 isoforms and calpain-cleaved α-spectrin increase only in association with irreversible brain injury (Siman et al., 2004; 2005). Indeed, the CSF level of 14-3-3β correlates with the magnitude of acute neurodegeneration as monitored by quantitative histopathology, and is an even better predictor of brain damage than the duration of the ischemia. Other forms of acute CNS damage such as traumatic brain injury also lead to marked increases in CSF levels of the two 14-3-3 isoforms, pNFH and calpain-derived fragments of α-spectrin and tau (Siman et al., 2004; Pike et al., 2004; Shaw et al., 2005). Calpain is a family of calcium-activated cysteine proteases that has been causally linked to the acute necrotic neurodegeneration that is triggered by intraneuronal calcium overload and occurs in response to ischemia, trauma, and excitotoxicity (Siman et al., 1989; Roberts-Lewis and Siman, 1993; Saatman et al., 1996; Zhang et al., 2003). Consequently, the rise in CSF levels of a calpain cleavage product of α-spectrin reported here (Figure 4), coupled with the coordinate increase in several neuron-enriched proteins, provides strong evidence that the circulation arrest-induced increases in these proteins result from irreversible acute CNS damage rather than reversible neuronal stress. It is also noteworthy that neuronal proteins both localized in cytoplasm (14-3-3 isoforms) and associated with the cytoskeleton (α-spectrin, pNFH) are markedly elevated, a finding consistent with pronounced neuronal structural disintegration. A close relationship between CSF increases in the protein panel and acute CNS injury is further supported by the striking finding that among the 12 surgical cases not involving DHCA, CSF marker elevations occurred preferentially in cases exhibiting acute neurological complications. Finally, the lag of 12–24 hours between the surgically-induced circulation arrest and the CSF elevations in the panel of proteins indicates that the marker changes are not simply associated with systemic surgical trauma, but instead develop in a protracted fashion over the known time course for the onset of delayed ischemic neuronal degeneration (Roberts-Lewis et al., 1994; Kirino, 2000).
On the basis of these findings, we hypothesize that a panel consisting of 14-3-3 β and ζ isoforms, pNFH, UCH-L1, and calpain-cleaved α-spectrin can serve as biomarkers for subtle injury to the CNS occurring following aortic aneurysm surgical repair with circulatory arrest. There is evidence for both transient acute neurologic dysfunction and long-term functional deficit following aortic surgery (Ergin et al., 1999; Shiiya et al., 2004). Coronary artery bypass graft surgeries with cardiopulmonary bypass also carry a major risk of long-term neurologic dysfunction (Roche et al., 1996; Arrowsmith et al., 2000; Selnes and McKhann, 2005). However, the magnitude and extent of lasting neurological abnormalities resulting from surgically-induced deep hypothermic circulatory arrest are uncertain, and there is currently no facile method for identifying the subset of patients at risk for CNS injury. Although most patients in the current study were released from hospital with normal scores on ASIA and the NIH stroke scale, additional and sensitive measures of cognitive and motor status were not evaluated in the current study. Additional research will be required to investigate relationships between the surgically-induced acute CSF alterations in this panel of protein biomarkers for CNS injury, the risk of developing lasting behavioral abnormalities, and the severity of the neurological dysfunction.
Within each case, the absolute magnitude of the CSF marker increases sometimes varies, and it is worth considering that these differences might be related to the severity or localization of the injuries. CSF marker changes varied most dramatically for case 15, an example of deep hypothermia circulatory arrest that produced a massive increase in CSF pNFH, a moderate rise in 14-3-3β, modest increases in 14-3-3ζ and UCH-L1, and a less than 2-fold change in calpain-cleaved α-spectrin. There are several factors that could contribute to differential changes in members of the marker panel, including differences in their brain regional, cellular, and subcellular localizations, as well as possible different rates of turnover in the lumbar CSF. For example, phosphorylated NFH is localized primarily in axons (Jung et al., 2000), where it is part of the cytoskeleton (Sternberger et al., 1982; Lasek et al., 1985), whereas 14-3-3 βand ζ are cytoplasmic proteins concentrated in perikarya (Martin et al., 1994; Aitken et al., 2003). The five proteins are also differentially expressed across the neuraxis. Whereas all are abundant in hippocampus and neocortex, their levels differ across the basal ganglia, thalamus, cerebellum and brainstem. The expression levels and localization of 14-3-3 isoforms and UCH-L1 in the spinal cord are largely unknown. Conceivably, not only might the magnitude of alterations for a marker panel reflect the extent of brain injury, but differences among a panel of biomarkers may provide useful information on the regional localization of acute CNS injuries or the neuronal elements undergoing injury, concepts that will require further and directed research. Whereas the vast majority of studies thus far of protein biomarkers for acute CNS injuries have focused on the measure of only one or two markers at a time, the simultaneous analysis of a large panel of protein markers such as the one described in the current study has the potential to improve the sensitivity and specificity of early detection of CNS injury, and provide additional information with clinical utility for the prognosis, acute management and therapeutic treatment of these injuries.
4. Experimental Procedures
Surgical procedures
This study received IRB approval and written informed consent was obtained from all study patients. All patients admitted to our institution for thoracoabdominal aortic aneurysm repairs from January 2003 through April 2004 and had lumbar CSF drains placed as part of the standard care were eligible for the study. Patients with significant preoperative neurologic deficits or acute preoperative neurologic events were excluded. Demographic data were collected preoperatively. Formal NIHSS and lower extremity ASIA scales were performed by a certified neurologist at baseline and 12, 24, and 48 hours postoperatively, as well as at discharge. Cases with acute neurologic events were subjected to further NIHSS and ASIA exams along with a detailed neurologic consultation and examination along with appropriate neuroimaging to confirm the existence of spinal or brain ischemia and exclude peripheral nerve or muscle injury. If an episode of spinal ischemia was detected intraoperatively or postoperatively, our institution’s standard protocol was followed to attempt to reverse ongoing ischemia (Cheung et al., 2002, 2005; McGarvey etal., 2007).
Clinical variables included age, race, gender, medical history, presentation status (emergent versus scheduled), whether deep hypothermic circulatory arrest (DHCA) was induced intraoperatively, whether intercostal vessels were re-implanted, presence of dissection or contained rupture, the repair type (cardiopulmonary bypass with DHCA, left atrium/femoral artery bypass (LA/FA), transthoracic endovascular repair (TEVAR)), cross-clamp time, circulation arrest time, bypass time, maximal change in intraoperative mean arterial pressure, and extent of repair. All patients received a narcotic-based anesthetic with inhaled isoflurane in oxygen. For open thoracoabdominal aneurysm repairs (TAAA), circulation management consisted of either distal aortic partial left heart bypass with core cooling to 32°C (LA/FA bypass) or a deep hypothermic technique utilizing full cardiopulmonary bypass via the left chest with an open proximal anastomosis (DHCA), which was used if a concomitant distal arch aneurysm required repair. For LA/FA management bypass flow rates averaged 2.5 L per minute, adjusted to achieve a target distal aortic perfusion pressure of at least 60 mm Hg, while maintaining proximal aortic pressure of at least 90 mm Hg. Repairs involving the distal arch repair required utilization of DHCA, systemic cooling on CPB until the EEG reached electrical cerebral silence (12–18°C), then termination of CPB to perform the open proximal anastomosis with total body retrograde cerebral perfusion (300–500 cm per minute at a central venous pressure of 12–15 mm Hg) via an unsnared superior vena cava. After completion, arterial circulation was reinitiated via the proximal descending Dacron graft, the distal anastomosis was performed, and the patient was rewarmed. For combined distal arch and type II and III TAAA repairs, arterial circulation was reinitiated with both proximal and distal femoral artery perfusion. Lumbar CSF drains were used in both LA/FA bypass and hypothermic cases. Intercostal arteries were reimplanted in all patients when possible. One patient in this cohort underwent descending thoracic aortic repair involving TEVAR without CPB involving general anesthesia, femoral artery cannulation, angiography, and aneurysm exclusion using an aortic stent graft.
Cerebrospinal fluid sample collection
CSF was drained intraoperatively and for the first 24 hours following the procedure to maintain intracerebral pressure (ICP) between 8–12 mm Hg. If no complications were observed, the CSF drain was clamped at 24 hours and removed at 48 hours; with complication, the drain was unclamped and again drained to an ICP of 8–12 mm Hg to enhance spinal cord perfusion. CSF samples were collected at the following time points: (A) lumbar drain placement immediately following induction; (B) at the time of aortic cross-clamp in cases involving LA/FA bypass, oe time of restart of CPB following DHCA, or aortic stent deployment in TEVAR procedures; (C) 1 hour following B, (D) 2 hours following B, (E) 12 hours following B, and (F) 24 hours following B. Further samples were collected at various time points if signs of paraparesis developed postoperatively. Samples were immediately centrifuged, and the supernatants free of cell debris were stored frozen at −80°C.
Western blot analysis of biomarker levels
Equivalent volumes of the CSF samples were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the polypeptides transferred to PVDF membranes using well established methods (e.g., Siman et al., 2001, 2004; Zhang et al., 2005). Five different candidate biomarker polypeptides were analyzed by immunoblotting, using antibodies that have been published with extensively: 14-3-3β (antibody dilution 1/1000; Santa Cruz), 14-3-3ζ (1/1000; Santa Cruz), 14-3-3γ (1/1000; Santa Cruz), tau (MAb Tau-1 @ 1/1000; Chemicon), UCH-L1 (1/2000; Chemicon), and α-spectrin (MAb1622 @ 1/1000; Chemicon). In addition, fragments of α-spectrin derived by calpain proteolysis were examined using cleavage site-specific antibodies reactive with the NH2- and COOH-terminal α-spectrin derivatives generated specifically by calpain (Abs 38 and 41, respectively; Roberts-Lewis et al., 1994; Saatman et al., 1996). Labeled polypeptides were detected using horseradish peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence substrate (Renaissance Plus kit, Perkin-Elmer), followed by exposure of the blots to X-ray film (Bio-Max Light, Kodak). For polypeptide quantification, band densities were determined from digitized images using Image Quant software (GE Healthcare), under conditions where band density varied in linear proportion with the volume of sample load. In all cases, only a single polypeptide of the expected molecular weight for the target protein of interest was labeled. Repeated measures of the same set of samples indicated interassay variability was low.
Enzyme Fluorescence Immunoassay for phosphorylated neurofilament H
A hypophosphorylated form of NFH, designated pNFH, was quantified by sandwich immunoassay, using a highly sensitive fluorescence-based procedure modified from the method of Petzold et al. (2003). Briefly, a mouse monoclonal antibody to a hypophosphorylated form of NFH (SMI35 at 1/5,000; Sternberger Monoclonals) was bound to 96 well microtiter plates in 50 mM sodium carbonate buffer overnight at 4°C. Wells were treated for 45 minutes with 0.5% bovine serum albumin (BSA) in Tris-buffered saline with Tween-20 (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween-20; TTBS), washed four times with TTBS, and the CSF samples added at 25 µl/well along with 75 µl/well BSA/TTBS for 90 minutes at 22°C. Wells were washed four times with TTBS, then incubated with the detecting antibody reactive with all forms of NFH (rabbit anti-NFH at 1/4000; Sigma) in BSA/TTBS for 60 minutes at 22°C. After washing, wells were incubated with mouse monoclonal anti-rabbit IgG-alkaline phosphatase (at 1/15,000; Sigma) in BSA/TTBS 60 minutes at 22°C. After washing, the wells were treated with an alkaline phosphatase fluorogenic substrate at 37°C consisting of 4-methylumbelliferylphosphate (250 µM) in 0.1 M Tris-HCl (pH 9.5)/ 1 mM MgCl2. The fluorescent product was measured over time in a microplate fluorimeter (Fluorocount; Perkin-Elmer). Negative controls included samples in which either the capture antibody or the CSF samples were omitted. Baseline fluorescence was determined from wells treated with the alkaline phosphatase substrate solution alone.
The pNFH immunoassay was standardized using mouse spinal cord extract. Cords from 2 adult CD-1 mice were excised rapidly and homogenized in 20 mM Tris-HCl (pH 7.4) containing protease inhibitor cocktail (Sigma). Following centrifugation for 30 minutes at 40,000 × g, the neurofilament-containing supernatant was frozen in small batches at −80°C. Standard curves were made on each microtiter plate using dilutions of the extract ranging from 0.3 – 10 nl/well, over which the fluorescence increased in linear proportion with the pNFH content. Fluorescence signal from all CSF samples was normalized to the standards, and is represented as the difference in relative fluorescence units compared with negative control lacking any added standard. Quantitative Western blot analysis was also performed on a subset of CSF samples using the pNFH-specific monoclonal antibody, and the relative intensities of the labeled ~200 kDa polypeptide matched the results obtained by the fluorescence enzyme immunoassay.
Analysis of multiple markers
For semiquantitative comparative analysis, biomarker changes were divided into quadrants: low (less than 1.5-fold above baseline average), moderate (1.5–3 fold above baseline average), high (3–5 fold above baseline average), or very high (>5 fold above baseline average). For graphical representation, the quadrants were color-coded as follows: low- blue; moderate- yellow; high- orange; very high- red.
Acknowledgements
We thank Dr. Robert Neumar for helpful discussions during the course of this work, and Lindsay Dressler for excellent technical assistance. The research was supported by NIH grant NS048234 to R.S.
Abbreviations
- pNFH
hypophosphorylated neurofilament H
- UCH-L1
ubquitin C-terminal hydrolase L1
- DHCA
deep hypothermic circulation arrest
- LA/FA
partial left heart bypass
- TEVAR
transthoracic endovascular repair
- TAAA
open thoracoabdominal aneurysm repair
- CSF
cerebrospinal fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken A, Jones D, Soneji Y, Howell S. 14-3-3 proteins: biological function and domain structure. Biochem. Soc. Trans. 2003;23:605–611. doi: 10.1042/bst0230605. [DOI] [PubMed] [Google Scholar]
- Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis. 2005;20:213–219. doi: 10.1159/000087701. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1258. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Arrowsmith JE, Grocott HP, Reves JG, Newman MF. Central nervous system complications of cardiac surgery. Br J Anaesth. 2000;84:378–393. doi: 10.1093/oxfordjournals.bja.a013444. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Betz, Dietrich . "Blood-brain barrier dysfunction in cerebral ischemia". In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular Disease. Oxford, UK: Blackwell; 1998. pp. 358–370. [Google Scholar]
- Boston P, Jackson P. Purification and properties of a brain-specific protein, human 14-3-3 protein. Biochem Soc Trans. 1980;8:617–618. doi: 10.1042/bst0080617. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Weiss SJ, McGarvey ML, Stecker MM, Hogan MS, Escherich A, Bavaria JE. Interventions for reversing delayed-onset postoperative paraplegia after thoracic aortic reconstruction. Ann Thorac Surg. 2002;74:413–419. doi: 10.1016/s0003-4975(02)03714-1. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Pochettino A, McGarvey ML, Appoo JJ, Fairman RM, Carpenter JP, Moser WG, Woo EY, Bavaria JE. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg. 2005;80:1280–1288. doi: 10.1016/j.athoracsur.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Coletti G, Torracca L, La Canna G, Maisano F, Sebastiano P, Fucci C, Berra P, Alfieri O. Diagnosis and management of cerebral malperfusion phenomena during aortic dissection repair by transesophageal Doppler echocardiographic monitoring. J Card Surg. 1996;11:355–358. doi: 10.1111/j.1540-8191.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40:1542–1547. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- Ergin MA, Uysal S, Reich DL, Apaydin A, Lansman SL, McCullough JN, Griepp RB. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg. 1999;67:1887–1890. doi: 10.1016/s0003-4975(99)00432-4. [DOI] [PubMed] [Google Scholar]
- Gao L, Taha R, Gauvin D, Othmen LB, Wang Y, Blaise G. Postoperative cognitive dysfunction after cardiac surgery. Chest. 2005;128:3664–3670. doi: 10.1378/chest.128.5.3664. [DOI] [PubMed] [Google Scholar]
- Green AJ, Thompson EJ, Stewart GE, Zeidler M, McKenzie JM, MacLeod MA, Ironside JW, Will RG, Knight RS. Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001;70:744–748. doi: 10.1136/jnnp.70.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DK, Walker AS, Kaukuntla H, Bracewell RM, Clutton-Brock TH, Faroqui M, Pagano D, Bonser RS. Selective antegrade cerebral perfusion attenuates brain metabolic deficit in aortic arch surgery: a prospective randomized trial. Circulation. 2004;110 Suppl 1:231–236. doi: 10.1161/01.CIR.0000138945.78346.9c. [DOI] [PubMed] [Google Scholar]
- Hasselblatt M, Mooren FC, von Ahsen N, Keyvani K, Fromme A, Schwarze-Eicker K, Senner V, Paulus W. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology. 2004;62:1634–1636. doi: 10.1212/01.wnl.0000123092.97047.b1. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR NINDS rt-PA Stroke Study Group. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- Jung C, Yabe JT, Lee S, Shea TB. Hypophosphorylated neurofilament subunits undergo axonal transport more rapidly than more extensively phosphorylated subunits in situ. Cell Motil Cytoskeleton. 2000;47:120–129. doi: 10.1002/1097-0169(200010)47:2<120::AID-CM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death. Neuropathology. 2000;20 Suppl:S95–S97. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Kleine TO, Benes L, Zofel P. Studies of the brain specificity of S100B and neuron-specific enolase (NSE) in blood serum of acute care patients. Brain Res Bull. 2003;61:265–279. doi: 10.1016/s0361-9230(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Phillips L, Katz MJ, Autilio-Gambetti L. Function and evolution of neurofilament proteins. Ann N Y Acad Sci. 1985;455:462–478. doi: 10.1111/j.1749-6632.1985.tb50429.x. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Dentcheva E, Curtis KM, Roberts VL, Siman R, Neumar RW. Neuroprotection with delayed initiation of prolonged hypothermia after in vitro transient global brain ischemia. Resuscitation. 2005;64:383–388. doi: 10.1016/j.resuscitation.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Leviton A, Dammann O. Brain damage markers in children. Neurobiological and clinical aspects. Acta Paediatr. 2002;91:9–13. doi: 10.1080/080352502753457851. [DOI] [PubMed] [Google Scholar]
- Lundar T, Froysaker T, Nornes H. Cerebral damage following open-heart surgery in deep hypothermia and circulatory arrest. Scand J Thorac Cardiovasc Surg. 1983;17:237–242. doi: 10.3109/14017438309099358. [DOI] [PubMed] [Google Scholar]
- Marchi N, Cavaglia M, Fazio V, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood-brain barrier damage. Clin Chim Acta. 2004;342:1–12. doi: 10.1016/j.cccn.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Martin H, Rostas J, Patel Y, Aitken A. Subcellular localisation of 14-3-3 isoforms in rat brain using specific antibodies. J Neurochem. 1994;63:2259–2265. doi: 10.1046/j.1471-4159.1994.63062259.x. [DOI] [PubMed] [Google Scholar]
- McGarvey ML, Mullen MT, Woo EY, Bavaria JE, Augoustides YG, Messe SR, Cheung AT. The treatment of spinal cord ischemia following thoracic endovascular aortic repair. Neurocrit Care. 2007;6:35–39. doi: 10.1385/NCC:6:1:35. [DOI] [PubMed] [Google Scholar]
- McKeating E, Andrews P, Mascia L. Relationship of neuron specific enolase and protein S-100 concentrations in systemic and jugular venous system to injury severity and outcome after traumatic brain injury. Acta Neurochir. 1998;71:117–119. doi: 10.1007/978-3-7091-6475-4_35. [DOI] [PubMed] [Google Scholar]
- Mussack T, Biberthaler P, Kanz K-G, Wiedemann E, Gippner-Steppert C, Mutschler W, Jochum M. Serum S100□ and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med. 2002;30:2669–2674. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Newman SP, Harrison MJ. Coronary-artery bypass surgery and the brain: persisting concerns. Lancet Neurol. 2002;1:119–125. doi: 10.1016/s1474-4422(02)00043-1. [DOI] [PubMed] [Google Scholar]
- Persson L, Hardemark H, Edner G, Ronne E, Mendel-Hartvig I, Pahlman S. S-100 protein in cerebrospinal fluid of patients with subarachnoid haemorrhage: a potential marker of brain damage. Acta Neurochir (Wien) 1988;93:116–122. doi: 10.1007/BF01402892. [DOI] [PubMed] [Google Scholar]
- Petzold A, Keir G, Green AJ, Giovannoni G, Thompson EJ. A specific ELISA for measuring neurofilament heavy chain phosphoforms. J Immunol Methods. 2003;278:179–190. doi: 10.1016/s0022-1759(03)00189-3. [DOI] [PubMed] [Google Scholar]
- Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Petzold A, Rejdak K, Belli A, Sen J, Keir G, Kitchen N, Smith M, Thompson EJ. Axonal pathology in subarachnoid and intracerebral hemorrhage. J Neurotrauma. 2005;22:407–414. doi: 10.1089/neu.2005.22.407. [DOI] [PubMed] [Google Scholar]
- Pike BR, Flint J, Dave JR, Lu XC, Wang KK, Tortella FC, Hayes RL. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004;24:98–106. doi: 10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- Pirim I. Ischemic rat brains contain immunoreactivity of 14-3-3 proteins. Int J Neurosci. 1998;95:101–106. doi: 10.3109/00207459809000653. [DOI] [PubMed] [Google Scholar]
- Pfeifer R, Borner A, Krack A, Sigusch HH, Surber R, Figulla HR. Outcome after cardiac arrest: predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation. 2005;65:49–55. doi: 10.1016/j.resuscitation.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Siman R. Spectrin proteolysis in the hippocampus: a biochemical marker for neuronal injury and neuroprotection. Ann N Y Acad Sci. 1993;679:78–86. doi: 10.1111/j.1749-6632.1993.tb18290.x. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci. 1994;14:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. NEJM. 1996;335:1857–1864. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- Rosen H, Rosengren L, Herlitz J, Blomstrand C. Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke. 1998;29:473–477. doi: 10.1161/01.str.29.2.473. [DOI] [PubMed] [Google Scholar]
- Routsi C, Stamataki E, Nanas S, Psachoulia C, Stathopoulos A, Koroneos A, Zervou M, Jullien G, Roussos C. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006;26:20–24. doi: 10.1097/01.shk.0000209546.06801.d7. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Bozyczko-Coyne D, Marcy V, Siman R, McIntosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemela O, Hillbom M. Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J Trauma. 2004;56:1229–1234. doi: 10.1097/01.ta.0000096644.08735.72. [DOI] [PubMed] [Google Scholar]
- Selnes OA, McKhann GM. Neurocognitive complications after coronary artery bypass surgery. Ann Neurol. 2005;57:615–621. doi: 10.1002/ana.20481. [DOI] [PubMed] [Google Scholar]
- Shaw G, Yang C, Ellis R, Anderson K, Parker Mickle J, Scheff S, Pike B, Anderson DK, Howland DR. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun. 2005;336:1268–1277. doi: 10.1016/j.bbrc.2005.08.252. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9:1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Flood DG, Thinakaran G, Neumar RW. Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer's disease-linked presenilin-1 knock-in mutation. J Biol Chem. 2001;276:44736–44743. doi: 10.1074/jbc.M104092200. [DOI] [PubMed] [Google Scholar]
- Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage in the rat. Neurobiol. Dis. 2004;16:311–320. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Shaw G, Yang C, Ellis R, Anderson K, Parker Mickle J, Scheff S, Pike B, Anderson DK, Howland DR. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun. 2005;336:1268–1277. doi: 10.1016/j.bbrc.2005.08.252. [DOI] [PubMed] [Google Scholar]
- Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol Dis. 2004;16:311–320. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Siman R, Zhang C, Roberts VL, Pitts-Kiefer A, Neumar RW. Novel surrogate markers for acute brain damage: cerebrospinal fluid levels correlate with severity of ischemic neurodegeneration in the rat. J Cereb Blood Flow Metab. 2005;25:1433–1444. doi: 10.1038/sj.jcbfm.9600138. [DOI] [PubMed] [Google Scholar]
- Singhal A, Baker AJ, Hare GM, Reinders FX, Schlichter LC, Moulton RJ. Association between cerebrospinal fluid interleukin-6 concentrations and outcome after severe human traumatic brain injury. J Neurotrauma. 2002;19:929–937. doi: 10.1089/089771502320317087. [DOI] [PubMed] [Google Scholar]
- Stapert S, de Kruijk J, Houx P, Menheere P, Twijnstra A, Jolles J. S-100B concentration is not related to neurocognitive performance in the first month after mild traumatic brain injury. Eur Neurol. 2005;53:22–26. doi: 10.1159/000083678. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Harwell LW, Sternberger NH. Neurotypy: Regional individuality in rat brain detected by immunocytochemistry with monoclonal antibodies. Proc Natl Acad Sci USA. 1982;79:1326–1330. doi: 10.1073/pnas.79.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Marumo T, Omura T, Yoshida S. Serum S100B indicates successful combination treatment with recombinant tissue plasminogen activator and MK-801 in a rat model of embolic stroke. Brain Res. 2007;1154:194–199. doi: 10.1016/j.brainres.2007.03.085. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, van Geel W, de Reus H, Biert J, Verbeek MM. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–1310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- Wunderlich MT, Ebert AD, Kratz T, Goertler M, Jost S, Herrmann M. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. 1999;30:1190–1195. doi: 10.1161/01.str.30.6.1190. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Jauch EC, Mulchahey JJ, Gabbita SP, Rosenberg WS, Speciale SG, Zuccarello M. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. doi: 10.1016/s0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- Zhang C, Siman R, Xu YA, Mills AM, Frederick JR, Neumar RW. Comparison of calpain and caspase activities in the adult rat brain after transient forebrain ischemia. Neurobiol Dis. 2002;10:289–305. doi: 10.1006/nbdi.2002.0526. [DOI] [PubMed] [Google Scholar]