Abstract
Using functional MRI (fMRI) we investigated 13 upper limb amputees with phantom limb pain (PLP) during hand and lip movement, before and after intensive 6-week training in mental imagery. Prior to training, activation elicited during lip purse showed evidence of cortical reorganization of motor (M1) and somatosensory (S1) cortices, expanding from lip area to hand area, which correlated with pain scores. In addition, during imagined movement of the phantom hand, and executed movement of the intact hand, group maps demonstrated activation not only in bilateral M1 and S1 hand area, but also lip area, showing a two-way process of reorganization. In healthy participants, activation during lip purse and imagined and executed movement of the non-dominant hand was confined to the respective cortical representation areas only. Following training, patients reported a significant reduction in intensity and unpleasantness of constant pain and exacerbations, with a corresponding elimination of cortical reorganization. Post hoc analyses showed that intensity of constant pain, but not exacerbations, correlated with reduction in cortical reorganization. The results of this study add to our current understanding of the pathophysiology of PLP, underlining the reversibility of neuroplastic changes in this patient population while offering a novel, simple method of pain relief.
Keywords: functional magnetic resonance imaging, phantom limb pain, mental imagery, cortical reorganization
Converging neuroanatomic, neurophysiological and clinical evidence suggests that amputation is associated with neuroplastic changes in the sensory and motor cortices (Flor et al., 2006). This change takes the form of a shift of cortical representation from neighbouring areas of the somatosensory (Mackert et al., 2003) and motor maps (Chen et al., 2002) to the deafferented cortical representation. The clinical manifestations of this change include phantom limb sensations and phantom limb pain (PLP). In upper limb amputees, the extent of somatotopic shift from the face area to the deafferented hand area has been shown to correlate with the incidence and severity of phantom pain (Lotze et al., 2001).
There is no clear understanding as to why deafferentation and subsequent expansion of the cortical representation of the face into the hand area causes pain, rather than just an abnormal perception. It has been hypothesized that it may be due to efferent motor cortical activity continuing without afferent sensory feedback to dampen motor commands (Harris, 1999). In other words, motor cortical activity is not inhibited by sensory cortical activation verifying that the required movement has indeed taken place. Clinical evidence that cortical deafferentation plays a role in phantom pain generation comes from observations that phantom pain may be relieved by imagining stretching movements of the hand, or by using a strategically placed mirror to give the illusion that the missing hand has returned and can be purposefully moved (Ramachandran and Hirstein, 1998). Other methods that compensate for sensory deafferentation may also have an analgesic effect, such as the regular use of a myoelectric prosthesis (Lotze et al., 1999; Weiss et al., 1999). Improvement of pain from pharmacological treatment has also been suggested to be associated with reduction of cortical reorganization (Huse et al., 2001).
This study was designed to test the analgesic effect of a mental imagery exercise while searching for answers to the generation and maintenance of phantom limb pain, as it relates to cortical reorganization. The challenge in this was to recognize the many different types of painful and non-painful sensations that patients with phantom limb pain describe. From a search of the relevant literature and our own extensive clinical practice we concluded that pertinent aspects of pain in the phantom were as follows: (i) Patients describe two types of pain; continuous pain of varying intensity (‘constant’ pain) and attacks of pain varying in duration from seconds to hours (‘exacerbations’ of pain); these appear to be independent of each other, (ii) While finding it difficult to describe the emotional content accurately they are able to estimate the degree of unpleasantness, (iii) The phantom or parts of the phantom can be perceived as well as its exposure to sensory and motor tasks, (iv) Touching the face (in the case of upper limb amputees) occasionally produces a projection of sensation into the phantom hand, (v) Severe pain in the phantom can extend beyond the phantom itself and (vi) Various manipulations aimed at changing the dominance of the phantom in an individual's personal experience (distraction, sensory stimulation of the stump using transcutaneous electric stimulation or spinal cord stimulation, use of a prosthesis, visual illusions using the mirror box and so on) are capable of reducing either or both types of pain. Based on these clinical observations, and results from previous brain imaging studies, we formulated four hypotheses we wished to test that link cortical reorganization to PLP (i) Cortical reorganization occurs so that representation of the face expands to invade some of the representation of the missing limb (cf. Flor et al., 2006), (ii) Cortical reorganization also occurs in the opposite direction with the representation of the missing limb expanding to the face and stump areas, (iii) These changes are associated with the intensity and/or the unpleasantness of PLP, (iv) A specific internally generated intervention, mental imagery to activate the sensory and motor cortices will reduce both cortical reorganization and reduce the intensity or unpleasantness of at least one of the pain types.
Studies based on the effects of interventions trying to establish causality between pathology and subjective symptoms are problematic because of the difficulty of controlling for the effects of the interventions per se. With this in mind, we chose to discard the use of any external interventions and rely on the patient's mental imagery as the sole intervention. Mental imagery is known to activate the motor and sensory cortices, and we hypothesized that, if practiced regularly, it would provide sufficient stimulation of the deafferented neurons and potentially alter the reorganization (Flor et al., 2006). Any change in cortical reorganization produced by this method could then be correlated with pain descriptors in a more straightforward manner than would be the case if a chemical or mechanical intervention (pharmacotherapy, neurostimulation, visual stimulation) were applied.
To measure change in cortical reorganization we adopted the method developed by Lotze and others for this patient population (Lotze et al., 2001). They showed that in patients with upper limb phantom pain there was, during the simple act of the individual pursing his or her lips, an inappropriate activation of the face area in the somatosensory cortex that was not seen in patients with amputations with no pain, or control participants. We used this lip purse to measure the cortical shift from the face area to the hand/arm area (F→H). To investigate the shift in the other direction (H→F) we exploited the fact that an imagined task will activate the cortical area activated by actual execution of the task (Jeannerod, 1995; Hesslow, 2002). We therefore used imagined movement of the phantom to explore the shift in this opposite direction.
To enhance the analgesic efficacy of mental imagery, we wanted participants to use a simple method of achieving a state of moderate relaxation while simultaneously being able to perceive vivid movement and sensation. We chose a mindfulness-based ‘body-scan’ meditation technique as a means of achieving a relaxed state, based on a pain management technique developed by Kabat-Zinn et al. (1985).
Methods
Participants
All participants gave written, informed consent. Thirteen participants (11 males, 2 females, age range 32–75 years, mean 52.92 years, SD 13.6), with unilateral, upper limb amputation at least above the wrist and phantom limb pain of at least one year's duration participated (see Table 1 for demographic details). Time since amputation, and duration of pain was in the range of 3–51 years (mean 24.54 years, SD 17.1). Only one subject regularly took analgesia, and he abstained for 12 h prior to scanning to eliminate any medication effects on activation maps. The other two participants who reported sporadic analgesic intake at assessment had not taken any analgesia for several weeks prior to scanning.
Table 1.
Demographic details of clinical participants
| ID | M/F | Age | L or R amputation | Above/below elbow | Reason for amputation | Years since amputation | Prosthesis use |
|---|---|---|---|---|---|---|---|
| 01 | M | 68 | Left | Below | Bone cancer | 51 | Myoelectric cosmetic |
| 02 | M | 52 | Left | Above | Trauma | 35 | None |
| 03 | M | 33 | Right | Above | Trauma | 12 | None |
| 04 | M | 58 | Right | Below | Trauma | 35 | None |
| 05 | M | 68 | Right | Above | Trauma | 42 | Cosmetic |
| 06 | M | 41 | Right | Above | Trauma | 22 | Cosmetic |
| 07 | F | 51 | Left | Below | Trauma | 4 | None |
| 08 | M | 59 | Right | Above | Trauma | 6 | Cosmetic |
| 09 | F | 42 | Right | Above | Trauma | 36 | Cosmetic |
| 10 | M | 62 | Right | Above | Trauma | 3 | Cosmetic |
| 11 | M | 75 | Right | Below | Trauma | 43 | Cosmetic |
| 12 | M | 32 | Left | Above | Trauma | 5 | None |
| 13 | M | 47 | Right | Above | Trauma | 25 | Cosmetic |
Participants were recruited from two local rehabilitation centres, a local pain clinic and the British Limbless Ex-Servicemen's Association (BLESMA). Six age- and gender-matched healthy volunteers (mean age 43 years, range 30–56) were also scanned to determine normal cortical responses to the tasks set. All participants were screened to ensure there were no MR contraindications. The study was approved by the local Research Ethics committee and the Research Governance committee at the Walton Centre for Neurology and Neurosurgery, Liverpool, UK and conducted according to the Declaration of Helsinki guidelines.
Clinical assessment
Before and after the fMRI scans all clinical participants underwent a detailed interview using the following clinical measures:
Demographics: current coping strategies, sleep pattern, duration of pain and medication use. The clinical interview was repeated at the final fMRI session.
Phantom limb pain questionnaire (Kooijman et al., 2000): to measure the incidence of stump pain, phantom limb phenomena (i.e. any phantom sensations not considered painful), phantom pain and prosthesis use.
Beck Depression and Anxiety Inventories (Beck et al., 1961, 1988): to exclude severe anxiety or depression which could be worsened by participation in deep relaxation classes.
Vividness of Imagery Scale (Lotze et al., 2001): to measure the vividness of each subject's perception of their ability to move the phantom limb, where 0 = no perception of any voluntary movement to 6 = very vivid perception of voluntary phantom movement.
Numerical rating scale (NRS): to measure intensity of pain (0 = no pain to 10 = worst pain imaginable) and unpleasantness (0 = not unpleasant at all, 10 = extreme unpleasantness). For statistical analysis, an average of diary scores over the past week was calculated. Participants completed daily pain diaries during the week following assessment, the 6 weeks of the intervention and the week prior to the final scan. Intensity and unpleasantness of constant pain and exacerbations of pain (number, duration, intensity and unpleasantness) were recorded, as all recruits stated that these unpredictable, severe bouts of pain were the most difficult to cope with.
Estimates of pain during the scanning session were taken immediately after the session was finished (‘contemporaneous’ pain).
fMRI study design and procedure
Participants first performed a simple lip purse during fMRI (before and after the therapy), measured against rest, to establish the extent of cortical reorganization from face to hand area in motor and somatosensory representational maps (Lotze et al., 2001). Secondly they performed rhythmic opening and closing of the intact fist (non-dominant left hand in the case of controls, to match movement of the left intact limb of the patient group), also measured against rest, to explore the function of the ipsilateral cortex in PLP patients and serve as a functional localiser. Thirdly, all participants imagined movement of both hands, alternating right dominant/phantom with left non-dominant/intact hand, measured against rest, to reveal other cortical reorganization that would extend beyond the face area.
Imagined movement of the intact hand was also used to determine the non-specific effects of the meditation process.
Participants rehearsed the task outside the scanner immediately before the scanning session, including having the opportunity to practise while listening to a CD of the noise of the EPI scans. They were instructed to perform the lip purse and the hand movements at a rate of 0.5 Hz and to keep to this pace during the scan, and they were monitored visually during scanning to ensure accuracy of performance without extraneous movement. The scanning paradigm consisted of a simple block design. After 30 s of rest, the task (lip purse, opening and closing of the fist or imagining movement of each hand) was performed for 30 s, followed by another 30 s of rest, for a period of 6.5 min. An investigator stayed in the scanning room with each subject, and they were cued by a light tap on the leg for ‘start’ and two taps for ‘stop’.
Clinical intervention
Following the first scanning session, each patient saw the therapist for six sessions of individual therapy, either once a week or once a fortnight, depending on his or her other commitments. Each session lasted one hour—40 minutes for the therapy and 20 minutes for debriefing. Recruits rested on a chair or couch, and the room was quiet (but not silent) and private. Therapy was a combination of the ‘body-scan’ exercise and imagined movement of and sensation in the phantom limb.
The body-scan facilitated relaxation and imagery. In particular recruits learned to concentrate on sensations from each area of the body consecutively, including the phantom arm and hand. Once a state of relaxation was achieved the recruit was encouraged to imagine comfortable and thorough movement and sensation in the phantom limb. More specifically recruits were encouraged to focus on sensation from each part of the phantom, for example imagining the sensation of the arm resting against the couch, whether the limb felt warm or cool, the position of each finger. Next, they were invited to imagine comfortable and thorough movement and sensation in the phantom limb, such that they could ‘stretch away the pain’, and finally to ‘allow the fingers, hand and arm to rest in a comfortable position’. The actual therapy of ‘moving’ and ‘feeling’ the limb lasted for ∼5 min.
Recruits were given a 40-min CD of the meditation and imagery exercises (personalized to take account of whether the right or left limb had been amputated, but otherwise adhering to a script) and were encouraged to practise daily. Recruits were also taught a short, 10 min form of the meditation/imagery exercise, which they could use without a CD.
Recruits kept a daily record of meditation practice recording the time spent practising, the immediate effect on pain and free space for any comments. By Week 3, all participants were competent in achieving the relaxation, and were then encouraged, in addition to the formalized CD practice, to move and feel the phantom several times daily, without having to resort to the relaxation exercise. All participants stated that adherence to the therapy was enhanced by the weekly visits to the therapist. Follow-up scans were repeated within 6 weeks of completion of the intervention. The healthy volunteers were also scanned twice but received no intervention.
Scanning procedure
MRI data were acquired using a 3T Siemen's Trio MR Scanner (Erlangen, Germany). FMRI was performed with a blood oxygenation level-dependent (BOLD) sensitive T2*-weighted multislice gradient echo EPI sequence (TE = 50 ms, TR = 3 s, flip angle = 90°, FOV = 19 cm, 128 × 128 matrix). Twenty-eight contiguous 4 mm thick axial slices were prescribed parallel to the AC–PC line and covered the whole brain. One hundred thirty three EPI volumes were collected in total (after saturation scans). For the purpose of anatomical referencing and visualization of brain activation, a high-resolution T1-weighted 3D inversion recovery prepared gradient echo (IRp-GRASS) sequence was acquired (TE = 5.4 ms, TR = 12.3 ms, TI = 450 ms, 1 mm slice thickness, FOV = 20 cm, 256 × 192 matrix), with 128 axial slices covering the whole brain.
Data analysis overview
FMRI data analysis was carried out using FEAT 3.3 software (FMRI Expert Analysis Tool, version 3.3, Oxford Centre for Functional Magnetic Resonance Imaging Analysis of the Brain—FMRIB – University of Oxford), part of the FMRIB software library (Smith et al., 2004). The following pre-statistics processing was applied; motion correction using MCFLIRT (Jenkinson and Smith, 2001); spatial smoothing using a Gaussian kernel of FWHM 5 mm; mean-based intensity normalization of all volumes by the same factor; non-linear high-pass temporal filtering (σ = 120 s Gaussian-weighted LSF straight line fitting). Statistical analysis was carried out using FILM (FMRIB's Improved Linear Model) with local autocorrelation correction of the data (non-linear spatial smoothing and prewhitening—Smith and Brady, 1997; Woolrich et al., 2001). In order to test for group level differences we defined the following contrasts at the first level—lip purse versus rest; executed movement of left intact arm versus rest; imagined movement of each hand versus rest. To accommodate inter-subject variability, the contrast images from all participants were then entered into a mixed effects group analysis carried out using FEAT 3.3 software (Beckmann et al., 2003; Woolrich et al., 2004). Statistical images were thresholded using clusters determined by Z = 2.3, P < 0.05 cluster-corrected and transformed into the stereotaxic space of the Montreal Neurological Institute (MNI), using FLIRT (FMRIB's Linear Image Registration Tool—Jenkinson and Smith, 2001). Additionally the raw data from the four left amputees was flipped so that all images of the left hemisphere would be contralateral to the amputated side, to allow group comparison of activation ipsi- and contralateral to the amputated side.
The following pain scores were also added to group General Linear Model (GLM) analysis of fMRI data as covariates of interest: intensity of pain experienced during the scan (‘contemporaneous pain’), constant pain intensity and unpleasantness, and exacerbation intensity and unpleasantness. Differences in activation before and after the intervention were compared with pairwise t-tests and whole brain maps inspected. The pain scores were used as a regressor within the GLM to confirm a positive covariance with the BOLD signal, allowing identification, voxel-by-voxel, of those areas of the brain where there was a reduction in activation relating to a reduction in pain scores.
Hand and lip M1 and S1 coordinates were identified by using the mean of coordinates from the brain imaging literature ±8 mm in each plane, plus mean group coordinates of both the clinical participants and the healthy volunteers.
[Lip M1 (mm): xyz ± 52, –8, 36. S1 (mm): xyz ± 58, –18, 24. Hand M1 (mm): xyz ± 34, –34, 52. S1 (mm) xyz ± 34,–30, 58. Porro et al., 2000; Lotze et al., 2000; Dettmers et al., 2001; Roux et al., 2001; Stippich et al., 2002; Ehrsson et al., 2003]. Individual brains were also inspected to locate the hand ‘knob’ on M1 and S1 (Yousry et al., 1997). In individuals and groups, activation maxima were obtained from the cluster lists provided within the FSL results, and FSLview was used to inspect and display images and to confirm the functional location. This location was confirmed with the Duvernoy brain atlas (Duvernoy, 1999).
The generous 8 mm latitude in all planes allowed for individual variations in representational maps, both normal and abnormal. Inspection of individual maps confirmed the presence of activation within these boundaries, therefore each individual contributed to the group analysis.
Healthy volunteers were scanned twice to address the role of possible non-specific effects due to increased thresholds for neural activation because of, e.g. familiarization of the tasks. No significant group differences were found between the first and the second scan (analysed at P < 0.05 uncorrected) in bilateral M1 and S1; and the similarity of neural activation patterns was confirmed by inspection of individual maps. We therefore proceeded to analyse patient data confident that changes in the neural activations of patients would reflect genuine effects from the intervention.
Results
Clinical results
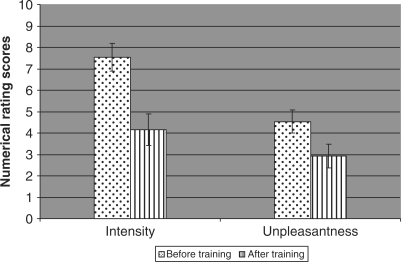
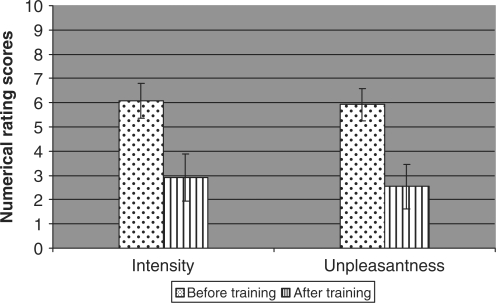
None of the recruits had raised psychometric scores on the Beck Anxiety and Depression Inventories at assessment or at follow up and all were either in full-time employment or pursuing an active, retired lifestyle. All had suffered from phantom pain since immediately after amputation (Table 2). Mean constant pain intensity was 7.5 before training (range 3–10, SD 2.3) with a mean unpleasantness score of 5 (range 2–9 SD 1.7); mean number of daily exacerbations was 9 (range 0–43 SD 12.0) at a mean intensity of 6 (range 0–9 SD 2.6 see Figs 1 and 2) and a mean unpleasantness score of 6 (range 0–9, SD 2.7). One patient had no exacerbations of pain. All participants successfully completed the course of therapy and by week 3 were competent at following the meditation and mental imagery exercises.
Table 2.
Numerical ratings of constant pain (intensity and perception of unpleasantness) and exacerbations (intensity and unpleasantness) before and after training in mental imagery
| ID | Constant pain |
Exacerbations of pain |
Analgesia |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensity |
Unpleasantness |
Intensity |
Unpleasantness |
|||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 01 | 3 | 2 | 2 | 1 | 6 | 0 | 5 | 0 | None | None |
| 02 | 5 | 1 | 5 | 1 | 9 | 0 | 6 | 0 | None | None |
| 03 | 10 | 8 | 6 | 6 | 9 | 8 | 7 | 7 | a b c d e f | ↓a b c d ↓e f |
| 04 | 10 | 6 | 8 | 3 | 8 | 5 | 8 | 0 | None | None |
| 05 | 10 | 5 | 6 | 3 | 6 | 0 | 8 | 0 | g | None |
| 06 | 5 | 5 | 4 | 3 | 6 | 6 | 6 | 4 | None | None |
| 07 | 8 | 3 | 5 | 2 | 3 | 1 | 4 | 0 | None | None |
| 08 | 9 | 8 | 6 | 5 | 7 | 8 | 8 | 8 | None | None |
| 09 | 5 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | None | None |
| 10 | 8 | 1 | 2 | 2 | 3 | 0 | 5 | 0 | None | None |
| 11 | 8 | 3 | 1 | 1 | 7 | 2 | 7 | 3 | None | None |
| 12 | 8 | 3 | 6 | 3 | 7 | 2 | 7 | 3 | g | None |
| 13 | 9 | 8 | 9 | 7 | 9 | 8 | 9 | 8 | None | None |
Analgesia: a = codeine phosphate; b = gabapentin; c = tramadol; d = lamotrigine; e = zopiclone; f = co-codamol; g = amitriptyline; ↓ = dose reduced.
Fig. 1.
Scores of constant pain intensity and unpleasantness before and after training, measured by daily pain diaries using numerical rating scores. Reduction in pain intensity was significant (P < 0.0005), as was reduction in pain unpleasantness (P < 0.01).
Fig. 2.
Changes in daily exacerbations of pain—intensity and unpleasantness of pain, using numerical rating scores. Reduction of intensity of exacerbations was significant (P < 0.005) as was the reduction in unpleasantness of exacerbations (P < 0.03).
At the end of training in therapy, 9 of the 13 participants had gained >50% pain relief (Figs 1 and 2). The most noticeable benefit for the participants was the reduction in the number and severity of exacerbations with six participants free from exacerbations of pain at the end of the study (Table 2 and Figs 1 and 2). Of the three patients taking analgesia, two had discontinued and one had reduced his requirements for codeine phosphate and zopiclone.
The group mean vividness of imagery scores at outset was 4 (range 2–6 SD 1.8) and at follow-up 5 (range 3–6 SD 1.1, P > 0.12, not statistically significant).
fMRI results
Lip purse
In patients, prior to training, lip purse resulted in bilateral activation in M1 and S1 lip/face area. In addition, in the left hemisphere (contralateral to the amputation) activation extended into M1 hand area [(mm) xyz –30, –36, 52] and, to a lesser extent into S1 hand/arm area [(mm) xyz –22, –36, 56]. Other significant activation sites, including bilateral insula, anterior cingulate cortex (ACC), thalamus and cerebellum are reported in Table 3 and see Fig. 3. In healthy volunteers, activation during lip purse was confined to bilateral M1 and S1 lip area only, without concomitant activation in M1/S1 hand area. Other significant activation sites are presented in Supplementary Table S1.
Table 3.
Lip purse: paired t-tests of activation (baseline minus follow up), with pain scores as covariates
| Anatomical site | Contemporaneous pain |
Constant pain NRS |
Constant unpleasantness |
Exacerbation Pain NRS |
Exacerbation unpleasantness |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | x | y | z | Z | x | y | z | Z | x | y | z | Z | x | y | z | Z | x | y | z | |
| L M1 hand/arm | × | × | × | × | 2.5 | −24 | −38 | 50 | × | × | × | × | × | × | × | × | × | × | × | × |
| L S1 hand/arm | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| R M1 hand/arm | 2.8 | 26 | −30 | 54 | 3.0 | 24 | −32 | 56 | × | × | × | × | × | × | × | × | 2.3 | 26 | −30 | 54 |
| R S1 hand/arm | 3.0 | 28 | −30 | 60 | 3.8 | 20 | −28 | 60 | × | × | × | × | × | × | × | × | 2.6 | 24 | −34 | 62 |
| R ACC | 3.4 | 2 | −22 | 42 | 4.1 | 6 | 10 | 32 | 3.6 | 4 | 12 | 32 | × | × | × | × | 3.5 | 4 | 12 | 32 |
| L ACC | 3.9 | −10 | 10 | 30 | 3.9 | −4 | 2 | 38 | 3.1 | −2 | −8 | 42 | 3.5 | −2 | −2 | 34 | 4.4 | 0 | −12 | 34 |
| L insula | 4.8 | −36 | 2 | 6 | × | × | × | × | 3.6 | −44 | 14 | –16 | 3.9 | −50 | 12 | −6 | 4.0 | −50 | 12 | −6 |
| R insula | × | × | × | × | 3.6 | 54 | −14 | 10 | × | × | × | × | × | × | × | × | 3.7 | 50 | 14 | −6 |
| L thalamus | 3.1 | −16 | −22 | 4 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| L superior temporal | 4.1 | −58 | 10 | −10 | 4.1 | −42 | 14 | −16 | × | × | × | × | 3.7 | −50 | 14 | −16 | 3.5 | −56 | 8 | −12 |
| R superior temporal | × | × | × | × | × | × | × | × | 3.6 | 8 | −78 | 24 | × | × | × | × | × | × | × | × |
| L S II | 3.6 | −32 | −30 | 42 | × | × | × | × | 3.5 | −12 | −42 | 48 | 3.7 | −10 | −42 | 50 | × | × | × | × |
| L inferior Frontal | 4.1 | −46 | −2 | 26 | × | × | × | × | × | × | × | × | × | × | × | × | 3.8 | 58 | 0 | 10 |
| R inferior Frontal | × | × | × | × | 3.7 | 54 | 4 | 10 | × | × | × | × | × | × | × | × | × | × | × | × |
| SMA | 4.2 | 0 | −2 | 50 | 3.9 | −10 | −8 | 56 | 3.1 | −4 | −28 | 62 | 3.7 | 0 | −6 | 42 | × | × | × | × |
| Premotor Area | 3.9 | −22 | −12 | 4 | 4.0 | 14 | −22 | 62 | × | × | × | × | × | × | × | × | × | × | × | × |
Brain areas in which reduction in activation at follow up covaried with pain reduction after therapy, measured by paired t-tests using the GLM within FSL software, with pain scores added as a regressor; × = no significant activation.
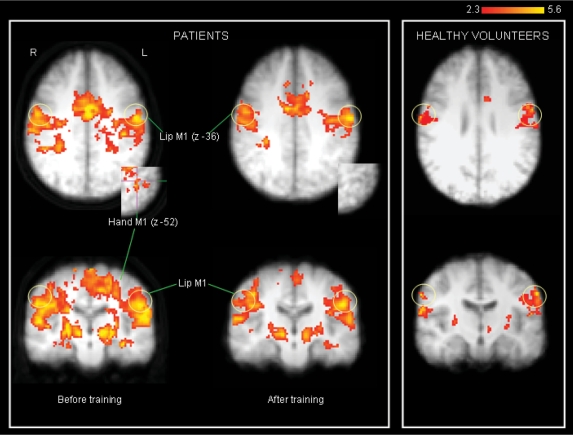
Fig. 3.
Activations in response to lip purse: patients before and after training: yellow circles indicate M1/S1 lip area. Activation in hand area M1/S1 is shown in axial view (inset). At follow-up activation is less diffuse and more confined to lip area. There is no activation in hand area M1/S1 (inset: shown in axial view). Healthy volunteers show activation in M1 lip area only.
After training, lip purse again produced bilateral activation of M1 and S1 in the lip/face area. However, there was no suprathreshold or sub-threshold activation of contralateral M1 and S1 hand area (Supplementary Table S1, Fig. 3).
Patients also differed from healthy participants in that activation in bilateral M1 lip area was more medial [contralateral to amputation (mm) xyz –52, –8, 52 versus –60, –12, 36; ipsilateral to amputation (mm) xyz 54, –12, 34 versus 66, –10, 32] (Fig. 3). This was also the case in bilateral S1 lip area [contralateral (mm) xyz –54, –16, 26 versus –62, –12, 24; ipsilateral 58, –12, 26 versus 62, –8, 24]. After training, M1 activation in the clinical group was more lateral in both hemispheres [contralateral (mm) xyz –58, –10, 33, ipsilateral 60, –8, 30, 6 mm lateral shift see Supplementary Table S1].
Before training, the extent of activation in contralateral M1 hand area covaried significantly with the severity of contemporaneous pain (i.e. pain experienced during the scan reported by the patient on NRS 0–10 immediately after the imaging session) but not intensity or unpleasantness of diary-based constant and exacerbation pain scores. Activation in SI did not covary with any pain attribute. In contrast, constant pain covaried with lip purse induced activation in ipsilateral thalamus and contralateral insula. (It should be noted that lip purse itself did not evoke any phantom pain during the scan.)
After training, reduction in constant pain scores covaried significantly with the reduction of activation in response to lip purse in contralateral M1 hand/arm area, ipsilateral M1 and S1 hand/arm area, as well as bilateral ACC (Table 3). Reduction in contemporaneous pain covaried with reduction in activation in ipsilateral M1 and S1 and contralateral ACC, insula and thalamus, but not contralateral MI or S1. Reduction in intensity of exacerbation pain did not covary with activations in primary sensory or motor cortices, although covariance was seen with contralateral ACC and insula. Reduction in scores of unpleasantness of constant pain and exacerbations of pain covaried with reduced activation in ipsilateral M1 and S1 hand areas with further covariance with bilateral ACC and contralateral insula.
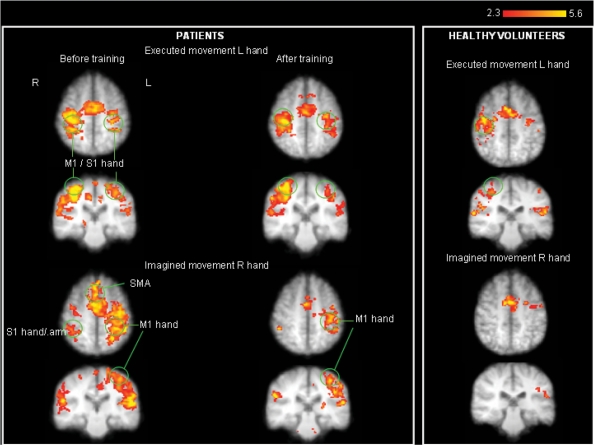
Imagined movement of hands (right) phantom movement
In both groups, activation was seen in contralateral MI and SI representing the hand [M1 (mm) xyz –38, –32, 52; S1 (mm) xyz –38, –32, 58] and SII and bilateral supplementary motor area (SMA) and ACC in accordance with other reports (Ehrsson et al., 2003). In patients, activation was also seen in the contralateral face area within the MI and S1 strip [M1 (mm) xyz –50, –10, 36; S1 (mm) xyz –48, –8, 28], ipsilateral SII, and bilateral thalamus, insula and cerebellum. After training, the excessive activations in bilateral face MI and ipsilateral hand SI were no longer present. No covariance was found between ipsi- or contralateral M1 and S1 activations and any pain scores either before or after training (see Fig. 4 and Supplementary Table S2).
Fig. 4.
Strong bilateral activation in response to left executed hand movement and right imagined hand movement seen in patients as opposed to healthy volunteers.
Imagined movement of intact (left) hand
In the patient group, there was activation in response to imagined movement of the left intact hand in bilateral hand area but not face area. There was no significant change in activation maps at follow up and no covariance with any pain scores. In the control group, activation in response to imagined movement of the left, non-dominant hand resulted in minimal activation in contralateral hand M1 and S1 only (see Fig. 4 and Supplementary Table S2).
Executed movement of intact (left) hand
Areas activated in common to both groups were contralateral M1 and S1 hand area, contralateral thalamus, midline SMA, bilateral insula and bilateral cerebellum. In patients, activation also occurred in ipsilateral hand area [MI (mm) xyz –30, –34, 52], ipsilateral lip area [M1 (mm) xyz –48, –20, 36], contralateral SII, contralateral inferior temporal and bilateral medial frontal gyrus (see Figs 4 and 5, Supplementary Table S3). After training, active movement of the hand failed to provoke activation in the ipsilateral lip motor cortex while activation in ipsilateral hand motor area appeared much reduced.
Fig. 5.
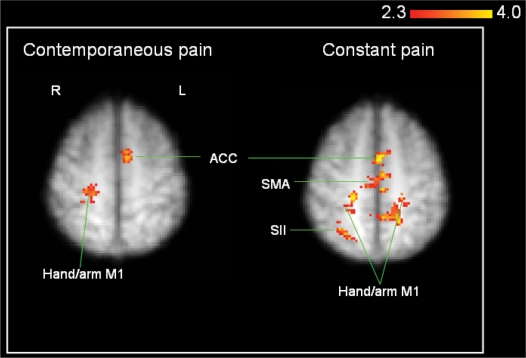
Lip purse: paired t-test of lip purse activations before and after training with pain scores added as covariates. Left map: illustration of activation remaining when follow-up lip purse is subtracted from baseline lip purse, with reduction in contemporaneous pain added as a covariate. Activation remains in right M1 hand area (ipsilateral to amputated side) and left ACC. Right map: illustration of activation remaining when follow-up lip purse is subtracted from baseline lip purse, with reduction in constant pain added as a covariate. Activation remains in bilateral M1 hand/arm area, supplementary motor area (SMA), ipsilateral SI and secondary somatosensory cortex (SII).
Before training, ipsilateral (i.e. contralateral to phantom) M1 and S1 hand area activation covaried significantly with constant pain intensity. After training, reduction in any form of recorded pain failed to covary with any changes in activation maps.
Discussion
In this study of PLP in upper limb amputees, the remarkably simple technique of imagining movement and sensation in the missing limb resulted in significant pain relief. All subjects found learning the body scan useful as a means of relaxation, regardless of whether their pain lessened, and they all felt that the body scan was a useful facilitator to imagining the return of the phantom limb.
Other researchers have reported the clinical benefit of imagined and virtual movement in patients with long-standing pain conditions. Moseley (2006) demonstrated significant pain relief in patients with complex regional pain syndrome (CRPS) and PLP when they first learned to improve laterality recognition using photographs of hands in varying positions, and then learned to imagine the injured hand in non-painful postures. Mirror therapy has also been reported to have an analgesic effect in PLP (Chan et al., 2007). So it seems that different ways of stimulating the motor and sensory cortices can be effective in relieving pain. Cortical reorganization patterns have also been shown to be a feature of CRPS, with changes in somatotopic cortical maps which normalize upon symptomatic recovery due to physiotherapy (Maihofner et al., 2004) or treatment with memantine (Sinis et al., 2007).
The primary focus of the present study was to evaluate the relationship between cortical reorganization, the various forms of pain in patients with phantom limb pain syndrome and the analgesic effect of mental imagery. We employed lip purse as a tool to demonstrate activation, measured by fMRI, in the hand area; such activation is not seen in healthy participants and is best explained by a change in the excitability of cortical neurons previously responsive to functions involving the hand or arm only. The relevance of this finding comes from the direct correlation between the hand area activation and contemporaneous pain (i.e. pain experienced during scanning), and is emphasized by the fact that, with significant pain reduction during the second scanning session, no such abnormal activation was elicited. That the association was seen in contemporaneous pain only and not with the pain scores obtained from pain diaries, suggests that in a given patient with PLP the at-present state of pain is a better indication of reorganization than is a general disposition of pain. Disappearance on the post-training scan of this abnormal activation in the patients, who for the major part experienced significant pain relief, points in the same direction. The contemporaneous pain scores on that occasion were so low that no correlation with them and the BOLD signal in the hand area could be reasonably expected; indeed none was found.
Another tool used in this study to measure cortical reorganization was that of imagined movement of the phantom. A novel finding in the present study was that cortical reorganization appears to have taken place in a more random fashion than previously thought, although such a finding supports clinical observations such as sensory experiences in the head reported by patients with a phantom arm (Ramachandran and Hirstein, 1998). Imagined movement of the phantom hand led to activation of the lip area which was not seen in healthy volunteers, which may be a form of ‘reverse’ functional reorganization (H→F). Interestingly, however, this change did not covary with any pain type. We conclude that this abnormality is likely to represent the effect of amputation per se and may not be critical for the development of pain. In line with this, movement of the intact hand resulted in activation of the ipsilateral lip area in the motor strip (i.e. contralateral to the phantom) and was also devoid of any covariance with pain scores.
Additionally, we were impressed by the consistent extensive activation of the M1 and S1 hand representation area contralateral to the phantom, irrespective of the task (lip purse, imagined movement of the phantom, imagined movement of the intact arm or executed movement of the intact arm). This kind of universal activation, exemplifying a lack of neural efficiency has been reported by others using motor evoked potentials (MEP; Cohen et al., 1991); transcranial magnetic stimulation (TMS; Roricht et al., 1999; Karl et al., 2001) and fMRI (Lotze et al., 2001). Interestingly, this excessive activation during intact arm movement, which was lacking in controls, covaried with constant pain, and adds to the significance of altered activity in M1/S1 region following nervous system injury (Navarro et al., 2007).
It has been proposed that the perception of phantom movement relies upon the preservation of a cortical representation of the missing limb, itself dependent upon intact neuronal connections (Mercier et al., 2006; Reilly et al., 2006). It is of interest that our patients recognized improvement in freedom of movement of the phantom as training progressed, suggesting they achieved better mental access to the deafferented cortical areas (Mercier et al., 2006). The decrease in activation post-training seen in this area is likely to reflect improved neural efficiency and precision, similarly to that seen after other forms of cognitive training (Kelly et al., 2006). A further interesting finding was the extensive bilateral activation in primary sensory and motor cortices observed in patients during every task.
Against this background it is of interest that during active movement of the intact limb, our patient group showed increased activation in the M1 and S1 hand areas contralateral to the phantom (ipsilateral to the intact arm) that at baseline correlated with the intensity of constant pain. Activation of ipsilateral S1 and M1 has been shown in patients with neuropathic pain in response to provocation of allodynic pain (Peyron et al., 2004). Our finding needs to be interpreted with caution, however. First, no patient reported any pain associated with the increased use of the intact arm and secondly, functional cortical reorganization, both inter- and intra-hemispheric, is well documented in the brain imaging literature in models of health and disease (Pascual-Leone et al., 2005). Although earlier primate studies suggest that cortical reorganization after amputation in primates is contralateral from neighbouring areas of the somatotopic map (Wu and Kaas, 1999), a later study using an animal model of extensive hind- and forepaw surgical peripheral denervation has established bilateral cortical–cortical reorganization (Pelled et al., 2007).
Bilateral cortical activation is reported in amputees without pain. Hamzei et al. (2001) studied seven participants who had been missing an arm since childhood (six amputees and one dysmelia)—all demonstrated bilateral structural and functional cortical changes [measured using MRI, fMRI and transcranial stimulation mapping (TMS)], including contralateral and ipsilateral M1 activation in response to finger tapping. Similar bilateral activation has been shown in lower limb amputees using TMS during movement of the intact limb (Schwenkreis et al., 2003). Kelly and Garavan (2005) suggest that in healthy volunteers, cortical plasticity in response to the challenge of learning a new task (in our case, mental imagery of the phantom) has a 2-fold physiological mechanism. Developing greater motor skill rests on increased neural efficiency (demonstrated in our participants by the reduction in activation shown after regular practice of imagined movement and sensation in the phantom limb), while developing a new strategy (for example, learning to be one-armed following amputation) relates to plastic change which usually presents itself in enhancement of activation.
Thus it seems that cortical reorganization following amputation is 2-fold—with intrahemispheric reorganization from the adjacent area on the homunculus, and interhemispheric reorganization from the recruitment of horizontal connections of the intact limb representation to the deafferented cortex. Why cortical reorganization of the kind we report here is associated with pain cannot be answered. The cortical reorganization we witnessed in patients during various tasks, prior to the clinical intervention, reduced in relation to the reduction in pain, but we cannot offer evidence that the link is causative. Nevertheless, reduction of cortical reorganization (i.e. reduction of activation in contralateral M1 and S1 hand area, induced by lip purse, to sub-threshold levels in group analysis) after training in our patients covaried with reduction of their constant pain scores. Reductions in activations in ipsilateral hand MI and SI covaried with intensity of contemporaneous and constant pain intensity, and unpleasantness of exacerbation scores. These findings are supportive of the concept of a relationship between cortical reorganization and PLP as previously suggested (Lotze et al., 2001; Flor et al., 2006). It is reinforced by the fact that we intentionally chose an intervention that was minimalist and aimed at repeatedly activating the primary motor and sensory cortices. It is intriguing that the relationship appears to be selective: it was condensed to F → H type reorganization, and primarily associated with ongoing and constant pain, and not exacerbations. For the latter, alternative mechanisms should be explored.
While the focus of this study was on the association of pain relief and reorganization of the sensory and motor cortices, we did observe a general reduction in all brain areas during the second scan after training. These more general activations could be explored in future studies using a control group of either healthy volunteers subjected to an intervention mimicking treatment, or patients with similar amputations not subjected to treatment, to establish whether they are a pathophysiological correlate of amputation or pain or mainly reflect natural fluctuation, familiarization with imagined movement or a similar non-specific effect. A further limitation of this study lies in the fact that we have reported group results with the danger of missing individual data due to normalization. However, inspection of individual activation maps showed little variability or deviation from the mean coordinates and boundaries described in the methodology. A future study where analysis of the individual relationship between BOLD response and pain measurements may be desirable, using a technique such as flatmapping to measure the definitive spatial extent of activation maps.
In conclusion, we have shown that, significant associations exist between different types of phantom limb pain and cortical reorganization, and that regularly practiced mental imagery results in pain relief, which is associated with a reduction in cortical reorganization. These results in part corroborate previous findings and add new important information, especially in the domain of neuroplasticity, suggesting that plastic changes may be surprisingly responsive to internally generated manipulation. Challenges that remain for future research include how to establish which aspect of reorganization is related to pain, whether reorganization drives the pain or vice versa, the role of any morphological changes and investigation of measures that might prevent the maladaptive effect of amputation on the cortex. Perhaps the most pivotal question relates to the exact mechanisms, whereby cortical reorganization is linked to PLP, which no study to date has unravelled. The therapeutic efficacy of the intervention in the present study was so impressive that a controlled trial seems warranted, encompassing a design that can bring together the various therapies (imagery, laterality recognition, mirror box), to determine which virtual therapy best suits which patient.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors wish to thank the clinical and academic staff at the Magnetic Resonance Imaging and Research Centre (MARIARC), University of Liverpool, UK, Steve Laird and Lynne Kacperek from the Preston Disablement Services Centre, The British Limbless Ex-Servicemen's Association, The Donald Tod Limb Fitting Centre, Liverpool, UK and the Pain Relief Foundation who provided funding for this project.
Glossary
Abbreviations:
- PLP
phantom limb pain
- NRS
Numerical rating scale
- BOLD
blood oxygenation level-dependent
- ACC
anterior cingulate cortex
- SMA
supplementary motor area
References
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:892–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multi-level linear modelling for group analysis in FMRI. NeuroImage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina F, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357:2206–7. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganisation following injury. Neuroscience. 2002;111:761–73. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganisation after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114:615–27. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Adler T, Rzanny R, van Schayck R, Gaser C, Weiss T, et al. Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neurosci Lett. 2001;307:109–12. doi: 10.1016/s0304-3940(01)01953-x. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. 2nd edn. New York: Springer Wien; 1999. The human brain: surface, blood supply and three-dimensional sectional anatomy. [Google Scholar]
- Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes and tongue activates corresponding body-part-specific motor representations. J Neurophysiol. 2003;90:3304–16. doi: 10.1152/jn.01113.2002. [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Jensen TS. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–81. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, Adler T, Kiebel S, Rijntjes M, et al. Structural and functional cortical abnormalities after upper limb amputation in childhood. NeuroReport. 2001;12:957–62. doi: 10.1097/00001756-200104170-00019. [DOI] [PubMed] [Google Scholar]
- Harris JA. Cortical origin of pathological pain. Lancet. 1999;354:1464–6. doi: 10.1016/S0140-6736(99)05003-5. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Conscious thought as simulation of behaviour. Trends Cogn Sci. 2002;6:242–7. doi: 10.1016/s1364-6613(02)01913-7. [DOI] [PubMed] [Google Scholar]
- Huse E, Larbig W, Flor H, Birbaumer N. The effect of opioids on phantom limb pain and cortical reorganisation. Pain. 2001;90:47–55. doi: 10.1016/s0304-3959(00)00385-7. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995;33:1419–32. doi: 10.1016/0028-3932(95)00073-c. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8:163–88. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganisation of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;21:3609–18. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehab. 2006;87:S20–9. doi: 10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- Kelly C, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kooijman CM, Dijkstra PU, Geertzen JHB, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87:33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 2000;11:473–81. doi: 10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain: an fMRI study in upper limb amputees. Brain. 2001;124:2268–77. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. Does use of a myoelectric prosthesis prevent cortical reorganisation and phantom limb pain? Nat Neurosci. 1999;2:501–2. doi: 10.1038/9145. [DOI] [PubMed] [Google Scholar]
- Mackert B-M, Sappok T, Grusser S, Flor H, Curio G. The eloquence of silent cortex. NeuroReport. 2003;14:409–12. doi: 10.1097/00001756-200303030-00022. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Cortical reorganisation during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- Mercier C, Reilly KT, Vargas CD, Aballea A, Sirigu A. Mapping phantom movement representations in the motor cortex of amputees. Brain. 2006;129:2202–10. doi: 10.1093/brain/awl180. [DOI] [PubMed] [Google Scholar]
- Moseley GL. Graded motor imagery for pathologic pain: a randomised controlled trial. Neurology. 2006;67:2129–34. doi: 10.1212/01.wnl.0000249112.56935.32. [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivi M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pelled G, Chuang K-H, Dodd SJ, Koretsky AP. Functional MRI detection of bilateral cortical reorganisation in the rodent brain following peripheral nerve deafferentation. NeuroImage. 2007;37:262–73. doi: 10.1016/j.neuroimage.2007.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Convers P, Barral F-G, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–46. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cettolo V, Francescato MP, Baraldi P. Ipsilateral involvement of primary motor cortex during motor imagery. Eur J Neurosci. 2000;12:3059–63. doi: 10.1046/j.1460-9568.2000.00182.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb Lecture. Brain. 1998;121:1603–30. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Mercier C, Schieber MH, Sirigu A. Persistent commands in the amputee's brain. Brain. 2006;129:2211–23. doi: 10.1093/brain/awl154. [DOI] [PubMed] [Google Scholar]
- Roricht S, Meyer B-U, Niehaus L, Brandt SA. Long-term reorganisation of motor cortex outputs after arm amputation. Neurology. 1999;53:106–11. doi: 10.1212/wnl.53.1.106. [DOI] [PubMed] [Google Scholar]
- Roux F-E, Ibarrola D, Lazorthes Y, Berry I. Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case report. Neurosurgery. 2001;48:681–8. doi: 10.1097/00006123-200103000-00050. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Pleger B, Cornelius B, Weyen U, Dertwinkel R, Zenz M, et al. Reorganisation in the ipsilateral motor cortex of patients with lower limb amputation. Neurosci Lett. 2003;349:187–90. doi: 10.1016/s0304-3940(03)00838-3. [DOI] [PubMed] [Google Scholar]
- Sinis N, Birbaumer N, Gustin S, Schwarz A, Berdanger S, Becker ST, et al. Memantine treatment of complex regional pain syndrome. Clin J Pain. 2007;23:237–43. doi: 10.1097/AJP.0b013e31802f67a7. [DOI] [PubMed] [Google Scholar]
- Smith SM, Brady JM. SUSAN - a new approach to low level image processing. Int J Comp Vis. 1997;23:45–78. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stippich C, Ochmann H, Sartor K. Somatotopic mapping of the human primary sensorimotor cortex during motor imagery and execution by functional magnetic resonance imaging. Neurosci Lett. 2002;331:50–4. doi: 10.1016/s0304-3940(02)00826-1. [DOI] [PubMed] [Google Scholar]
- Weiss T, Miltner WHR, Adler T, Bruckner L, Taub E. Decrease in phantom limb pain associated with prosthesis-induced increased use of an amputation stump in humans. Neurosci Lett. 1999;272:131–4. doi: 10.1016/s0304-3940(99)00595-9. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckman CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich M, Brady M, Smith SM. In Seventh International Conference on Functional Mapping of the Human Brain. 2001. Hierarchical fully Bayesian spatio-temporal analysis of FMRI data. [Google Scholar]
- Wu CW-H, Kaas JH. Reorganisation in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci. 1999;19:7679–97. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid H, Alkhadi H, Schmidt D, Peraud A, Buettner A, et al. Localisation of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–57. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.