Abstract
OBJECTIVE—High serum uric acid levels lead to gout and have been reported to be associated with an increased risk of hypertension, obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Recently, the putative fructose transporter SLC2A9 was reported to influence uric acid levels. The aim of the present study was to examine the association of four single nucleotide polymorphisms within this gene with uric acid levels and to determine whether this association is modified by obesity.
RESEARCH DESIGN AND METHODS—Four single nucleotide polymorphisms within SLC2A9 (rs6855911, rs7442295, rs6449213, and rs12510549) were genotyped in the population-based prospective Bruneck Study (n = 800) and in a case-control study from Utah including 1,038 subjects recruited for severe obesity and 831 control subjects.
RESULTS—We observed highly significant associations between all four polymorphisms and uric acid levels in all study groups. Each copy of the minor allele decreased age- and sex-adjusted uric acid levels by 0.30–0.35 mg/dl on average, which translates to a relative decrease of 5–6% with P values ranging from 10−9 to 10−11 in the combined analysis. An extended adjustment for BMI, creatinine, gout medication, and alcohol intake improved P values to a range of 10−14 to 10−20. The association was more pronounced in women and the population-based Bruneck Study and was significantly modified by BMI, with stronger effect sizes in individuals with high BMI.
CONCLUSIONS—Genetic variants within SLC2A9 have significant effects on uric acid levels and are modified by sex and BMI.
In humans, uric acid is the end product of purine metabolism, and hyperuricemia might be caused by an overproduction of or by disturbances in the elimination of uric acid. Although some experimental evidence supports the idea of a beneficial role of uric acid because of its strong antioxidative properties (1), a number of epidemiological studies reported an association between high uric acid levels and cardiovascular disease, hypertension, kidney disease, metabolic syndrome, and even total mortality (2,3). Uric acid levels are, furthermore, positively associated with serum glucose in healthy subjects (4), and subjects with higher uric acid levels have a higher risk of developing type 2 diabetes or metabolic syndrome (5). However, the mechanisms underlying this association are still unclear.
The regulation of uric acid levels is under strong genetic control, with heritability estimates ranging from 25 to70% (6,7). The elucidation of the genetic contributors to uric acid levels as an intermediate phenotype for various diseases might shed some light on the pathogenesis of these complex phenotypes and might help identify new targets for treating undesirably high uric acid levels.
Recent genome-wide association studies identified a strong association of uric acid levels with genetic variants within SLC2A9 (8–11), a gene located in the chromosomal region 4p16.1 encoding a putative fructose transporter. There is strong evidence from both animal models and human studies supporting fructose as a highly lipogenic nutrient that contributes to tissue insulin insensitivity, metabolic disturbances, and the development of a prediabetic state when consumed in high quantities (12).
In the present study we aimed to replicate the recently discovered association between genetic variation within the SLC2A9 gene and uric acid concentrations in the population-based Bruneck Study. This analysis was extended by a large case-control study of severely obese individuals from Utah who showed increased uric acid levels compared with those in control subjects to explore whether the genetic association is modified by obesity.
RESEARCH DESIGN AND METHODS
Bruneck Study
The Bruneck Study is a prospective population-based survey designed to investigate the epidemiology and pathogenesis of atherosclerosis (13). Briefly, the study population was recruited as a sex- and age-stratified random sample of all inhabitants of Bruneck, Italy (125 women and 125 men in each decade of age from the fifth to the eighth, n = 1,000). At the 1990 baseline, 93.6% of recruited subjects participated, with data assessment completed in 919 subjects. Follow-up examinations were performed in 1995, 2000, and 2005. Detailed information on prevalent and incident metabolic syndrome components, diabetes, and cardiovascular events is available from all examinations. The present analysis focuses on the 1995 reexamination and on the follow-up period for clinical events between 1995 and 2005. In 1995, the study population consisted of 826 subjects (96.5% of those alive). Sufficient DNA was available for 800 participants.
Obesity case-control study from Utah
The study included 1,869 individuals from two groups of subjects gathered in the state of Utah. The study population was composed of 1,038 subjects recruited for severe obesity (“severe obesity group” with a BMI between 33 and 92 kg/m2) and a general population sample of 831 individuals of the same ethnicity (“control subjects”). The two groups of subjects were described in detail elsewhere (14). In brief, the 1,038 subjects with severe obesity were either seeking gastric bypass surgery or were randomly chosen from a population-based sample of severely obese participants. The examination of patients undergoing gastric bypass surgery was done before the intervention. The control group consisted of 831 individuals from the same geographical region and was found to be representative of the Utah population, spanning the entire BMI range.
Metabolic syndrome and type 2 diabetes
Prevalent type 2 diabetes was considered to be present if a prior physician diagnosis had been made, if the fasting blood glucose upon screening was ≥126 mg/dl, or if insulin-sensitizing agents or diabetes medications were being taken by the individual. Metabolic syndrome was defined according to the scientific statement from the American Heart Association and the National Heart, Lung, and Blood Institute (15). The incidence of type 2 diabetes and metabolic syndrome was assessed in the Bruneck Study from the 1995 examination to the 2005 examination.
Laboratory methods
In both study populations, blood samples were collected after an overnight fasting period. Uric acid levels were measured using enzymatic-colorimetric methods (Bruneck Study: Merck, Vienna, Austria; Utah study: Roche, Indianapolis, IN) with intra-assay and interassay coefficients of variation <2%. Other clinical chemical parameters were measured as described recently (14).
Four single nucleotide polymorphisms (SNPs) with genome-wide P values <10−7 in the data from Li et al. (8) and Döring et al. (10) were selected for genotyping using a 5′ nuclease allelic discrimination (TaqMan) assay (Applied Biosystems, Foster City, CA): rs6855911, rs7442295, rs6449213, and rs12510549. Genotyping was done within the Genotyping Unit of the Gene Discovery Core Facility at Innsbruck Medical University (Innsbruck, Austria).
Statistical and bioinformatic analysis
To compare characteristics between individual groups, we applied t tests, Wilcoxon tests, and Pearson χ2 tests. Spearman correlation coefficients were used to describe the correlation between uric acid levels and components of the metabolic syndrome. A χ2 test for violation of the Hardy-Weinberg equilibrium was performed. General linear regression models were used to estimate the association of any of the four SNPs with uric acid levels adjusted for various covariates, assuming an additive model. The additive model was applied because of a priori evidence from previous studies in various populations (8,10,11) and from the inspection of the genotype-specific means of uric acid levels. Interactions between the SNPs investigated and BMI on uric acid levels were tested by adding an interaction term (SNP × BMI) and the main covariates (sex, SNP, and BMI) to the model. The effect modification was graphically illustrated for three BMI classes (<30, 30–40, and >40 kg/m2) and the three genotype levels using interaction plots applying PROC GENMOD. All statistical analyses were performed with SPSS (version 15.0; SPSS, Chicago, IL) or SAS (version 9.1; SAS Institute, Cary, NC).
Because all polymorphisms that we investigated were located in noncoding regions, their possible effect on transcription factor binding sites was evaluated in silico using different components of the Genomatix Software Suite (Genomatix, Munich, Germany). Analyses included a search for the presence of known functional genetic elements (e.g., promoter sequences or microRNAs) using Eldorado (release 4.5), a search for unknown promoter-specific sequences using Promoter Inspector (release 4.6), and an investigation of possible effects of the polymorphisms on single transcription factor binding sites using SNPInspector (release 4.6).
RESULTS
Baseline clinical characteristics and laboratory data of the study groups from Bruneck and Utah are reported in Table 1. Analysis of the Utah group was also stratified by case-control status (severe obesity versus control).
Table 1.
Clinical and laboratory data for participants in the Bruneck and Utah study groups with stratification in patients with severe obesity and control subjects
| Bruneck | Utah
|
||
|---|---|---|---|
| Control | Severe obesity | ||
| n | 800 | 831 | 1,038 |
| Age (years) | 62.7 ± 11.1 | 52.8 ± 8.5 | 44.4 ± 11.4* |
| Sex: male/female | 398/402 (49.8/50.2) | 403/428 (48.5/51.5) | 193/845 (18.6/81.4)* |
| rs12510549: TT/TC/CC | 506/243/35 (64.5/31.0/4.5) | 519/265/33 (63.5/32.4/4.1) | 633/335/43 (62.6/33.1/4.3) |
| MAF (%) | 20.0 | 20.3 | 20.8 |
| rs6449213: TT/TC/CC | 517/237/30 (65.9/30.2/3.8) | 539/259/20 (65.9/31.7/2.4) | 648/319/40 (64.3/31.7/4.0) |
| MAF (%) | 18.9 | 18.3 | 19.8 |
| rs6855911: AA/AG/GG | 462/283/43 (58.6/35.9/5.5) | 471/304/43 (57.6/37.2/5.2) | 526/412/69 (52.2/40.9/6.9) |
| MAF (%) | 23.4 | 23.8 | 27.3 |
| rs7442295: AA/AG/GG | 494/257/34 (62.9/32.7/4.3) | 501/287/28 (61.4/35.2/3.4) | 595/366/49 (58.9/36.2/4.9) |
| MAF (%) | 20.7 | 21.0 | 23.0 |
| Uric acid (mg/dl) | 4.7 ± 1.3 | 5.5 ± 1.5 | 6.3 ± 1.5* |
| BMI (kg/m2) | 25.6 ± 3.8 | 27.6 ± 4.9 | 46.0 ± 7.6* |
| Creatinine (mg/dl) | 0.94 ± 0.19 | 0.92 ± 0.31 | 0.81 ± 0.22* |
| Total cholesterol (mg/dl) | 230.0 ± 42.6 | 187.1 ± 34.0 | 186.8 ± 36.2 |
| LDL cholesterol (mg/dl) | 145.5 ± 37.9 | 105.1 ± 27.7 | 108.2 ± 27.6† |
| HDL cholesterol (mg/dl) | 58.7 ± 16.2 | 50.1 ± 15.0 | 45.9 ± 11.0* |
| Triglycerides (mg/dl) (25th, 50th, 75th percentile) | 131.7 ± 71.9 (81, 111, 158) | 156 ± 105 (86, 121, 169) | 186 ± 106 (164, 119, 223)* |
| Systolic blood pressure (mmHg) | 148.3 ± 20.7 | 121.5 ± 16.7 | 127.3 ± 18.5* |
| Diastolic blood pressure (mmHg) | 87.1 ± 9.2 | 73.0 ± 10.5 | 71.9 ± 10.8† |
| Glucose (mg/dl) | 102 ± 24 | 91 ± 18 | 104 ± 33* |
| Metabolic syndrome‡ | 261 (32.6) | 267 (32.1) | 766 (73.8)* |
| Metabolic factors, median§ | 2.0 (2) | 2.0 (2) | 3.0 (2)* |
| Diabetes | 76 (9.5) | 55 (6.6) | 225 (21.7)* |
| Use of gout medication | 13 (1.6) | 7 (0.8) | 11 (1.1)† |
| Use of lipid-lowering drugs | 25 (3.1) | 64 (7.7) | 122 (11.9)* |
Data are means ± SD or n (%) unless otherwise indicated.
P < 0.001;
P < 0.05 for comparison between severely obese subjects and control subjects from Utah.
Definition according to the scientific statement from the American Heart Association and the National Heart, Lung, and Blood Institute. Three of the following five parameters had to be present: elevated waist circumference (≥102 cm in men and ≥88 cm in women); elevated triglycerides (≥150 mg/dl [1.7 mmol/l]) or receiving drug treatment for elevated triglycerides; reduced HDL cholesterol (<40 mg/dl [1.03 mmol/l] in men and <50 mg/dl [1.3 mmol/l] in women) or receiving drug treatment for reduced HDL cholesterol; hypertension (≥130 mmHg systolic blood pressure or ≥85 mmHg diastolic blood pressure) or receiving antihypertensive drug treatment in a patient with a history of hypertension; or elevated fasting glucose (≥100 mg/dl) or receiving drug treatment for elevated glucose.
Metabolic factors: average number of factors considered in the definition of metabolic syndrome (see preceding footnote), measured as median (interquartile range). MAF, minor allele frequency.
All four SNPs were found to be in Hardy-Weinberg equilibrium in all groups analyzed (P > 0.1), and the genotyping efficiency ranged between 97 and 98.5%. We observed no difference in genotype frequencies between the Bruneck and Utah groups (P > 0.1).
Association of genetic variation within SLC2A9 and uric acid levels
Age- and sex-adjusted linear regression models revealed a strong and highly significant association of each of the four SNPs with uric acid levels (Table 2) in all study groups, which was strongest in the population-based Bruneck Study. This association can clearly be described by an additive model: each copy of the minor allele lowered uric acid levels by 0.30–0.35 mg/dl on average, representing a relative decrease of 5–6%, with P values ranging from 10−9 to 10−11 in the combined analysis.
Table 2.
Association between uric acid levels and SNPs in SLC2A9 in the Bruneck and Utah study groups
| SNP | Study | Men and women
|
Men
|
Women
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uric acid (mg/dl)*
|
β† | P† | β* | P* | ||||||||
| AA | Aa | aa | β‡ | P‡ | β‡ | P‡ | ||||||
| rs12510549 | Bruneck | 4.86 ± 0.047 | 4.43 ± 0.068 | 4.21 ± 0.180 | −0.319 | 1.86 × 10−5 | −0.381 | 1.51 × 10−8 | −0.428 | 2.93 × 10−5 | −0.352 | 4.74 × 10−5 |
| Utah: entire group§ | 6.20 ± 0.044 | 5.93 ± 0.061 | 5.23 ± 0.179 | −0.300 | 1.31 × 10−7 | −0.350 | 2.79 × 10−8 | −0.225 | 0.068 | −0.397 | 5.81 × 10−8 | |
| Control | 5.52 ± 0.068 | 5.11 ± 0.096 | 4.79 ± 0.330 | −0.263 | 9.47 × 10−4 | −0.402 | 9.91 × 10−5 | −0.212 | 0.186 | −0.611 | 2.44 × 10−5 | |
| Obese | 6.43 ± 0.054 | 6.23 ± 0.075 | 5.45 ± 0.209 | −0.326 | 4.39 × 10−5 | −0.324 | 2.09 × 10−5 | −0.196 | 0.278 | −0.397 | 2.74 × 10−5 | |
| Combined§ | 5.71 ± 0.033 | 5.39 ± 0.047 | 4.87 ± 0.132 | −0.306 | 3.67 × 10−9 | −0.357 | 5.80 × 10−14 | −0.309 | 9.78 × 10−5 | −0.392 | 3.21 × 10−11 | |
| rs6449213 | Bruneck | 4.86 ± 0.046 | 4.44 ± 0.068 | 3.99 ± 0.192 | −0.403 | 8.60 × 10−8 | −0.427 | 3.54 × 10−10 | −0.384 | 1.79 × 10−4 | −0.492 | 2.00 × 10−8 |
| Utah: entire group§ | 6.21 ± 0.043 | 5.87 ± 0.062 | 5.07 ± 0.191 | −0.335 | 1.40 × 10−8 | −0.423 | 5.58 × 10−11 | −0.311 | 0.015 | −0.459 | 9.25 × 10−10 | |
| Control | 5.55 ± 0.066 | 5.00 ± 0.097 | 4.57 ± 0.422 | −0.281 | 9.55 × 10−4 | −0.537 | 8.34 × 10−7 | −0.431 | 0.014 | −0.688 | 2.04 × 10−8 | |
| Obese | 6.45 ± 0.054 | 6.19 ± 0.076 | 5.30 ± 0.216 | −0.373 | 4.12 × 10−6 | −0.384 | 6.74 × 10−7 | −0.205 | 0.258 | −0.459 | 9.44 × 10−7 | |
| Combined§ | 5.72 ± 0.032 | 5.35 ± 0.047 | 4.68 ± 0.141 | −0.357 | 2.33 × 10−11 | −0.428 | 1.13 × 10−18 | −0.326 | 5.18 × 10−5 | −0.489 | 5.84 × 10−16 | |
| rs6855911 | Bruneck | 4.90 ± 0.048 | 4.49 ± 0.062 | 3.99 ± 0.161 | −0.383 | 5.50 × 10−8 | −0.432 | 1.13 × 10−11 | −0.411 | 1.64 × 10−5 | −0.479 | 8.00 × 10−9 |
| Utah: entire group§ | 6.26 ± 0.048 | 5.93 ± 0.056 | 5.30 ± 0.141 | −0.340 | 1.84 × 10−10 | −0.407 | 4.08 × 10−12 | −0.289 | 0.012 | −0.445 | 6.05 × 10−11 | |
| Control | 5.56 ± 0.072 | 5.11 ± 0.089 | 4.85 ± 0.253 | −0.280 | 2.11 × 10−4 | −0.411 | 1.54 × 10−5 | −0.303 | 0.047 | −0.548 | 1.95 × 10−6 | |
| Obese | 6.51 ± 0.060 | 6.22 ± 0.068 | 5.51 ± 0.166 | −0.389 | 1.62 × 10−7 | −0.401 | 1.70 × 10−8 | −0.262 | 0.118 | −0.445 | 4.90 × 10−8 | |
| Combined§ | 5.76 ± 0.035 | 5.40 ± 0.043 | 4.81 ± 0.109 | −0.319 | 5.74 × 10−11 | −0.413 | 1.76 × 10−20 | −0.332 | 7.86 × 10−6 | −0.463 | 6.39 × 10−17 | |
| rs7442295 | Bruneck | 4.88 ± 0.047 | 4.45 ± 0.066 | 4.04 ± 0.182 | −0.382 | 2.45 × 10−7 | −0.424 | 2.34 × 10−10 | −0.366 | 3.36 × 10−4 | −0.503 | 4.00 × 10−9 |
| Utah: entire group§ | 6.23 ± 0.046 | 5.90 ± 0.059 | 5.28 ± 0.172 | −0.301 | 1.15 × 10−7 | −0.385 | 6.48 × 10−10 | −0.264 | 0.029 | −0.428 | 3.59 × 10−9 | |
| Control | 5.56 ± 0.069 | 5.04 ± 0.092 | 4.82 ± 0.346 | −0.260 | 1.27 × 10−3 | −0.482 | 3.23 × 10−6 | −0.371 | 0.023 | −0.674 | 1.44 × 10−7 | |
| Obese | 6.46 ± 0.057 | 6.22 ± 0.072 | 5.50 ± 0.198 | −0.335 | 2.08 × 10−5 | −0.349 | 3.33 × 10−6 | −0.148 | 0.402 | −0.428 | 3.03 × 10−6 | |
| Combined§ | 5.74 ± 0.034 | 5.37 ± 0.045 | 4.82 ± 0.129 | −0.308 | 2.61 × 10−9 | −0.401 | 1.76 × 10−17 | −0.299 | 1.52 × 10−4 | −0.465 | 2.01 × 10−15 | |
Data are means ± SEM.
Adjusted for age, sex, BMI, creatinine, alcohol consumption, and gout medication.
Adjusted for age and sex.
Adjusted for age, BMI, creatinine, alcohol consumption, and gout medication.
Additionally adjusted for group status (severely obese versus control subjects).
Sex-specific associations between SLC2A9 and uric acid levels
A sex-stratified analysis revealed much stronger associations between the four SNPs and uric acid levels in women than in men (Table 2). This observation was most pronounced in the population-based Bruneck Study, in which the effect estimates for each minor allele were up to twice as high in women than in men. Considering the fact that women have on average 20% lower uric acid levels than men, the effect differences get even more meaningful.
Interaction with BMI
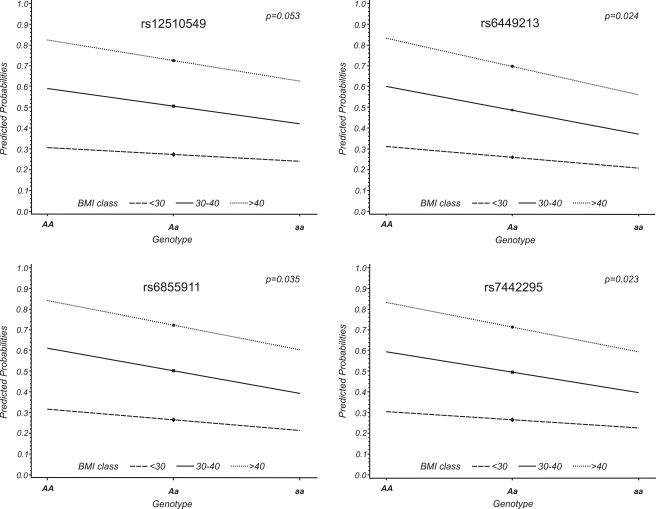
The associations of the four SNPs with uric acid levels became stronger after additional adjustments for BMI, creatinine, gout medication, and alcohol intake, with P values ranging from 10−14 to 10−20 (Table 2). BMI was the variable that most contributed to the change in P values. Thus, we investigated whether BMI was an effect modifier of the SNP–uric acid association by introducing an interaction term BMI × SNP in addition to BMI and SNP to the model. This analysis was done by combining all three groups, but with adjustments for age, sex, and population (Bruneck versus Utah). The interaction was significant for three of the four SNPs (P = 0.023–0.035) and borderline significant for rs12510549 (P = 0.053). Figure 1 graphically illustrates these interactions for BMI groups <30, 30–40, and >40 kg/m2.
Figure 1.
Interaction plots describing the effect modification of BMI on the association of the four investigated SLC2A9 SNPs with uric acid levels. The x-axis displays the allelic status of the respective SNP; the y-axis corresponds to the predicted probability that an individual with the respective genotype exceeds the sex-specific median of uric acid levels given the specific BMI class. The quantitative interactions are represented as lines, with statistically significant differing slopes in all but one SNP (rs12510549). AA, Aa, and aa denote the three possible genotypes: wild-type, heterozygote carriers, and homozygotes for the minor allele, respectively.
Association with metabolic syndrome and type 2 diabetes
We observed significant correlations between uric acid levels and each singular metabolic syndrome component, as well as with the sum of the components (Table 3). These correlations were seen in all three groups of subjects. Age- and sex-adjusted uric acid levels were significantly higher in subjects with metabolic syndrome compared with those without. With the exception of the severe obesity group, uric acid levels were also significantly higher in subjects with type 2 diabetes compared with those without (Table 3). Uric acid levels were significantly associated with the development of either metabolic syndrome and/or type 2 diabetes in the Bruneck Study from the 1995 examination to the 2005 examination. However, no association between the four SNPs and prevalent or incident metabolic syndrome or type 2 diabetes could be established.
Table 3.
Correlation of uric acid levels with parameters of the metabolic syndrome and type 2 diabetes
| Bruneck | Utah
|
||
|---|---|---|---|
| Control | Severe obesity | ||
| n | 800 | 831 | 1,038 |
| Correlation coefficients between uric acid and | |||
| BMI | 0.29* | 0.44* | 0.17* |
| Glucose | 0.28* | 0.31* | 0.09† |
| Systolic blood pressure | 0.15* | 0.25* | 0.08‡ |
| Diastolic blood pressure | 0.11† | 0.29* | 0.01 |
| HDL cholesterol | −0.29* | −0.44* | −0.20* |
| Triglycerides | 0.38* | 0.33* | 0.14* |
| Metabolic syndrome components | 0.31* | 0.39* | 0.18* |
| Uric acid concentrations in subjects | |||
| With and without metabolic syndrome | 5.3 ± 1.4 vs. 4.3 ± 1.2* | 6.2 ± 1.4 vs. 5.2 ± 1.4* | 6.4 ± 1.5 vs. 6.0 ± 1.5* |
| With and without type 2 diabetes | 5.0 ± 1.5 vs. 4.7 ± 1.3‡ | 6.1 ± 1.4 vs. 5.5 ± 1.5† | 6.3 ± 1.7 vs. 6.3 ± 1.5 |
Data are correlation coefficients and means ± SD.
P < 0.001;
P < 0.01;
P < 0.05.
Bioinformatics
Putative transcription factor binding sites were predicted for three of the four polymorphisms (five for rs12510549, two for rs6449213, and one for rs7442295), although no biologically obvious candidates could be found. The predicted candidates belonged mainly to pathways not directly connected with the phenotypes investigated, such as cellular growth (E2F) or immune response (PAX5, BCL6, NFAT, and NR2F) (data not shown).
For the polymorphism rs12510549, the generation or disruption of five different putative binding sites was predicted. Among these, a putative binding site for NFAT factors was recognized. NFAT factors have been implicated in various roles during development and adaptation of several mammalian cell types outside the immune system (16).
CONCLUSIONS
We observed a strong sex-specific association between genetic variation within the SLC2A9 gene and uric acid concentrations. The finding was most pronounced in the population-based Bruneck Study and was replicated in severely obese and control individuals from Utah. This association was modified by BMI such that increasing BMI amplified effects of genetic variants on uric acid levels.
SLC2A9 was recently identified by four independent genome-wide association studies to be strongly associated with uric acid levels (8–11). SLC2A9 encodes a putative hexose transporter whose probable substrate is fructose (17). Fructose intake has been described as an important contributor to uric acid levels and gout (18,19), as the ADP generated during the phosphorylation of fructose is used for rapid production of uric acid (20). Epidemiological data showed that increased total fructose intake correlated with increasing incidence of obesity, metabolic syndrome (21), and gout (19). Over the past decades, a general increase in uric acid levels was observed, and it was hypothesized that fructose-induced hyperuricemia might be in part responsible for the rise in metabolic syndrome (12,22,23). The detection of genes that determine uric acid levels by influencing fructose metabolism would therefore be of interest. The actual mechanism of how genetic variation within SLC2A9 modulates uric acid levels has not been fully elucidated. One possibility would be an influence on the hepatic uptake of fructose and production of uric acid. On the other hand, SLC2A9 variants were associated with low fractional excretion of uric acid in various population samples, and experiments in Xenopus laevis oocytes showed that SLC2A9 has not only fructose but also strong uric acid transport activity (11).
It is important to note that we did not find an association between the genetic variants within the SLC2A9 gene and prevalent or incident metabolic syndrome or type 2 diabetes. This finding is intriguing given the pronounced association between the genetic variants and uric acid levels and the strong association of uric acid levels and these diseases in our study and in earlier studies. This may by explained, on the one hand, by lack of power: the variance in serum uric acid levels related to the genotypes investigated in population-based studies was about 1.2% in men and 6% in women (10), and the fraction of these two diseases explained by uric acid levels was also small. On the other hand, the possibility that uric acid level is a surrogate marker of the disease without being in the causal pathway cannot be ruled out. However, recent studies in rats showed that fructose-induced metabolic syndrome is partially prevented by lowering uric acid levels and that the reduction in endothelial nitric oxide bioavailability caused by uric acid may be a mechanism for insulin resistance and hypertension (23). A proof of a causal association of uric acid with disease end points might be possible by the application of a Mendelian randomization approach. However, this proof will probably require examination of several thousand individuals. Homozygotes of the wild type and of the rare allele differ in uric acid levels by 0.64–0.81 mg/dl, which corresponds to 11–13% of the mean levels. Based on the findings in earlier studies, such a difference in uric acid levels would change the rate of cardiovascular events by 1%.
A recent study with a systematic investigation of sex-specific differences of literature-reported genetic effects on various phenotypes documented that only one of 432 sex difference claims was consistently replicated in at least two other studies (24). The association of genetic variants within SCL2A9 with uric acid levels clearly adds to this list, as much stronger associations were found in women than in men in five population samples in previous studies (10,11) and in three population samples in this study. How obesity modulates the association between SLC2A9 variants and uric acid levels remains to be determined. It could be related to the higher fructose intake in obese subjects (22) and a different saturation capacity of fructose transport depending on the genotype.
In summary, our study shows a strong association of genetic variants within the SLC2A9 gene and uric acid levels that is modified by sex and BMI.
Acknowledgments
This research was funded by grants from the “Genomics of Lipid-associated Disorders–GOLD” of the Austrian Genome Research Programme GEN-AU to F.K., by a grant from the German National Genome Research Net to the GSF-Institute of Epidemiology, and by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-55006) to S.C.H.
We thank Anke Gehringer and Markus Haak for excellent laboratory work.
Published ahead of print at http://care.diabetesjournals.org on 16 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ames BN, Cathcart R, Schwiers E, et al.: Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 78:6858–6862, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanellis J, Kang DH: Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 25:39–42, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Li C, Cook S, et al.: Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115:2526–2532, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Facchini F, Chen YD, Hollenbeck CB, et al.: Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 266:3008–3011, 1991 [PubMed] [Google Scholar]
- 5.Dehghan A, van Hoek M, Sijbrands EJ, et al.: High serum uric acid as a novel risk factor for type 2 diabetes mellitus. Diabetes Care 2007 [DOI] [PubMed]
- 6.Wilk JB, Djousse L, Borecki I, et al.: Segregation analysis of serum uric acid in the NHLBI Family Heart Study. Hum Genet 106:355–359, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Guo CY, Cupples LA, et al.: Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism 54:1435–1441, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Li S, Sanna S, Maschio A, et al.: The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3:e194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace C, Newhouse SJ, Braund P, et al.: Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 82:139–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döring A, Gieger C, Mehta D, et al.: SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40:430–436, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Vitart V, Rudan I, Hayward C, et al.: SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40:437–442, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Miller A, Adeli K: Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol 24:204–209, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kiechl S, Willeit J, Mayr M, et al.: Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity and 10-year cardiovascular outcomes: prospective results from the Bruneck Study. Arterioscler Thromb Vasc Biol 27:1788–1795, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Schoenborn V, Heid IM, Vollmert C, et al.: The ATGL gene is associated with free fatty acids, triglycerides and type 2 diabetes. Diabetes 55:1270–1275, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, et al.: Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112:2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Horsley V, Pavlath GK: NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol 156:771–774, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joost HG, Thorens B: The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 18:247–256, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Stirpe F, Della CE, Bonetti E, et al.: Fructose-induced hyperuricaemia. Lancet 2:1310–1311, 1970 [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Curhan G: Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 336:309–312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallfrisch J: Metabolic effects of dietary fructose. FASEB J 4:2652–2660, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Segal MS, Sautin Y, et al.: Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86:899–906, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Bray GA, Nielsen SJ, Popkin BM: Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Hu H, Zharikov S, et al.: A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290:F625–F631, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Patsopoulos NA, Tatsioni A, Ioannidis JP: Claims of sex differences: an empirical assessment in genetic associations. JAMA 298:880–893, 2007 [DOI] [PubMed] [Google Scholar]