Abstract
OBJECTIVE—To determine whether development of insulin requirement in patients with latent autoimmune diabetes in adults (LADA) is accompanied with the emergence of a type 1 diabetes–like autoimmune response.
RESEARCH DESIGN AND METHODS—We correlated β-cell–specific autoimmunity reflected in autoantibodies to the 65-kDa isoform of GAD (GAD65) with insulin requirement. We determined GAD65Ab epitope specificities in type 1 diabetic patients, LADA patients without insulin requirement (nonprogressed), and LADA patients that had developed insulin requirement (progressed).
RESULTS—Recognition of a type 1 diabetes–specific GAD65Ab epitope was more pronounced in type 1 diabetic patients than in nonprogressed (P < 0.001) or progressed (P < 0.01) LADA patients, with no significant differences between the two LADA cohorts. These differences were particularly pronounced in samples with GAD65Ab titers <1,000 units/ml, with no differences in epitope specificities in samples with higher GAD65Ab titers. Disease duration (initial diabetes diagnosis until sample collection or development of insulin requirement) in nonprogressed and progressed LADA patients, respectively, was not correlated with epitope specificity, suggesting lack of epitope maturation. This was supported by epitope analyses of longitudinal samples from LADA patients during progression to insulin requirement.
CONCLUSIONS—First, the GAD65Ab-specific autoimmune reaction in type 1 diabetic patients with low and moderate GAD65Ab titers differs from that in LADA patients, irrespective of insulin requirement. Second, the GAD65Ab-specific autoimmune response in LADA patients does not change after their initial diabetes diagnosis. Finally, LADA patients with high GAD65Ab titers resemble type 1 diabetic patients in their GAD65Ab epitope specificity.
Latent autoimmune diabetes in adults (LADA) consists of a subgroup (∼10%) of adult patients initially diagnosed with type 2 diabetes, who show signs of β-cell autoimmunity and eventually develop insulin requirement (1,2). Signs of β-cell autoimmunity, such as the well-characterized insulin autoantibodies, glutamate decarboxylase (GAD65), and the tyrosine phosphatase–like protein insulinoma-associated protein-2, indicate significant damage of the β-cells and subsequent development of insulin requirement in these patients (1). While autoantibodies to insulin and insulinoma-associated protein-2 antibody (Ab) are inversely correlated with age at onset, GAD65Ab shows no, and in some studies even a positive, correlation with age at onset and is therefore a particularly attractive marker for autoimmune diabetes in the adult population (3,4). Moreover, GAD65Ab can be detected years after the clinical onset of the disease, indicating that these autoantibodies may be permanent markers for the autoimmune response (5,6).
Notably, not all LADA patients progress to insulin requirement, raising the possibility that the autoimmune response in these patients resembles that in autoantibody-positive healthy individuals, with no significant risk for development of insulin requirement (7,8). A better understanding of the autoimmune response is necessary to predict insulin requirement in LADA patients, which is important to prevent escalation of blood glucose levels and subsequent complications.
In previous studies, we have investigated the humoral immune response toward GAD65 as a reflection of islet cell destruction (9). It remains unclear whether the autoimmune response in LADA patients and type 1 diabetic patients differs or whether only the duration of the prodomal period distinguishes between the two groups (10). Therefore, we compared the GAD65-specific humoral autoimmune response in type 1 diabetic patients with that in LADA patients who had or had not progressed to insulin requirement.
RESEARCH DESIGN AND METHODS
Patients and sera
Sera of GAD65Ab-positive type 1 diabetic patients were collected at the Saitama Social Insurance Hospital, Urawa City, Japan (n = 119). All type 1 diabetic patients required insulin treatment at the time of diabetes diagnosis. Sera were collected between 1989 and 2005 and were taken at various times after onset of disease (0–27 years of disease duration [median 1 year]).
Patients classified as LADA patients were admitted to the Saitama Social Insurance Hospital, Urawa City, Japan. Diagnosis of LADA was made according to the commission of Immunology of Diabetes Society (2) (patients were diagnosed with type 2 diabetes and tested positive for GAD65Ab with an onset age ≥30 years). None of these patients required isulin treatment within the first 6 months after the initial diagnosis.
We differentiated two groups of LADA patients, based on their insulin requirements. Nonprogressed LADA patients (n = 56) did not require insulin treatment for over 5 years after diagnosis with type 2 diabetes. Six of these samples were collected at Keio University. Some of the samples were taken earlier (see Table 1); however, all patients were followed to ensure that they did not require insulin treatment for over 5 years past type 2 diabetes diagnosis. Progressed LADA patients (n = 58) developed insulin requirement after the initial LADA classification and had low fasting serum C-peptide levels (≤0.4 ng/ml). Insulin treatment was started at an A1C of ≥8% despite usage of the maximum dose of glibenclamide (5 mg) and observation of a strict diet.
Table 1.
Patient characteristics
| Age at onset (years) | GAD65Ab titer (World Health Organization units/ml) | Duration since diabetes onset (years) | Duration until insulin requirement (years) | n | |
|---|---|---|---|---|---|
| Type 1 diabetes | 26 (6–84) | 489 (52–4,668) | 1 (0–12) | NA | 114 (61 female) |
| Nonprogressed LADA | 49 (32–78) | 176 (54–129,334) | 7 (0.5–27) | NA | 56 (35 female) |
| Progressed LADA | 45 (30–67) | 657 (55–150,990) | 7 (0.5–26) | 2 (0–18) | 58 (27 female) |
Data are median (range), unless otherwise indicated. NA, not applicable.
Longitudinal samples were obtained from nine individuals (five male subjects, median age 34 years) who were classified as LADA patients and developed insulin requirement during follow-up. Local institutional ethics committee approval was obtained before collection of all serum samples. Informed consent was obtained from all patients or their legal guardians. The age at onset of diabetes (type 1 or type 2), GAD65Ab titer, duration of diabetes, requirement of insulin, and other clinical relevant information are summarized in Table 1.
GAD65Ab titer determination
GAD65Ab positivity of the serum samples was initially evaluated using a commercial radioimmunoprecipitation assay (Cosmic, Tokyo, Japan) and the manufacturer's suggested cutoff level of 1.5 units/ml. GAD65Ab positivity was confirmed using a radioligand binding assay (RBA) (described below). The World Health Organization standard for GAD65Ab (11) and negative samples were included in every assay to correct for interassay variation and to express immunoglobulin binding levels as units per milliliter (units/ml). Cutoff levels for positivity (34 units/ml) were calculated as the 98th percentile from a healthy control group (n = 50). Samples with a GAD65Ab >1,000 units/ml in the initial screen were diluted to determine their half-maximal binding concentration. Subsequent epitope mapping experiments were carried out at this half-maximal binding concentration. In the Diabetes Antibody Standardization Program 2005 workshop, the GAD65Ab analysis ranked at 80% sensitivity and 91% specificity.
Recombinant Fab used in this study
Monoclonal antibodies used in this study were previously described (12). Recombinant Fabs (rFabs) were produced in Escherichia coli 25F2 cells as previously described (9). Briefly, DPA and DPD were derived from a type 1 diabetic patient and recognize epitopes located at amino acids 483–499 plus 556–586 and 96–173, respectively. Monoclonal antibody b96.11, derived from a patient with autoimmune polyendocrine syndrome type 2, recognizes a conformational epitope involving amino acids located in both the middle and the C-terminus of the molecule (13). Monoclonal antibody MICA-3, isolated from a patient with type 1 diabetes, recognizes an epitope located at amino acid residues 451–585.
Epitope-specific RBA
Recombinant human [35S]-GAD65 was produced in an in vitro–coupled transcription/translation system with SP6 RNA polymerase and nuclease-treated rabbit reticulocyte lysate (Promega, Madison, WI) as described previously (14). The in vitro–translated [35S]-antigen was kept at −70°C and used within 2 weeks.
The capacity of the rFab to inhibit GAD65 binding by human serum GAD65Ab was tested in a competitive epitope-specific RBA using protein A Sepharose (Zymed Laboratories) as described (9). The rFab were added at a concentration sufficient to compete binding of the originating intact mAb to GAD65 by at least 80% (0.7–1 μg/ml). The background competition for each rFab was established in competition experiments with normal control sera. The background was subtracted before calculation of percent binding. The cutoff for specific competition was determined as >10% by using a negative control rFab D1.3 (a kind gift from Dr. J. Foote, Arrowsmith Technologies, Seattle), specific to an irrelevant target, hen-egg lysozyme, at 5 μg/ml.
Statistical analyses
Binding of GAD65Ab to GAD65 in the presence of rFab was expressed as follows: counts per minute of [35]S-GAD65 bound in the presence of rFab/counts per minute of [35S]-GAD65 bound in the absence of rFab × 100.
All samples were analyzed in triplicate determinations, and the intra-assay average coefficient of variation was 5% (range 13–0.04). Median ages, GAD65Ab titers, and competition levels between groups were analyzed using the nonparametric ANOVA (Kruskal-Wallis) followed by Dunn's multiple comparisons test. Competition levels within each group were tested for significance using the nonparametric Wilcoxon matched-pair test. A P value <0.05 was considered significant.
RESULTS
Autoantibody status and clinical parameters
The type 1 diabetic cohort had a significantly lower median age compared with the nonprogressed and progressed LADA patients (P < 0.0001) (Table 1). No significant difference between the median ages of the nonprogressed LADA patients and the progressed LADA patients was observed. We emphasize that samples from progressed LADA patients were taken after they developed insulin requirement. No significant differences in GAD65Ab levels between the three groups were observed.
GAD65Ab response in relation to insulin requirement
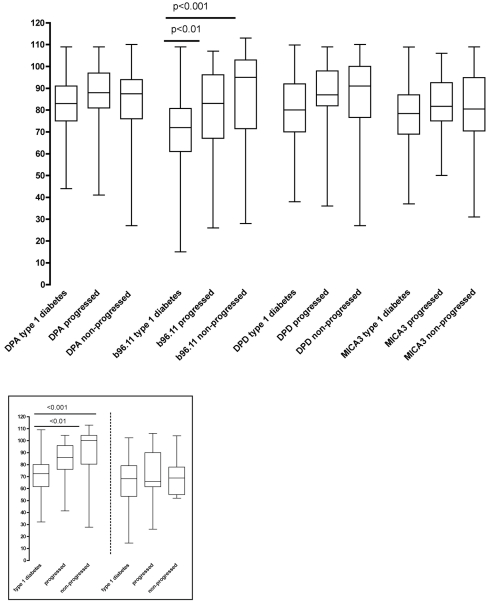
All serum samples were analyzed for their binding to GAD65 in the presence of GAD65-specific rFab DPA, b96.11, DPD, and MICA-3 (Fig. 1). We observed significant reduction in median binding to GAD65 in the presence of rFab DPA, DPD, b96.11, and MICA-3 in all groups. No correlation between GAD65Ab titer and reduction of binding conferred by any of the rFab was observed in the type 1 diabetic and progressed LADA patients. In the nonprogressed LADA patients, we observed a significant correlation of GAD65Ab titer and reduction of binding conferred by rFab b96.11 (P = 0.005) and DPD (P = 0.004) (data not shown). No correlation between GAD65Ab epitope specificity and sex or age was observed in any of the groups.
Figure 1.
GAD65Ab epitope specificities in patients with different forms of autoimmune diabetes. Sera of type 1 diabetic patients, progressed LADA, and nonprogressed LADA patients were analyzed for their capacity of binding to GAD65 in the presence of the indicated rFab. Results are presented as binding to GAD65 in the presence of rFab related to uncompeted binding (set at 100%). Median, interquartile range, and upper and lower extremes are shown. P values are indicated. Inset: GAD65Ab epitope specificities in LADA patients with high GAD65Ab titers resemble those in type 1 diabetic patients. Serum samples of type 1 diabetic, progressed LADA, and nonprogressed LADA patients with GAD65Ab titers >1,000 units/ml (left panel) and GAD65Ab titers <1,000 units/ml (right panel) were analyzed for their binding to GAD65 in the presence of rFab b96.11. Results are presented as binding to GAD65 in the presence of rFab related to uncompeted binding (set as 100%). Median, interquartile range, and upper and lower extremes are shown. P values are indicated.
To determine whether the epitope recognition differed between the groups, we compared the differences in reduction in median binding to GAD65 conferred by the different rFab (Fig. 1). We found that the reduction in binding conferred by rFab b96.11 was significantly more pronounced in type 1 diabetic patients as compared with progressed and nonprogressed LADA patients (P < 0.01 and P < 0.001, respectively).
GAD65Ab response in relation to GAD65Ab titer
Based on our above findings of a correlation between GAD65Ab titer and epitope recognition in the nonprogressed LADA patients and our previous observation that high GAD65Ab titers predict progression to insulin requirement (15), we divided the analysis between samples with GAD65Ab titers > and <1,000 units/ml (Fig. 1, inset). We found that sera exhibiting high GAD65Ab titer samples in both LADA groups showed strong inhibition of GAD65 binding by rFab b96.11,which was similar to that observed in sera obtained from type 1 diabetic patients. For both insulin-requiring patient groups (type 1 diabetes and progressed LADA), no significant differences in inhibition levels observed in the presence of rFab b96.11 were observed when comparing sera with high and low GAD65Ab titers. However, in nonprogressed LADA patients, binding levels in the presence of rFab b96.11 in sera with high GAD65Ab titers were significantly lower compared with sera with low GAD65Ab titers (P < 0.001). Consequently, samples with GAD65Ab titers below the 1,000 units/ml cutoff showed significant differences in the GAD65 binding in the presence of rFab b96.11 between type 1 diabetic patients and progressed (P < 0.01) and nonprogressed LADA patients (P < 0.001).
GAD65Ab response in relation to disease duration
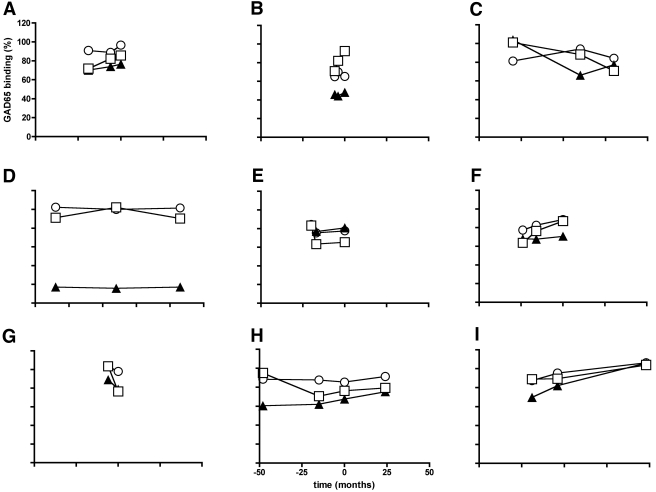
We tested whether the GAD65Ab epitope specificities may change longitudinally toward progression to insulin requirement. Therefore, we correlated epitope specificities with disease duration (initial diabetes diagnosis) in nonprogressed LADA patients and time from initial diabetes diagnosis to insulin requirement in progressed LADA patients. No correlation with GAD65Ab titer or GAD65Ab epitope specificity was observed, indicating no longitudinal changes over time. Longitudinal samples obtained from LADA patients (n = 9) during their progression to insulin requirement were analyzed for their epitope specificities (Fig. 2). While some patients showed longitudinal changes over time (Fig. 2A–C), no overall trend in the change of epitope specificities was obvious.
Figure 2.
No overall longitudinal trend in GAD65Ab epitope specificities suggests lack of epitope maturation. GAD65Ab epitope specificity was analyzed in longitudinal samples from LADA patients who progressed to insulin requirement in the follow-up period. Longitudinal samples obtained from LADA patients (n = 9) were analyzed for their GAD65Ab epitope specificities. GAD65 binding in the presence of rFab DPA (○), b96.11 (▴), and MICA-3 (□) is reported in relation to uncompeted binding (set as 100%). The zero time point indicates time of insulin requirement, negative values refer to time before insulin requirement, and positive values to samples obtained after initiation of insulin requirement.
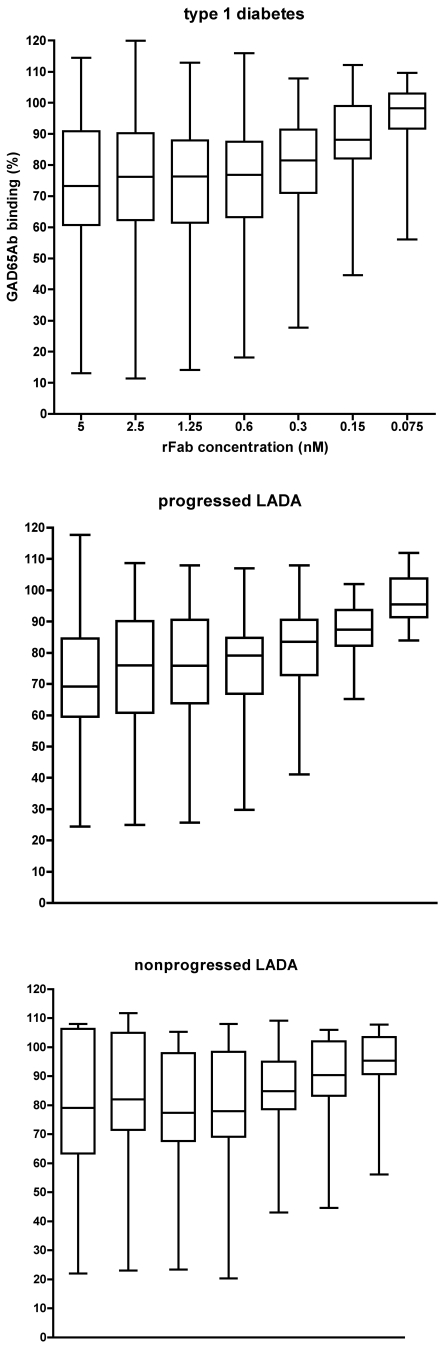
rFab concentration needed for maximal inhibition is identical in the three groups
Disparities in inhibition levels of GAD65 binding exerted by a rFab could be caused by different affinities, or differences in binding specificities. Therefore we established the rFab b96.11 concentration necessary to achieve maximal inhibition in all three groups (Fig. 3). The median rFab concentration to reach 50% inhibition (EC50) was 0.39 nmol/l for all three groups. Serum samples whose GAD65 binding was not inhibited by rFab concentrations of 2.5 nmol/l, were also not inhibited at 12.5 nmol/l rFab. This confirms that the assay conditions are optimal, as the rFab concentration used (12 nmol/l) exceeded the rFab concentration necessary to achieve maximal competition. These results also suggest that the observed differences between the groups were not caused by different binding capacities to the GAD65Ab epitope defined by b96.11.
Figure 3.
Binding capacity of the three patient cohorts to the b96.11-defined epitope. Binding to GAD65 by serum samples of type 1 diabetic, progressed LADA, and nonprogressed LADA patients in the presence of the indicated concentrations of rFab b96.11 was analyzed. Results are presented as binding to GAD65 in the presence of rFab related to uncompeted binding (set as 100%). Median, interquartile range, and upper and lower extremes are shown.
CONCLUSIONS
Our results confirmed previous observations of different GAD65-specific humoral immune responses in type 1 diabetic patients and nonprogressed LADA patients (9,16). Progressed LADA patients exhibited a GAD65Ab epitope pattern intermediate between type 1 diabetic patients and nonprogressed LADA patients. This may indicate that the GAD65Ab response matures in patients as they progress toward insulin requirement. We tested this possibility by analyzing longitudinal samples obtained from a small group of LADA patients during their development of insulin requirement. While some of the patients showed changes in their epitope binding specificities, no overall trend was observed.
In the progressed LADA patients the disease duration before insulin requirement varies from 0.5 to 27 years. We analyzed whether LADA patients who progressed faster to disease showed different GAD65Ab epitope specificities from patients that progressed slower. However, no correlation between GAD65Ab epitope specificities and length of the prodomal period was observed. These findings together with our earlier observations of longitudinal changes in GAD65Ab epitope specificities in healthy adult individuals during their progression to type 2 diabetes (17) lead to our hypothesis that the autoimmune response in LADA patients remains constant after type 2 diabetes onset.
Some of the nonprogressed LADA patients showed a very long disease duration without developing insulin requirement (up to 27 years since initial diabetes diagnosis). While the presence of GAD65Ab in LADA patients is considered as a risk factor for subsequent insulin requirement (1), one autoantibody alone confers only a low risk for progression in the general population (7). One could therefore assume that some LADA patients show signs of β-cell autoimmunity but are unlikely to develop insulin requirement. To test this hypothesis, we analyzed GAD65Ab epitope specificities in correlation with disease duration. However, no correlation between disease duration and epitope specificity was observed. These data are in agreement with the longitudinal study of LADA patients in the U.K. Prospective Diabetes Study 77, reporting stagnant GAD65Ab epitope reactivities (18).
The observed differences in GAD65Ab epitope specificities were particularly pronounced in the samples with medium to low GAD65Ab titers, while high GAD65Ab titer samples in the three groups recognized the type 1 diabetes–associated b96.11 epitope to similar degrees. This may indicate that the type 1 diabetes–associated autoimmune response is more emphasized in LADA patients with high GAD65Ab titers. While these unexpected findings need to be confirmed in a larger study cohort, previous studies (15,19) report that LADA patients with high GAD65Ab titers progress to insulin requirement more often than LADA patients with low GAD65Ab titers. Moreover, a recent study (20) reported that LADA patients with high GAD65Ab titers resemble type 1 diabetic patients with respect to clinical characteristics, genetic susceptibility, and other autoimmune components. However, no correlation between GAD65Ab titer and aggressiveness of β-cell autoimmunity was found in the recent U.K. Prospective Diabetes Study 77 (18). These differences may be caused by different distribution of GAD65Ab titers, as our LADA cohorts included serum samples with very high GAD65Ab titers, while the sera in the U.K. Prospective Diabetes Study 77 cohort showed more moderate GAD65Ab titers.
The observed differences in GAD65Ab epitope specificities between high and low GAD65Ab titer samples within the nonprogressed LADA patients suggest a heterogeneous autoimmune response in this group. The disease progression in high titer LADA patients with type 1 diabetes–like GAD65Ab epitope specificity needs to be analyzed in future studies to address this hypothesis.
We conclude that the autoimmune responses in LADA and type 1 diabetic patients show different GAD65-specific immune responses, particularly in the samples with moderate GAD65Ab titers. The particular GAD65Ab characteristics remain stable and do not mature during progression to insulin requirement, which may suggest a distinct autoimmune response in the pathogenesis for LADA patients.
Acknowledgments
The study was performed as independent research sponsored by National Institutes of Health Grant DK53456, as well as DK53004, DK26190 (to Å.L.), and DK17047.
Published ahead of print at http://care.diabetesjournals.org on 16 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Turner R, Stratton I, Horton V, et al.: UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 350:1288–1293, 1997. [erratum in Lancet 351:376, 1998] [DOI] [PubMed] [Google Scholar]
- 2.Fourlanos S, Dotta F, Greenbaum CJ, et al.: Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 48:2206–2212, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Graham J, Hagopian WA, Kockum I, et al.: Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 51:1346–1355, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Falorni A, Brozzetti A: Diabetes-related antibodies in adult diabetic patients. Best Pract Res Clin Endocrinol Metab 19:119–133, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Borg H, Gottsater A, Fernlund P, et al.: A 12-year prospective study of the relationship between islet antibodies and β-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 51:1754–1762, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Decochez K, Tits J, Coolens JL, Van Gaal L, et al.: High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age: the Belgian Diabetes Registry. Diabetes Care 23:838–844, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bingley PJ, Bonifacio E, Williams AJK, et al.: Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 46:1701–1710, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Verge CF, Gianani R, Kawasaki E, et al.: Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45:926–933, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Padoa CJ, Banga JP, Madec AM, et al.: Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes 52:2689–2695, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Gale EA: Latent autoimmune diabetes in adults: a guide for the perplexed. Diabetologia 48:2195–2199, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mire-Sluis A, Gaines Das R, Lernmark A: Standardization of antibody preparations for use in immunogenicity studies: a case study using the World Health Organization International Collaborative Study for Islet Cell Antibodies. Dev Biol (Basel) 112:153–163, 2003 [PubMed] [Google Scholar]
- 12.Maruyama T, Oak S, Hall TR, et al.: Autoantibody epitopes to the smaller isoform of glutamate decarboxylase do not differ in Swedish and Japanese type 1 diabetes patients and may be associated with high-risk human leucocyte antigen class II alleles. Clin Exp Immunol 150:416–421, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenalti G, Hampe CS, Arafat Y, et al.: C-terminal clustering of autoantibody and T-cell determinants on the structure of GAD65 provide insights into the molecular basis of autoreactivity. Diabetes 57:1293–301, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Grubin C, Daniels T, Karlsen AE, et al.: The cDNA-directed, in vitro-synthesized nascent peptide of glutamic acid decarboxylase (GAD2) is the autoantigen in insulin-dependent diabetes (Abstract). Clin Res 40:299A, 1992 [Google Scholar]
- 15.Kasuga A, Maruyama T, Nakamoto S, et al.: High-titer autoantibodies against glutamic acid decarboxylase plus autoantibodies against insulin and IA-2 predicts insulin requirement in adult diabetic patients. J Autoimmun 12:131–135, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Falorni A, Calcinaro F: Autoantibody profile and epitope mapping in latent autoimmune diabetes in adults. Ann N Y Acad Sci 958:99–106, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hampe CS, Hall TR, Agren A, et al.: Longitudinal changes in epitope recognition of autoantibodies against glutamate decarboxylase 65 (GAD65Ab) in prediabetic adults developing diabetes. Clin Exp Immunol 148:72–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai M, Cull CA, Horton VA, et al.: GAD autoantibodies and epitope reactivities persist after diagnosis in latent autoimmune diabetes in adults but do not predict disease progression: UKPDS 77. Diabetologia 50:2052–2060, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lohmann T, Kellner K, Verlohren HJ, et al.: Titre and combination of ICA and autoantibodies to glutamic acid decarboxylase discriminate two clinically distinct types of latent autoimmune diabetes in adults (LADA). Diabetologia 44:1005–1010, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Buzzetti R, Di Pietro S, Giaccari A, et al.: High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 30:932–938, 2007 [DOI] [PubMed] [Google Scholar]