Abstract
OBJECTIVE—In patients with type 2 diabetes, left ventricular hypertrophy (LVH) predicts cardiovascular events, and the prevention of LVH is cardioprotective. We sought to compare the effect of ACE versus non-ACE inhibitor therapy on incident electrocardiographic (ECG) evidence of LVH (ECG-LVH).
RESEARCH DESIGN AND METHODS—This prespecified study compared the incidence of ECG-LVH by Sokolow-Lyon and Cornell voltage criteria in 816 hypertensive type 2 diabetic patients of the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT), who had no ECG-LVH at baseline and were randomly assigned to at least 3 years of blinded ACE inhibition with trandolapril (2 mg/day) or to non-ACE inhibitor therapy. Treatment was titrated to systolic/diastolic blood pressure <130/80 mmHg. ECG readings were centralized and blinded to treatment.
RESULTS—Baseline characteristics of the two groups were similar. Over a median (interquartile range) follow-up of 36 (24–48) months, 13 of the 423 patients (3.1%) receiving trandolapril compared with 31 of the 376 patients (8.2%) receiving non-ACE inhibitor therapy developed ECG-LVH (hazard ratio [HR] 0.34 [95% CI 0.18–0.65], P = 0.0012 unadjusted, and 0.35 [0.18–0.68], P = 0.0018 adjusted for predefined baseline covariates). The HR was significant even after adjustment for follow-up blood pressure and blood pressure reduction versus baseline. Compared with baseline, both Sokolow-Lyon and Cornell voltages significantly decreased with trandolapril but did not change with non-ACE inhibitor therapy.
CONCLUSIONS—ACE inhibition has a specific protective effect against the development of ECG-LVH that is additional to its blood pressure–lowering effect. Because ECG-LVH is a strong cardiovascular risk factor in people with hypertension and diabetes, early ACE inhibition may be cardioprotective in this population.
Left ventricular hypertrophy (LVH), a cardinal manifestation of preclinical cardiovascular disease, strongly predicts myocardial infarction, stroke, and cardiovascular death in patients with hypertension (1) or coronary artery disease (2), as well as in the general population (3). In the Framingham Study, electrocardiographic (ECG) evidence of LVH (ECG-LVH) was associated with a twofold increase in mortality over that resulting from hypertension alone (4).
Studies have consistently shown that antihypertensive therapy may effectively limit the incidence of ECG-LVH, regardless of the treatments used to reduce blood pressure (5). However, the Heart Outcomes Prevention Education (HOPE) (6) and the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trials (7) showed that, in patients with ECG-LVH at inclusion, the ACE inhibitor ramipril and the angiotensin receptor blocker (ARB) losartan, respectively, regressed LVH more effectively than drugs that do not directly interfere with the renin-angiotensin-aldosterone system (RAAS). The finding in both trials that this benefit was significant even after adjustments for the small differences in blood pressure between the two treatment groups provided consistent evidence that RAAS inhibitor therapy has a specific cardioprotective effect that exceeds expectations based on changes in blood pressure alone. However, the HOPE trial (6) was not powered to assess the treatment effect on new-onset LVH in the subgroup with no ECG-LVH at baseline, and the LIFE trial (7) included only patients with LVH. Thus, whether RAAS inhibitor therapy may also prevent new-onset LVH in subjects with normal left ventricular mass to start with is unknown. To formally explore this issue, we compared the effect of ACE versus non-ACE inhibitor therapy on incident ECG-LVH in patients from the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) (8–11) who had no ECG-LVH at inclusion.
RESEARCH DESIGN AND METHODS
BENEDICT (8) was a prospective, randomized, double-blind, parallel group study that evaluated the possibility of preventing the onset of persistent microalbuminuria in 1,204 patients with type 2 diabetes (World Health Organization criteria) and arterial hypertension (systolic or diastolic blood pressure >130 or 85 mmHg or concomitant antihypertensive therapy) but normal urinary albumin excretion rate (<20 μg/min in at least two of three consecutive overnight urine collections) who were randomly assigned to at least 3 years of treatment with one of the following study drugs: 1) a nondihydropyridine calcium channel blocker (240 mg/day verapamil SR), 2) an ACE inhibitor (2 mg/day trandolapril), 3) a fixed-dose combination (180 mg/day verapamil SR plus 2 mg/day trandolapril [VeraTran]), and 4) placebo. The target blood pressure after random assignment and throughout the whole study period was <130/80 mmHg for all of the treatment groups. Other antihypertensive drugs (with the exception of RAAS inhibitors and nondihydropyridine calcium channel blockers different from the study drugs) could be used to achieve and maintain the target blood pressure according to predefined guidelines (8). The study protocol was in accordance with the Declaration of Helsinki and was approved by the institutional review board at each center and by the safety committee of BENEDICT. All patients gave written informed consent. Patients were eligible to enter the ECG-LVH substudy if they had no ECG evidence of LVH at baseline and had at least 1 year of follow-up.
Aims
The primary aim of analyses was to compare the incidence of ECG-LVH in patients randomly assigned to ACE inhibitor or non-ACE inhibitor therapy who had no ECG evidence of LVH at baseline. Secondarily, we evaluated the relationships between incidence of ECG-LVH and baseline and follow-up variables, including treatable risk factors such as blood pressure and A1C.
ECG-LVH and other outcome variables
The main outcome variable was ECG-LVH defined as Sokolow-Lyon (SV1 + RV5/6) voltage ≥3.5 mV (12) and/or Cornell (RaVL + SV3) voltage ≥2.0 mV (women) or ≥2.4 mV (men) (13). Secondary outcomes were Sokolow-Lyon and Cornell voltages considered as continuous variables. Standard 12-lead ECGs were recorded at 25 mm/s and 1 mV/cm calibration at baseline and every year thereafter. They were centrally and independently evaluated by two investigators who were blinded to treatment allocation and patient data. ECGs with inconsistent readings were evaluated by a third independent cardiologist, and his diagnosis was considered for data analysis. Trough systolic and diastolic (Korotkoff phase I/V) blood pressure was measured in the morning before treatment administration (8).
Data were reported in dedicated case report forms and doubly entered in an ad hoc database that was eventually merged with the BENEDICT database. Before analyses, all data were monitored by the Monitoring Unit of the Clinical Research Center for Rare Diseases “Aldo & Cele Daccò” of the Mario Negri Institute for Pharmacological Research.
Sample size
We assumed a 10% incidence of ECG-LVH in the non-ACE inhibitor group and a 60% reduction (from 10 to 4%) in the ACE inhibitor group. The expected incidence of ECG-LVH was assumed to be higher than that in the HOPE control subjects (6) because, different from the HOPE trial, all BENEDICT patients were hypertensive and diabetic at inclusion (8), and they also had additional risk factors such as older age, systolic hypertension, obesity, and, conceivably, insulin resistance. The 60% risk reduction was assumed on the basis of experimental evidence that ACE inhibition fully prevents LVH if treatment is started before the induction of arterial hypertension (14). Because our patients were already hypertensive at study entry and had other risk factors, we considered a conservative assumption of 60% risk reduction as appropriate. Thus, we calculated that 400 patients per group gave the study a 90% power to detect as statistically significant (α = 0.05, two-tailed test) the expected between-group difference in incidence of ECG-LVH.
Statistical analyses
The analyses were performed by the Laboratory of Biostatistics of the Clinical Research Center. Between-group comparisons were performed on continuous variables by unpaired t test or Wilcoxon's rank-sum test and on categorical variables by a χ2 test or Fisher's exact test. Within-group comparisons were performed on continuous variables by paired t test or Wilcoxon's signed-rank test and on categorical variables by the McNemar test. Predefined (8) baseline covariates were considered: site, age, sex, smoking status (patients who had never smoked versus former smokers and current smokers), diastolic blood pressure, and log-transformed urinary albumin excretion (median of three readings) at baseline.
The main study results were reported by a Cox regression model. For graphic representation, Kaplan-Meier curves were plotted for each group considered. Exploratory analyses were also conducted, including follow-up systolic and diastolic blood pressure measurement and absolute reductions from baseline for follow up of blood pressure measurement to help data interpretation. All statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC). P < 0.05 was considered statistically significant. No P value adjustment was carried out for multiple comparisons.
RESULTS
Of 905 patients with readable ECG at baseline and at least 1 year of follow-up, 816 (433 receiving ACE and 383 receiving non-ACE inhibitor therapy) had no ECG evidence of LVH. Of these, 799 patients (423 receiving ACE and 376 receiving non-ACE inhibitor therapy) were available for analyses (see study profile in Online Appendix 2 [available at http://dx.doi.org/10.2337/dc08-0371]). Baseline characteristics and Sokolow-Lyon and Cornell voltages were similar between treatment groups with the exception of the percentage of never smokers and of serum cholesterol levels that were higher in the non-ACE inhibitor group (Table 1).
Table 1.
Baseline characteristics of hypertensive patients with type 2 diabetes and normal urinary albumin excretion in the study group as a whole and according to ACE inhibitor therapy (yes or no)
| Overall | ACE inhibitor yes | ACE inhibitor no | |
|---|---|---|---|
| n | 799 | 423 | 376 |
| Age (years) | 61.6 ± 8.0 | 61.5 ± 7.8 | 61.7 ± 8.2 |
| Male sex | 443 (55.4) | 238 (56.3) | 205 (54.5) |
| BMI (kg/m2) | 29.1 ± 4.8 | 29.2 ± 5.1 | 29.1 ± 4.6 |
| Known duration of diabetes (years) | 7.5 ± 6.5 | 7.5 ± 6.6 | 7.6 ± 6.3 |
| Smokers | |||
| Never | 435 (54.4) | 213 (50.4) | 222 (59.0)* |
| Former | 257 (32.2) | 147 (34.7) | 110 (29.3) |
| Current | 107 (13.4) | 63 (14.9) | 44 (11.7) |
| A1C (%)† | 5.8 ± 1.4 | 5.7 ± 1.4 | 5.7 ± 1.3 |
| Glucose (mg/dl) | 160.4 ± 45.7 | 159.1 ± 45.6 | 161.9 ± 45.9 |
| Trough blood pressure (mmHg) | |||
| Systolic | 150.4 ± 14.0 | 150.0 ± 14.0 | 150.9 ± 14.0 |
| Diastolic | 87.6 ± 7.5 | 87.5 ± 8.0 | 87.8 ± 7.0 |
| Mean arterial pressure | 108.6 ± 8.3 | 108.3 ± 8.5 | 108.9 ± 8.0 |
| Urinary albumin excretion (μg/min) | 5.2 (3.6–8.9) | 5.2 (3.6–9.3) | 5.2 (3.6–8.8) |
| Serum creatinine (μmol/l) | 79.6 ± 17.7 | 79.6 ± 17.7 | 79.6 ± 17.7 |
| Triglycerides (mmol/l) | 1.65 ± 0.91 | 1.63 ± 0.83 | 1.66 ± 1.00 |
| Cholesterol (mmol/l) | 5.41 ± 0.93 | 5.33 ± 0.93 | 5.50 ± 0.93* |
| Sokolow-Lyon voltage (mV) | 19.2 ± 5.8 | 19.2 ± 5.9 | 19.2 ± 5.7 |
| Cornell voltage (mV) | 13.4 ± 4.2 | 13.2 ± 4.2 | 13.6 ± 4.2 |
Data are means ± SD, n (%), or median (interquartile range).
P < 0.05 versus ACE inhibitor yes.
A1C was measured by ion-exchange high-performance liquid chromatography (normal range 3.5–5.2%).
Incidence of ECG-LVH
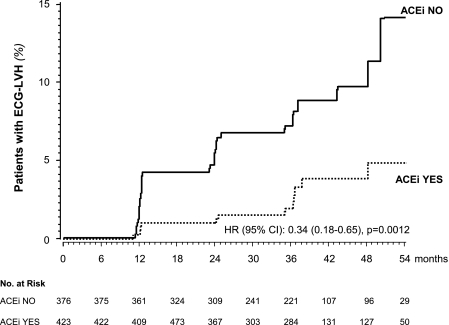
Over a median (interquartile range) of 36 (24–48) months of follow-up, LVH developed in 44 patients (5.5%), 13 (3.1%) receiving ACE and 31 (8.2%) receiving non-ACE inhibitor therapy (Fig. 1). The unadjusted hazard ratio (HR) [95% CI] for ECG-LVH was 0.34 [0.18–0.65] (P = 0.0012). The HR (0.35 [0.18–0.68]) was statistically significant even after adjustment for predefined baseline characteristics (P = 0.0018) and baseline and follow-up systolic and diastolic blood pressure, as well as systolic and diastolic blood pressure reduction versus baseline (Table 2).
Figure 1.
Kaplan-Meier curves for the percentages of subjects receiving ACE inhibitor therapy with trandolapril (ACEi YES) or receiving non-ACE inhibitor therapy (ACEi NO) who developed ECG-LVH. The difference in ECG-LVH adjusted for prespecified baseline covariates was significant (P = 0.0018).
Table 2.
HR (95% CI) of the incidence of ECG-LVH in patients randomly assigned to ACE inhibitor therapy compared with patients randomly assigned to non-ACE inhibitor therapy
| HR (95% CI) | P value | |
|---|---|---|
| Unadjusted | 0.34 (0.18–0.65) | 0.0012 |
| Adjusted | ||
| Baseline predefined | 0.35 (0.18–0.68) | 0.0018 |
| Baseline SBP | 0.36 (0.18–0.68) | 0.0019 |
| Baseline DBP | 0.36 (0.19–0.69) | 0.0020 |
| Follow-up SBP | 0.38 (0.20–0.73) | 0.0036 |
| Follow-up DBP | 0.34 (0.18–0.67) | 0.0016 |
| SBP reduction | 0.35 (0.18–0.68) | 0.0018 |
| DBP reduction | 0.34 (0.18–0.66) | 0.0013 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Compared with baseline, both Sokolow-Lyon and Cornell voltages significantly decreased at different years on follow-up in the study group as a whole and in the subgroup receiving ACE inhibitor therapy (Table 3). In the non-ACE inhibitor group, changes in Sokolow-Lyon voltage were not significant, and changes in Cornell voltage achieved statistical significance only at 2 and 3 years (Table 3).
Table 3.
Changes in Sokolow-Lyon and Cornell voltages at different years versus baseline in the study group as a whole (overall) and according to ACE or non-ACE inhibitor therapy
| 1 year | 2 years | 3 years | 4 years | |
|---|---|---|---|---|
| Solokow-Lyon (mV) | ||||
| Overall | −0.5 ± 0.1* | −1.1 ± 0.2† | −1.2 ± 0.2† | −1.3 ± 0.3 |
| ACE inhibitor | −0.6 ± 0.2* | −1.4 ± 0.2‡ | −1.4 ± 0.2† | −1.7 ± 0.4 |
| non-ACE inhibitor | −0.3 ± 0.2 | −0.7 ± 0.2 | −1.0 ± 0.3 | −0.8 ± 0.4 |
| Cornell (mV) | ||||
| Overall | −0.4 ± 0.1* | −0.5 ± 0.2‡ | −0.4 ± 0.1‡ | −0.3 ± 0.2‡ |
| ACE inhibitor | −0.5 ± 0.1† | −0.8 ± 0.1‡ | −0.6 ± 0.2‡ | −0.4 ± 0.3‡ |
| non-ACE inhibitor | −0.2 ± 0.2 | −0.2 ± 0.3† | −0.3 ± 0.2* | −0.2 ± 0.4 |
Data are means ± SEM.
P < 0.001,
P < 0.01,
P < 0.0001 versus baseline.
Blood pressure and metabolic control
Follow-up systolic (138.4 ± 9.4 mmHg) and diastolic (80.8 ± 5.2 mmHg) blood pressure was 12.7 and 7.1 mmHg lower than at baseline, respectively (P < 0.0001 for both). Follow-up systolic (137.2 ± 9.2 vs. 139.7 ± 9.5 mmHg, respectively, P = 0.045) and diastolic (80.0 ± 5.2 vs. 81.7 ± 5.0 mmHg, P = 0.005) blood pressure was lower in the ACE than in the non-ACE inhibitor group. Follow-up A1C levels were lower in the ACE than in the non-ACE inhibitor group (5.76 ± 1.17 vs 5.91 ± 1.23%, P = 0.03), whereas blood glucose was similar in the two treatment groups (156.1 ± 36.2 vs. 161.3 ± 39.1 mg/dl, P = 0.31) (see blood pressure, A1C, and blood glucose profiles in Online Appendix 3 [available at http://dx.doi.org/10.2337/dc08-0371]).
In multivariable analyses, known diabetes duration, baseline BMI, and Sokolow-Lyon and Cornell voltages were associated with the incidence of ECG-LVH on follow-up, whereas baseline and follow-up systolic and diastolic blood pressure, A1C, and blood glucose as well as systolic and diastolic blood pressure, A1C, and blood glucose changes versus baseline had no predictive value. No significant correlation was found between changes in Sokolow-Lyon or Cornell voltages on follow-up and concomitant changes in blood pressure, A1C, and blood glucose (versus baseline).
Comparative analyses between randomization arms
LVH developed in 4 patients receiving trandolapril (1.9%), 9 receiving VeraTran (4.2%), 16 receiving verapamil (8.9%), and 15 receiving placebo (7.6%). After adjustment for predefined covariates, the risk of LVH was significantly lower with trandolapril than with verapamil (HR 0.22 [95% CI 0.07–0.65)], P = 0.007) or placebo (0.25 [0.08–0.78], P = 0.017) and with VeraTran compared with verapamil (0.42 [0.19–0.97], P = 0.0.043). Risk reduction with VeraTran compared with placebo was not significant (0.49 [0.21–1.16], P = 0.10). Risk was not significantly different between VeraTran and trandolapril (1.97 ([0.60–6.49], P = 0.26) and between verapamil and placebo (1.21 [0.58–2.52], P = 0.61).
CONCLUSIONS
In the present study, ACE inhibition with trandolapril significantly reduced the incidence of ECG-LVH in patients with arterial hypertension and type 2 diabetes compared with non-ACE inhibitor therapy. The protective effect of trandolapril against ECG-LVH was already evident at 1 year after random assignment and progressively increased on follow-up. Sokolow-Lyon and Cornell voltages consistently decreased with trandolapril therapy, whereas they did not change appreciably with non-ACE inhibitor therapy.
The reduced incidence of ECG-LVH with trandolapril was not explained by the small differences in blood pressure control between the two treatment groups, because risk reduction was highly significant even after adjustments were made for blood pressure control achieved on follow-up and for blood pressure reductions observed versus baseline. Baseline factors potentially involved in LVH development and progression, such as age, BMI, blood pressure, and Sokolow-Lyon and Cornell voltages were similar in the two groups. A low-salt diet was prescribed for all patients, and no patient performed vigorous physical activities. Altogether, the above data indicate that trandolapril had a specific protective effect against the development of LVH that was additional to the benefit of blood pressure reduction.
Both hemodynamic and nonhemodynamic factors most likely contributed to the cardioprotective effect of trandolapril therapy. ACE inhibitors increase vessel wall compliance and reduce arterial wave reflection amplitude and thus reduce aortic and left ventricular blood pressure even more consistently than peripheral artery blood pressure, that is, the brachial blood pressure as measured at the arm (15). Thus, at comparable peripheral blood pressure, ACE inhibitors may reduce central pressures and left ventricular afterload more effectively than antihypertensive drugs that do not directly interfere with the RAAS (16). These hemodynamic effects probably contributed to the regression of LVH observed with RAAS inhibitor therapy in the HOPE (6) and LIFE trials (7) and might also explain why in BENEDICT trandolapril prevented LVH more effectively than non-ACE inhibitor therapy, even at comparable brachial blood pressure (9).
ACE inhibitors may prevent LVH also through direct inhibition of cardiac RAAS. Angiotensin II promotes the growth of myocytes independently of loading conditions (17), and ACE inhibitors may prevent the hypertrophic effect of angiotensin II even at doses that do not affect the blood pressure (14). Renin expression and tissue-converting enzyme activity are increased in hypertrophied hearts of spontaneously hypertensive rats (18,19), and angiotensin II type 1 (AT1) receptors are overexpressed in cardiomyocytes of mice with cardiac hypertrophy and normal blood pressure (20). Consistently, in rats, cardiac expression of the AT1 receptor antisense transgene attenuated cardiac hypertrophy without affecting the blood pressure (21). However, recent data that cardiac AT1 receptors cannot sustain angiotensin II-dependent hypertension and cardiac hypertrophy after knocking-out renal AT1 receptors (22) suggest that kidney RAAS activation is also involved and that its inhibition may explain at least part of the cardioprotective effect of ACE inhibitors and ARBs. Unlike ARBs, ACE inhibitors may directly prevent myocardial hypertrophy also by increasing local bradykinin bioavailability through inhibition of the myocardial kallikrein-kinin pathway (23).
Vascular stiffness and RAAS activation (24) are common in patients with type 2 diabetes, and this may explain the high prevalence and severity of LVH in this population, even at “normal” blood pressure (8,9). The synergistic effect on left ventricular structure of arterial hypertension and of the above hemodynamic and metabolic abnormalities most likely explained the relatively high incidence of ECG-LVH we observed in our present control subjects receiving non-ACE inhibitor therapy. On the other hand, all of the above risk factors for LVH can be controlled by ACE inhibitor therapy. Indeed, in addition to inhibiting angiotensin II and aldosterone production, ACE inhibitors may also ameliorate arterial compliance (25). Thus, the coexistence of several abnormalities that can be ameliorated by RAAS inhibitors may also explain the remarkable protective effect of trandolapril against ECG-LVH we observed here, an effect that appears to exceed that of ramipril in the HOPE trial (6).
Post hoc analyses according to the four original randomization arms showed that trandolapril alone prevented LVH more effectively than verapamil alone or placebo, whereas trandolapril combined with verapamil was more cardioprotective than verapamil alone. Trandolapril alone tended to be more effective than trandolapril combined with verapamil, whereas the effect of verapamil was similar to that of placebo. Within the limitations of the limited power and multiple comparisons, these data show, consistent with the results of primary outcome analyses, that the ACE inhibitor is the effective component of the trandolapril-verapamil combination and that verapamil has no specific protective effect against LVH.
A possible limitation of the present study is that, as in previous large studies (4,6,7), LVH was assessed by electrocardiography. This probably resulted in reduced precision of outcome data (ECG may underestimate LVH in obese subjects) that reduced the power of the analyses but probably was not likely to introduce a systematic bias. Actually, a posterior power analyses showed that the probability of a false-positive finding was <0.2%.
In summary, hypertensive patients with type 2 diabetes receiving trandolapril had a significantly lower incidence of ECG-LVH by Sokolow-Lyon and Cornell voltage than control subjects receiving non-ACE inhibitor therapy, an effect that was highly significant even after adjustments for blood pressure control achieved in the two treatment groups throughout the study. These findings and the observation that ECG-LVH strongly predicts cardiovascular morbidity and mortality in people with hypertension and type 2 diabetes support the use of early ACE inhibitor therapy for effective prevention of left ventricular hypertrophy and, conceivably, cardiovascular morbidity and mortality (6,7) in this population.
Supplementary Material
Acknowledgments
Abbott (Ludwigshafen, Germany) partially supported the study.
We thank Maria Ganeva and Laura Gallizioli for technical support, the doctors and nurses of the BENEDICT study group for patient care, and Manuela Passera for help in preparing the manuscript.
Published ahead of print at http://care.diabetesjournals.org on 28 April 2008. Clinical trial reg. no. NCT00235014.
A complete list of the members of the BENEDICT Study Group can be found in Online Appendix 1 (available at http://dx.doi.org/10.2337/dc08-0371).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH: Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114:345–352, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS: The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med 117:831–836, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP: Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB: Prevalence and natural history of electrocardiographic left ventricular hypertrophy. Am J Med 75:4–11, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Hypertension Detection and Follow-up Program Cooperative Group: Five-year findings of the Hypertension Detection and Follow-up Program: prevention and reversal of left ventricular hypertrophy with antihypertensive drug therapy. Hypertension 7:105–112, 1985 [PubMed] [Google Scholar]
- 6.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S: Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 104:1615–1621, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Dahlof B: Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Circulation 108:684–690, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G: Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351:1941–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bella JN, Devereux RB, Roman MJ, Palmieri V, Liu JE, Paranicas M, Welty TK, Lee ET, Fabsitz RR, Howard BV: Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study). Am J Cardiol 87:1260–1265, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Dinneen SF, Gerstein HC: The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: a systematic overview of the literature. Arch Intern Med 157:1413–1418, 1997 [PubMed] [Google Scholar]
- 11.Ruggenenti P, Perna A, Ganeva M, Ene-Iordache B, Remuzzi G: Impact of blood pressure control and angiotensin-converting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: a post hoc analysis of the BENEDICT Trial. J Am Soc Nephrol 17:3472–3481, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Sokolow M, Lyon TP: The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads: 1949. Ann Noninvasive Electrocardiol 6:343–368, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G: Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 104:2039–2044, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Linz W, Schaper J, Wiemer G, Albus U, Scholkens BA: Ramipril prevents left ventricular hypertrophy with myocardial fibrosis without blood pressure reduction: a one year study in rats. Br J Pharmacol 107:970–975, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell GF, Pfeffer MA, Finn PV, Pfeffer JM: Equipotent antihypertensive agents variously affect pulsatile hemodynamics and regression of cardiac hypertrophy in spontaneously hypertensive rats. Circulation 94:2923–2929, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Topouchian J, Asmar R, Sayegh F, Rudnicki A, Benetos A, Bacri AM, Safar ME: Changes in arterial structure and function under trandolapril-verapamil combination in hypertension. Stroke 30:1056–1064, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Aceto JF, Baker KM: [Sar1]angiotensin II receptor-mediated stimulation of protein synthesis in chick heart cells. Am J Physiol 258:H806–813, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Passier RC, Smits JF, Verluyten MJ, Daemen MJ: Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am J Physiol 271:H1040–1048, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Alfonso MS, Kreutz R, Zeh K, Liu Y, Ganten D, Paul M: Differential regulation of vascular angiotensin I-converting enzyme in hypertension. Hypertension 24:280–286, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M: Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA 97:931–936, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pachori AS, Numan MT, Ferrario CM, Diz DM, Raizada MK, Katovich MJ: Blood pressure-independent attenuation of cardiac hypertrophy by AT1R-AS gene therapy. Hypertension 39:969–975, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103:17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linz W, Scholkens BA: A specific B2-bradykinin receptor antagonist HOE 140 abolishes the antihypertrophic effect of ramipril. Br J Pharmacol 105:771–772, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacchetti G, Sechi LA, Rilli S, Carey RM: The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab 16:120–126, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Safar ME, Laurent SL, Bouthier JD, London GM, Mimran AR: Effect of converting enzyme inhibitors on hypertensive large arteries in humans. J Hypertens Suppl 4:S285–S289, 1986 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.