Abstract
OBJECTIVE—The purpose of this study was to quantify the effects of glycemic index on postprandial glucose excursion (PPGE) in children with type 1 diabetes receiving multiple daily injections and to determine optimal insulin therapy for a low–glycemic index meal.
RESEARCH DESIGN AND METHODS—Twenty subjects consumed test breakfasts with equal macronutrient contents on 4 consecutive days; high–and low–glycemic index meals (glycemic index 84 vs. 48) were consumed with preprandial ultra-short-acting insulin, and the low–glycemic index meal was also consumed with preprandial regular insulin and postprandial ultra-short-acting insulin. Each child's insulin dose was standardized. Continuous glucose monitoring was used.
RESULTS—The PPGE was significantly lower for the low–glycemic index meal compared with the high–glycemic index meal at 30–180 min (P < 0.02) when preprandial ultra-short-acting insulin was administered. The maximum difference occurred at 60 min (4.2 mmol/l, P < 0.0001). Regular insulin produced a 1.1 mmol/l higher PPGE at 30 min compared with ultra-short-acting insulin (P = 0.015) when the low–glycemic index meal was consumed. Postprandial ultra-short-acting insulin produced a higher PPGE at 30 and 60 min compared with preprandial administration when the low–glycemic index meal was consumed. The maximum difference was 2.5 mmol/l at 60 min (P < 0.0001).
CONCLUSIONS—Low–glycemic index meals produce a lower PPGE than high–glycemic index meals. Preprandial ultra-short-acting insulin is the optimal therapy for a low–glycemic index meal.
The results of the Diabetes Control and Complications Trial (DCCT) established that intensive insulin therapy optimizes glycemic control and reduces the risk of long-term complications in people with type 1 diabetes (1). Subjects who matched carbohydrate amount and insulin dose demonstrated a further improvement in glycemic control (2). Consequently, carbohydrate amount is considered to be the most important dietary determinant of postprandial glucose control (3).
Published educational programs regarding intensive insulin therapy do not consider the influence of carbohydrate type on metabolic control (4–7). Glycemic index ranks carbohydrate-containing foods on the basis of their ability to raise blood glucose levels (BGLs) for a standardized amount of carbohydrate (8). Glycemic index is dependent on the chemical structure of the carbohydrate and preparation methods, which influence the speed of carbohydrate digestion and absorption.
Established dietary recommendations for children with type 1 diabetes advocate the consideration of glycemic index (9). Some evidence suggests that a low–glycemic index diet may improve the long-term glycemic control of people with type 1 diabetes (10) and that postprandial glucose excursion (PPGE) is improved when children receiving treatment with conventional insulin regimens consume low–glycemic index meals (11,12). A recent article demonstrated improved daily glycemic profiles when children receiving intensive therapy consumed low–glycemic index diets (13). However, the effect of glycemic index on the postprandial glucose response requires further exploration in children receiving intensive insulin therapy.
Newer intensive regimens using ultra-short-acting insulin analogs have been shown to improve postprandial glycemic rise (14). The potential additional benefits of low–glycemic index meals are uncertain within this context. Moreover, the time action profile of ultra-short-acting insulin may be inappropriate for the prolonged and lower glycemic rise of low–glycemic index meals.
Preprandial regular insulin, which has a more delayed onset, lower peak concentration, and longer duration of action compared with ultra-short-acting insulin (15,16), may better match the absorption profile of low–glycemic index foods. However, postprandial ultra-short-acting insulin has been demonstrated to produce higher PPGEs compared with preprandial administration (17,18), and this may be another alternative approach to optimizing insulin therapy for a low–glycemic index meal, as suggested by the British Dietetics Association in its 2005 consensus statement (19).
Therefore, the primary aim of this study was to determine the effect of altering the glycemic index of a meal on postprandial glucose control in children with type 1 diabetes receiving multiple daily injection therapy. The secondary aim was to assess whether preprandial ultra-short-acting insulin remains the optimal insulin therapy for a low–glycemic index meal.
RESEARCH DESIGN AND METHODS
Children and adolescents with type 1 diabetes diagnosed for ≥1 year and using multiple daily injection therapy (≥4 injections/day) for >6 months were recruited from the John Hunter Children's Hospital Diabetes Clinic. Eligibility criteria included age between 7 and 17 years inclusive, that subjects not be obese, and recent A1C ≤8.5% (Primus PDQ A1c Analyzer; Primus, Kansas City, MO). Exclusion criteria included complications of diabetes such as gastroparesis or coexisting medical disorders such as celiac disease. Recruitment continued until a sample size of 20 was reached.
Ethics approval was obtained by the Hunter New England Human Research Ethics Committee in December 2006. Written consent was obtained from all participants and/or their parents.
Participants and their families were contacted by telephone 2 weeks before study commencement to review BGLs and adjust insulin therapy to optimize waking BGLs (4–8 mmol/l) and minimize the risk of hypoglycemia, defined as symptomatic or BGL <3.5 mmol/l. Four test conditions were administered to each child at breakfast on 4 consecutive days in a randomized way using a permuted block method with block size of four, stratified by test condition: 1) low–glycemic index meal (glycemic index 48), preprandial ultra-short-acting insulin; 2) high–glycemic index meal (glycemic index 84), preprandial ultra-short-acting insulin; 3) low–glycemic index meal (glycemic index 48), preprandial regular insulin; and 4) low–glycemic index meal (glycemic index 48), postprandial ultra-short-acting insulin.
Because of an increased risk of sensor failure on day 4, test conditions 1 and 2 were randomly assigned on the first 2 days to ensure conservation of these results. Test conditions 3 and 4 were randomly assigned on the final 2 days.
The preprandial injection of insulin was administered immediately before meal consumption. Postprandial insulin was administered 15 min after meal commencement. A kitchen timer was supplied to each participant to ensure accuracy. All children received their normal breakfast insulin dose based on the amount of carbohydrate in the test meal. If the preprandial BGL was >16 mmol/l, the subject was instructed to add a correction dose of insulin and was asked to repeat the study.
Participants were provided with four premade test meals. Each meal was consumed at breakfast after a minimum 10-h overnight fast. Evening meals and suppers the preceding nights were standardized for carbohydrate amount and type to limit the impact of these meals on the analysis of breakfast PPGE. Participants were supplied with measuring cups to facilitate compliance with preparation of these meals.
Children were required to fast and standardize their activity during the 4-h postprandial period. A food and activity diary was kept by each subject/parent to determine adherence to this protocol.
Test meals
The low–glycemic index test meal (glycemic index 48) and the high–glycemic index test meal (glycemic index 84) consisted of a ham sandwich and a drink (Table 1). Sandwiches with a concentrated yeast extract spread (Vegemite) were offered as a vegetarian option, which two participants accepted. The carbohydrate, fat, protein, and fiber amounts were standardized for both meals (Table 1). The glycemic index of each food was obtained from published tables (20) where available and confirmed by manufacturers. The glycemic index of each meal was calculated using methods described previously (21).
Table 1.
Nutritional information for low–and high–glycemic index test meals
| Weight (g) | Energy (kcal) | Carbohydrate (g)* | Fat (g) | Protein (g) | Fiber (g) | GI | |
|---|---|---|---|---|---|---|---|
| Low-GI meal | |||||||
| Low-GI white bread (Tip Top UP EnerGI white bread) | 75 | 186.4 | 33.6 | 2.3 | 6.2 | 3 | 54 |
| Apple juice (Berri) | 250 ml | 107.1 | 26.5 | <1 | <1 | <1 | 40 |
| Low-fat ham | 26 | 26.7 | Tr (<1) | 0.8 | 4.4 | 0.0 | NA |
| Margarine | 9 | 55.7 | Tr (<1) | 6.3 | Tr (<1) | 0.0 | NA |
| Total | 375.9 | 60.1 | 9.4 | 10.6 | 3 | 48 | |
| High-GI meal | |||||||
| Regular white bread (Tip Top Sunblest white bread) | 60 | 148.1 | 27.1 | 1.4 | 5.6 | 1.8 | 71 |
| Glucose-based energy drink (Lucozade Energy) | 184 ml | 133.2 | 32.9 | 0.0 | Tr | 0.0 | 95 |
| Low-fat ham | 30 | 30.8 | Tr (<1) | 0.9 | 5.0 | 0.0 | NA |
| Margarine | 10 | 61.9 | Tr (<1) | 7.0 | Tr (<1) | 0.0 | NA |
| Total | 359.0 | 60 | 9.3 | 10.6 | 1.8 | 84 |
Available carbohydrate including sugars and starch and excluding fiber. GI, glycemic index; NA, not applicable; Tr, trace amounts.
Food was weighed using Salter kitchen scales (model 323; Salter, Kent, U.K.), which measure to ±1 g. Liquids were measured using a 250-ml measuring jug and a 10-ml disposable syringe.
All meals were supplied in an insulated bag the afternoon before study commencement. Sandwiches and drinks were individually wrapped and labeled with the day of consumption and instructions to refrigerate the drinks and freeze the sandwiches, defrosting them the night before consumption.
Blood glucose measurement
A continuous glucose monitoring system was used (Medtronic; MiniMed, Northridge, CA). The sensor was inserted in the abdominal subcutaneous tissue the afternoon before study commencement. Subjects were instructed to enter four BGLs per day into the monitor at a time when BGLs were stable for calibration. They were asked to enter a “meal marker” into the continuous glucose monitoring system immediately before meal consumption.
Only subjects who had acceptable traces for each of the four test conditions were accepted for analysis. If sensor failure occurred, then the test condition was repeated using a new sensor.
Statistical analysis
The 4-h postprandial period was analyzed using SPSS software (version 15; SPSS, Chicago, IL). A one-way repeated-measures ANOVA was used to analyze the following parameters: 1) blood glucose excursions at 30-min increments, 2) incremental area under the curve (AUC) as described previously (22), 3) peak blood glucose level, 4) time to peak BGL, and 5) time to return to baseline.
If significance was reached (P < 0.05) the test of least significant differences (LSD) was applied as the post hoc test. Sensitivity analysis was performed using Bonferroni correction to adjust for multiple pairwise comparisons. Results are presented as means with 95% CIs.
RESULTS
Thirty-four participants were recruited. Two participants withdrew before study commencement for personal reasons, and one participant was excluded because of an inappropriate A1C. Of the 31 children who participated, the results of 11 participants were excluded from analysis because of sensor failure (n = 4), inappropriate starting BGL on test days (n = 2), prolonged hypoglycemia (n = 2), failure to comply with the protocol (n = 1), diagnosis during the study period of a medical condition that would interfere with study protocol (n = 1), and sensor misplaced (n = 1).
The mean age of participants was 13.6 ± 2.7 (range 8.3–17.7) years. The subjects had been diagnosed with type 1 diabetes for 5.2 ± 3.8 (1.0–13.6) years and had good metabolic control (A1C 7.4 ± 0.7 [6.1–8.5] %). The starting BGL for each of the four test conditions was not significantly different (one-way repeated-measures ANOVA P > 0.05) (Table 2).
Table 2.
Fasting BGL, peak BG excursion, time to peak BGL, time to fasting BGL, and AUC for each test condition
| Fasting BGL (mmol/l) | Peak BG excursion (mmol/l) | Time to peak BGL (min) | Time to fasting BGL (min) | AUC (mmol · h−1 · l−1) | |
|---|---|---|---|---|---|
| Low-GI meal, preprandial ultra-short-acting insulin | 8.8 (7.3–10.4) | 4.6 (3.0–6.2) | 70 (45–95) | 137 (99–175) | 2.5 (−2.9–7.8) |
| High-GI meal, preprandial ultra-short-acting insulin | 7.7 (6.3–9.1) | 8.1 (6.2–10.1)* | 75 (61–88)* | 179 (150–209) | 13.8 (6.4–21.2)* |
| Low-GI meal, preprandial regular insulin | 9.1 (7.0–11.2) | 5.7 (4.3–7.1) | 73 (54–92) | 136 (100–172) | 6.4 (1.9–10.9)* |
| Low-GI meal, postprandial ultra-short-acting insulin | 8.9 (7.5–10.2) | 6.3 (5.1–7.5)* | 63 (52–74) | 160 (133–186) | 5.9 (0.7–11.1) |
Data are means (95% CI).
Statistically different when compared with result for the low-GI meal, preprandial ultra-short-acting insulin (LSD, P < 0.05).
Low–glycemic index versus high–glycemic index meal with preprandial ultra-short-acting insulin
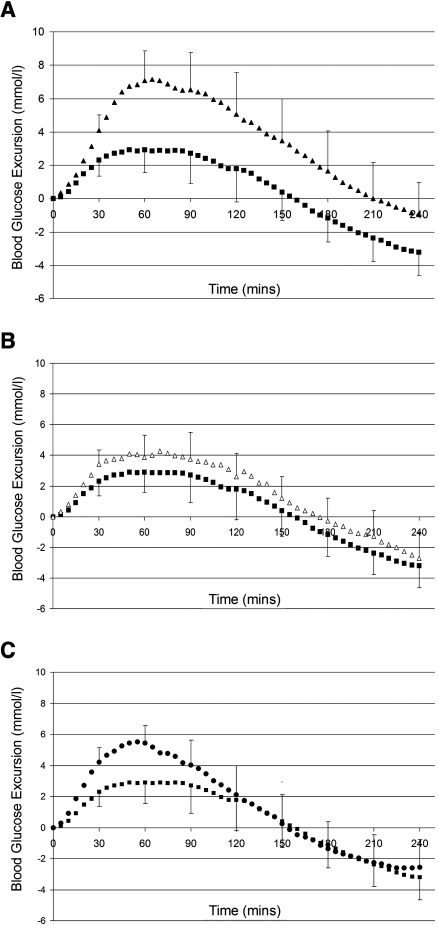
The high–glycemic index meal resulted in a significantly higher PPGE at all time points between 30 and 180 min compared with the low–glycemic index meal (LSD, P < 0.02) (Fig. 1A). The maximum difference between the PPGE after each test condition occurred at 60 min; the high–glycemic index meal was 4.2 mmol/l higher than the low–glycemic index meal (7.1 [range 5.2–9.0] vs. 2.9 [1.5–4.3] mmol/l; LSD, P < 0.0001).
Figure 1.
A: Low–versus high–glycemic index meal with preprandial ultra-short-acting insulin. ▪, low–glycemic index meal, preprandial ultra-short-acting insulin; ▴, high–glycemic index meal, preprandial ultra-short-acting insulin. Mean PPGEs of 20 patients after a low–glycemic index meal and high–glycemic index meal with preprandial ultra-short-acting insulin. Error bars represent 95% CI. The results are significantly different for 30–180 min (LSD, P < 0.02). B: Preprandial ultra-short-acting insulin versus preprandial regular insulin for a low–glycemic index meal. ▪, low–glycemic index meal, preprandial ultra-short-acting insulin; ▵, low–glycemic index meal, preprandial regular insulin. PPGEs after administration of ultra-short-acting insulin and regular insulin before a low–glycemic index meal. The results are significantly different at 30 min only (LSD, P < 0.02). C: Preprandial versus postprandial ultra-short-acting insulin for a low–glycemic index meal. ▪, low–glycemic index meal, preprandial ultra-short-acting insulin. •, low–glycemic index meal, postprandial ultra-short-acting insulin. PPGEs after administering preprandial and postprandial ultra-short-acting insulin with a low–glycemic index meal. The results were significantly different at 30 and 60 min (LSD, P < 0.02).
The AUC and peak blood glucose excursion were greater for the high–glycemic index meal than for the low–glycemic index meal (LSD, P < 0.0001) (Table 2). After the high–glycemic index meal, the return to baseline BGL took significantly longer (LSD, P = 0.011) (Table 2). There was no difference in time to peak BGL for the two test conditions (Table 2).
Preprandial ultra-short-acting insulin versus preprandial regular insulin for a low–glycemic index meal
Administering preprandial regular insulin compared with ultra-short-acting insulin resulted in a 1.1 mmol/l higher blood glucose excursion at 30 min only (3.4 [range 2.4–4.4] vs. 2.3 [1.3–3.3] mmol/l; LSD, P = 0.015) (Fig. 1B). Ultra-short-acting insulin reduced the AUC compared with administration of regular insulin (LSD, P = 0.046) (Table 2). There was no difference in the peak blood glucose excursion, time to peak, or time to baseline between the two test conditions (Table 2).
Preprandial versus postprandial ultra-short-acting insulin for a low–glycemic index meal
Administration of preprandial ultra-short-acting insulin resulted in a significantly lower PPGE compared with postprandial administration at 30 and 60 min (Fig. 1C). At 60 min the blood glucose excursion was 2.5 mmol/l lower after administration of preprandial insulin compared with postprandial insulin (2.9 [range 1.5–4.3] vs. 5.4 [4.2–6.6] mmol/l; LSD, P < 0.0001).
Preprandial administration resulted in a lower AUC for 0–1 h (1.9 [range 1.0–2.7] vs. 3.5 [2.8–4.2] mmol · h−1 middot; l−1; LSD, P < 0.0001) and 0–2 h (4.4 [1.8–7.0] vs. 7.1 [4.8–9.4] mmol · h−1 · l−1; LSD, P = 0.012); however, the total AUC was not significantly different. The peak BGL was 1.7 mmol/l lower when preprandial insulin was administered (LSD, P = 0.003) (Table 2). There was no difference in the time to peak BGL or time to return to baseline between the two test conditions (Table 2).
Adjusting for multiple comparisons using the Bonferroni correction led to a P = 0.02. Almost all of the P values in the analysis remained significant using this threshold, and this adjustment did not change the conclusions.
CONCLUSIONS
To our knowledge, this study is the first to examine whether changing the glycemic index of a mixed meal alters the PPGE in children receiving multiple daily injections. The low–glycemic index meal produced a significantly lower PPGE for 30–180 min, a lower AUC, a smaller peak blood glucose excursion, and reduced time to reach baseline BGLs compared with the high–glycemic index meal when preprandial ultra-short-acting insulin was administered (LSD, P < 0.02).
Previous studies in children receiving conventional insulin therapy and in adults receiving intensive insulin therapy (11,23,24) support our findings. Furthermore, Nansel et al. (13) recently postulated that reductions in postprandial excursions may be responsible for the improved daytime glycemic control they found in youths consuming low–glycemic index diets. However, one study in children failed to show a difference in the PPGE when the glycemic index of a test breakfast was altered (25). This study was influenced by numerous confounders, including failure to standardize fiber and macronutrient content. Additionally, the results were not applicable to children receiving intensive insulin therapy.
It may be hypothesized that the substantial differences demonstrated in the postprandial glucose excursion and AUC between the low–and high–glycemic index test meals in this study should extrapolate to improved A1C. This theory is supported by Brand-Miller's meta-analysis, which detected a 10.6% reduction in the A1C of adults and children with type 1 diabetes when a low–glycemic index diet was consumed over a 2- to 52-week period (10).
The test meals in this study had significantly different glycemic index values (glycemic index 48 vs. 84). A limitation of the study is that more moderate differences in glycemic index values may result in less significant alterations in PPGE. In addition, as discussed by Mohammed and Wolever (24), the shape of the postprandial blood glucose curve may be different if a starchy food of the same glycemic index value was substituted for the fruit juice given in this study. Further studies in mixed meals are warranted to explore these issues.
After consideration of the effect of glycemic index on PPGEs, the question of whether insulin type (regular insulin or ultra-short-acting insulin) or timing of insulin administration should be altered when a low–glycemic index meal is consumed remains. Administration of preprandial ultra-short-acting insulin and regular insulin produced clinically similar PPGEs. Administration of regular insulin produced a 1.1 mmol/l higher blood glucose excursion at 30 min (P = 0.015). Therefore, the use of preprandial regular insulin did not offer an advantage over that of preprandial ultra-short-acting insulin when a low–glycemic index meal was consumed.
This study demonstrated that postprandial administration of ultra-short-acting insulin produced significantly higher postprandial blood glucose levels. These findings are supported by the results of other studies in adults (17,18). Thus, we believe that postprandial administration of ultra-short-acting insulin should not be recommended, as it potentates deviations from normoglycemia.
Dietary focus remains on carbohydrate counting as a strategy to reduce the risk and progression of long-term complications in the context of intensive insulin therapy. The current study supports the integration of low–glycemic index dietary advice into medical nutrition therapy for children and adolescents with type 1 diabetes receiving multiple daily injections. Preprandial ultra-short-acting insulin remains the optimal insulin therapy when a low–glycemic index meal is consumed and postprandial injection of insulin is not recommended as a standard management technique.
Acknowledgments
We thank the Australian Diabetes Educators Association for delivering the independent grant from Eli Lilly that supported this study.
We thank Kathy Hodge for her assistance throughout the study and Dr. Brent McSharry for his assistance in data collation.
A pilot study was previously published in abstract form in the proceedings of the 33rd Annual Scientific Meeting for the International Society of Pediatric and Adolescent Diabetes, Berlin, Germany, 26–29 September, 2007 (Pediatr Diabetes 8 [Suppl. 7]:60, 2007).
Published ahead of print at http://care.diabetesjournals.org on 5 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Diabetes Control and Complications Trial Research Group: The relationship of glycemic exposure (HbA1c) to risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 44:968–984, 1995 [PubMed] [Google Scholar]
- 2.Delahanty LM, Halford BN: Role of diet behaviours in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care 16:1453–1458, 1993 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association: Nutrition recommendations and interventions for diabetes–2006: a position statement of the American Diabetes Association. Diabetes Care 29:2140–2157, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Samann A, Muhlhauser I, Bender R, Kloos C, Muller UA: Glycaemic control and severe hypoglycemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation program. Diabetologia 48:1965–1970, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Mühlhauser I, Bott U, Overmann H, Wagener W, Bender R, Jörgens V, Berger M: Liberalized diet in patients with type 1 diabetes. J Intern Med 237:591–597, 1995 [DOI] [PubMed] [Google Scholar]
- 6.DAFNE Study Group: Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 325:746–748, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowles J, Waller H, Eiser C, Heller S, Roberts J, Lewis M, Wilson K, Hutchinson T, Willan M, Bavelja P, Bennet G, Price K: The development of an innovative education curriculum for 11–16 yr old children with type 1 diabetes mellitus. Pediatr Diabetes 7:322–328, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV: Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 34:362–366, 1981 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association: Care of children and adolescents with type 1 diabetes. Diabetes Care 28:186–212, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Brand-Miller JC, Petocz P, Hayne S, Colagiuri S: Meta-analysis of low-glycemic index diets in the management of diabetes. Diabetes Care 26:2261–2267, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Collier GR, Giudici S, Kalmusky J, Wolever TMS, Helman G, Wesson V, Ehrlich R, Jenkins D: Low glycaemic index starchy foods improve glucose control and lower serum cholesterol in diabetic children. Diabetes Nutr Metab 1:11–19, 1988 [Google Scholar]
- 12.Birnbacher R, Waldhor T, Schneider U, Schober E: Glycaemic responses to commonly ingested breakfasts in children with insulin-dependent diabetes mellitus. Eur J Pediatr 154:353–355, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Nansel TR, Gellar L, McGill A: Effect of varying glycemic index meals on blood glucose control assessed with continuous glucose monitoring in youth with type 1 diabetes on basal-bolus regimens. Diabetes Care 31:695–697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen HB, Lindholm A, Olsen BS, Hylleberg B: Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr 159:483–488, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR: [Lys(B28), Pro(B29)]-human insulin: a rapidly absorbed analogue of human insulin (LYSPRO). Diabetes 43:396–402, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Heinemann L, Heise T, Jorgensen LN, Starke AA: Action profile of the rapid acting insulin analogue: human insulin B28Asp. Diabet Med 10:535–539, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Brunner GA, Hirschberger S, Sendlhofer G, Wutte A, Ellmerer M, Balent B, Schaupp L, Krejs GJ, Pieber TR: Post-prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabet Med 17:371–375, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Strachan MWH, Frier BM: Optimal time of administration of insulin lispro. Diabetes Care 21:26–31, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan L: Suggested Good Practice for Dieticians Involved in the Dietetic Management of Adults, with Type 1 Diabetes, Treated with Insulin Analogues. Professional consensus statement of the Diabetes Management and Education Group of the British Dietetic Association. Birmingham, British Dietetic Association, 2005
- 20.Foster-Powell K, Holt SHA, Brand-Miller JC: International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76:5–56, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Wolever TM, Jenkins DJ: The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 43:167–172, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Le Floch J, Escuyer P, Baudon D, Perlemuter L: Blood glucose area under the curve: methodological aspects. Diabetes Care 13:172–175, 1990 [DOI] [PubMed] [Google Scholar]
- 23.La France L, Rabasa-Lhoret R, Poisson D, Ducros F, Chiasson JL: Effects of different glycemic index foods and dietary fibre intake on glycaemic control in type 1 diabetic patients on intensive insulin therapy Diabet Med 15:972–978, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Mohammed N, Wolever T: Effect of carbohydrate source on post-prandial blood glucose in subjects with type 1 diabetes treated with insulin Lispro. Diabetes Res Clin Pract 65:29–35, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Weyman-Daum M, Fort P, Recker B, Lanes R, Lifshitz F: Glycemic response in children with insulin-dependent diabetes mellitus after high- or low-glycemic-index breakfast. Am J Clin Nutr 46:798–803, 1987 [DOI] [PubMed] [Google Scholar]