Abstract
OBJECTIVE—To assess the efficacy of 1-h plasma glucose concentration and the metabolic syndrome in predicting future risk of type 2 diabetes.
RESEARCH DESIGN AND METHODS—A total of 1,611 subjects from the San Antonio Heart Study, who were free of type 2 diabetes at baseline; who had plasma glucose and insulin concentrations measured at time 0, 30, 60, and 120 min during the oral glucose tolerance test (OGTT); and who had their diabetes status determined with an OGTT after 7–8 years of follow-up, were evaluated. Two models, based on glucose tolerance status, 1-h plasma glucose concentration, and presence of the metabolic syndrome, were tested in predicting the risk for type 2 diabetes at 7–8 years of follow-up.
RESULTS—A cutoff point of 155 mg/dl for the 1-h plasma glucose concentration during the OGTT was used to stratify subjects in each glucose tolerance group into low, intermediate, and high risk for future type 2 diabetes. A model based upon 1-h plasma glucose concentration, Adult Treatment Panel (ATP) III criteria for the metabolic syndrome, and fasting plasma glucose, independent of 2-h plasma glucose, performed equally well in stratifying nondiabetic subjects into low, intermediate, and high risk for future type 2 diabetes and identified a group of normal glucose-tolerant subjects who were at very high risk for future type 2 diabetes.
CONCLUSIONS—The plasma glucose concentration at 1 h during the OGTT is a strong predictor of future risk for type 2 diabetes. A plasma glucose cutoff point of 155 mg/dl and the ATP III criteria for the metabolic syndrome can be used to stratify nondiabetic subjects into three risk groups: low, intermediate, and high risk.
Clinical trials have demonstrated that lifestyle intervention and pharmacological therapy in high-risk individuals reduce the incidence of type 2 diabetes (1). Thus, reliable models for identification of individuals at high risk for future type 2 diabetes are essential and have important clinical implications for intervention programs. Subjects with impaired glucose tolerance (IGT) are at increased risk for future type 2 diabetes (2), and the oral glucose tolerance test (OGTT) has become the standard method for identifying individuals at risk for type 2 diabetes. Indeed, all clinical trials that have assessed strategies for type 2 diabetes prevention have recruited subjects with IGT. Although IGT subjects have increased risk for type 2 diabetes, only ∼50% convert to type 2 diabetes within 10 years of follow-up (2), indicating that the future risk for diabetes is not similar among all individuals with IGT. Furthermore, in longitudinal epidemiological studies, ∼40% of subjects who develop type 2 diabetes have normal glucose tolerance (NGT) at baseline, indicating that there is a population of NGT subjects who are at risk for future type 2 diabetes (2). Recently, we demonstrated that subjects with NGT, despite having relatively low risk for type 2 diabetes, can be stratified into low- and high-risk categories based upon the relationship between their postload and fasting plasma glucose (FPG) concentrations (3).
Several models have been proposed to improve the predictive ability for future type 2 diabetes (4–7). These models are based upon established risk factors for type 2 diabetes (e.g., obesity, FPG, lipid profile, and blood pressure). All of these risk factors are components of the metabolic or insulin resistance syndrome, which is itself a predictor of future type 2 diabetes in nondiabetic individuals (8). In a recent publication (9), we demonstrated that the 1-h plasma glucose concentration is a better predictor for future type 2 diabetes than either the FPG or 2-h plasma glucose concentration. Furthermore, the addition of the 1-h plasma glucose concentration to a prediction model based on clinical parameters significantly improved the ability of the model to predict future type 2 diabetes (9). In this study, we have used the classification tree model (10) to stratify the risk for future type 2 diabetes in nondiabetic subjects based upon their 1-h plasma glucose concentration during the OGTT and the Adult Treatment Panel (ATP) III criteria for the metabolic syndrome. We demonstrate that a model based on the combination of 1-h plasma glucose concentration during the OGTT and the ATP III criteria for the metabolic syndrome improves the ability to predict the future risk for type 2 diabetes.
RESEARCH DESIGN AND METHODS
All subjects were participants of the San Antonio Heart Study (11–13), which is a population-based, epidemiological study of type 2 diabetes and cardiovascular disease. A total of 2,616 eligible participants, who were free of type 2 diabetes at baseline, completed a 7- to 8-year follow-up examination and had their diabetes outcome determined with a repeat OGTT. Of 2,616 participants, 1,610 subjects had plasma glucose measurements at 0, 30, 60, and 120 min during the baseline OGTT and constitute the study population. The study was approved by the institutional review board of University of Texas Health Science Center at San Antonio. All subjects gave their written informed consent before participation.
Definition of variables and outcomes
All studies were performed in a mobile clinic following a 12-h overnight fast. A standard 75-g glucose OGTT was performed, and blood was obtained at 0, 30, 60, and 120 min for determination of plasma glucose and serum insulin concentrations. Plasma glucose and serum lipids were measured with an Abbott Bichromatic Analyzer (South Pasadena, CA). The diagnosis of diabetes was based upon World Health Organization criteria (14). Subjects on insulin or oral antihyperglycemic medications also were considered to have diabetes. The metabolic syndrome was diagnosed according to ATP III criteria (15).
Classification tree
Recursively partitioned classification trees (16) were used to model the relationship between the future risk of type 2 diabetes and 1) 1-h plasma glucose concentration during the OGTT and 2) presence or absence of the metabolic syndrome. Sequential partitioning of the individuals based upon their 1-h plasma glucose concentration relative to 155 mg/dl (above or below) and the presence or absence of the metabolic syndrome produced subgroups or compartments of individuals with homogenous risk for future type 2 diabetes. Subgroups with annual risk for future type 2 diabetes <0.5% (<3.5% risk in 7–8 years) were considered as having low risk for future type 2 diabetes. Annual risk between 1 and 2% (7–15% risk in 7–8 years) was considered intermediate risk. Annual risk >4% (>30% risk in 7–8 years) was considered high risk.
Statistical methods
Variables are presented as the means ± SD. The significance of the mean differences was tested with ANOVA. Differences between categorical variables were tested with the χ2 test. Statistical significance was considered at the level of P < 0.05. Assessment of the predictive discrimination of the various models was made using the receiver-operating characteristic curve by plotting the sensitivity against the corresponding false-positive rate. Statistical analysis was performed with the SPSS software package.
RESULTS
Table 1 presents the anthropometric, laboratory, and clinical characteristics of the study population. Of 1,611 study participants, 1,301 had NGT, 90 had impaired fasting glucose (IFG), and 221 had IGT at baseline, respectively. Fifty-one of 221 subjects with IGT also had IFG and were designated as having combined glucose intolerance (CGI). The conversion rate to type 2 diabetes over the study period (7–8 years) was 5.0, 26.1, 30.9, and 82.3% for NGT, IFG, IGT, and CGI subjects, respectively. We previously demonstrated that the 1-h plasma glucose concentration during the OGTT is a good predictor for future type 2 diabetes (9). A plasma glucose cutoff point of 155 mg/dl has the maximal sum of sensitivity and specificity (0.75 and 0.79 for sensitivity and specificity, respectively) and for predicting future type 2 diabetes. Similarly, the ideal cutoff point for fasting plasma glucose concentration in predicting future type 2 diabetes was 94.5 mg/dl. Therefore, we have used these values as cutoff points to test the prediction of future type 2 diabetes with two tree models.
Table 1.
Anthropometric, clinical, and laboratory characteristics of the study population
| NGT | IFG | IGT | CGI | ANOVA | |
|---|---|---|---|---|---|
| n | 1,301 | 90 | 169 | 51 | |
| Sex (% female) | 57 | 39 | 63 | 53 | <0.0001 |
| Age (years) | 42 ± 11 | 47 ± 10 | 49 ± 10 | 51 ± 2 | <0.0001 |
| BMI (kg/m2) | 27.1 ± 5.1 | 29.8 ± 5.9 | 30.3 ± 5.6 | 31.1 ± 0.8 | <0.0001 |
| Waist circumference (cm) | 87.9 ± 1.3 | 96.3 ± 1.2 | 96.4 ± 1.6 | 102.5 ± 1.8 | <0.0001 |
| FPG (mg/dl) | 83 ± 8 | 106 ± 5 | 93 ± 12 | 109 ± 1 | <0.0001 |
| 2-h plasma glucose (mg/dl) | 95 ± 23 | 105 ± 22 | 165 ± 16 | 173 ± 5 | <0.0001 |
| Subjects with 1-h plasma glucose > 155 mg/dl | 217 | 52 | 171 | 50 | <0.0001 |
| Metabolic syndrome (%) | 14.3 | 66.7 | 49.3 | 84.3 | <0.0001 |
| Subjects converted to diabetes | 65 | 23 | 52 | 42 | <0.0001 |
Data are means ± SD, unless otherwise indicated.
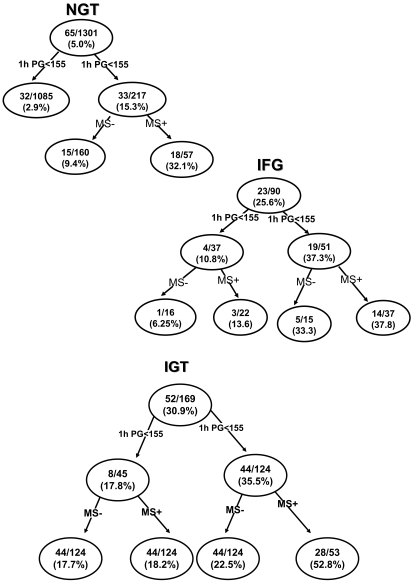
The first tree model is based upon the glucose tolerance status (17), 1-h plasma glucose value, and presence of the metabolic syndrome. The receiver-operating characteristic for this model was 86.7%. In this model, individuals were divided, according to the American Diabetes Association criteria (17), into four groups (NGT, IFG, IGT, and CGI) based upon their fasting and 2-h plasma glucose concentration. Individuals in each group were further divided into two subgroups based upon their 1-h plasma glucose concentration (above or below 155 mg/dl). Figure 1 depicts the incidence of type 2 diabetes based upon 1-h plasma glucose concentration. Although, as a whole, subjects with NGT had a low risk for type 2 diabetes (5.0%), normal glucose-tolerant subjects with 1-h plasma glucose >155 mg/dl had significantly increased risk (15.3%) for future type 2 diabetes compared with nor subjects with 1-h plasma glucose <155 mg/dl (2.9%) (P < 0.0001). Further division of this group based upon the presence or absence of the metabolic syndrome demonstrated that NGT subjects with 1-h plasma glucose >155 mg/dl and the metabolic syndrome had a 32.1% incidence rate of type 2 diabetes compared with a 9.4% incidence rate for subjects without the metabolic syndrome.
Figure 1.
Tree model based on the glucose tolerance status of the subjects, 1-h plasma glucose concentration, and presence or absence of the metabolic syndrome. The numbers in each nodule represent the number of subjects converting to diabetes/total number of subjects in each particular group and the incidence rate of conversion to diabetes over 8 years. 1-h PG, 1-h plasma glucose concentration during the OGTT; MS+, metabolic syndrome present; MS−, metabolic syndrome absent.
Subjects with IFG and a 1-h plasma glucose >155 mg/dl had a 37.3% incidence of type 2 diabetes, while IFG subjects with a 1-h plasma glucose concentration <155 mg/dl had a 10.8% incidence rate. Table 2 presents the odds ratio for having diabetes for the various glucose tolerance groups. Subjects with IGT and a 1-h plasma glucose >155 mg/dl had a 35.5% diabetes incidence rate, while IGT subjects with a 1-h plasma glucose <155 mg/dl had a 17.8% diabetes incidence rate.
Table 2.
Odds ratio and 95% CI for the risk of developing type 2 diabetes for the prediction models
| Odds ratio (95% CI) | |
|---|---|
| Model 1 | |
| NGT, 1-h plasma glucose <155 mg/dl | 1 |
| NGT, 1-h plasma glucose >155 mg/dl without metabolic syndrome | 3.4 (1.8–6.4) |
| NGT, 1-h plasma glucose >155 mg/dl with metabolic syndrome | 15.2 (7.8–29.3) |
| IFG, 1-h plasma glucose <155 mg/dl | 4.0 (1.3–11.9) |
| IFG, 1-h plasma glucose >155 mg/dl | 19.5 (10.0–38.0) |
| IGT, 1-h plasma glucose <155 mg/dl | 7.1 (3.0–16.5) |
| IGT, 1-h plasma glucose >155 mg/dl | 18.1 (10.8–30.1) |
| Model 2(A) | |
| 1-h plasma glucose <155 mg/dl without metabolic syndrome | 1 |
| 1-h plasma glucose <155 mg/dl with metabolic syndrome | 2.4 (1.2–4.8) |
| 1-h plasma glucose >155 mg/dl without metabolic syndrome and FPG <95 mg/dl | 3.6 (2.0–6.2) |
| 1-h plasma glucose >155 mg/dl with metabolic syndrome or FPG >95 mg/dl | 30.0 (19.4–46.3) |
| Model 2(B) | |
| 1-h plasma glucose <155 mg/dl and triglyceride–to–HDL cholesterol ratio <3.5 | 1 |
| 1-h plasma glucose < 155 mg/dl and triglyceride–to–HDL cholesterol ratio >3.5 | 2.3 (1.3–4.2) |
| 1-h plasma glucose >155 mg/dl and triglyceride–to–HDL cholesterol ratio <3.5 and FPG <95 mg/dl | 4.3 (2.4–7.9) |
| 1-h plasma glucose >155 mg/dl and triglyceride–to–HDL cholesterol ratio >3.9 or FPG >95 mg/dl | 22.4 (14.2–35.3) |
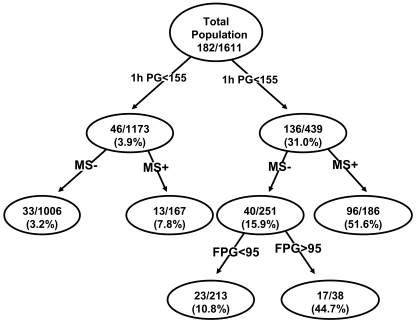
The second model includes the 1-h plasma glucose concentration, the metabolic syndrome, and fasting plasma glucose concentration. The receiver-operating characteristic for this model was 85.4%. In this model, subjects were divided into two groups based upon their 1-h plasma glucose concentration (above or below 155 mg/dl) and each group was further divided into two subgroups based upon the presence or absence of the metabolic syndrome. Figure 2 depicts the 7- to 8-year risk for type 2 diabetes for each subgroup. In general, nondiabetic subjects with 1-h plasma glucose <155 mg/dl had a low risk (3.9%) for future development of type 2 diabetes compared with subjects with a 1-h plasma glucose >155 mg/dl (31.0%) (P < 0.0001). When subjects with 1-h plasma glucose <155 mg/dl were divided according to the presence or absence of the metabolic syndrome, subjects with a 1-h plasma glucose <155 mg/dl without the metabolic syndrome had a 3.2% risk for future type 2 diabetes, while those with the metabolic syndrome had 7.8% risk for future diabetes. Subjects with a 1-h plasma glucose concentration >155 mg/dl and the metabolic syndrome had a 51.6% risk for future diabetes. Subjects with a 1-h plasma glucose >155 mg/dl without the metabolic syndrome, but with a fasting plasma glucose >95 mg/dl, had a 44.7% risk for future diabetes, while subjects with a 1-h plasma glucose >155 mg/dl without the metabolic syndrome and fasting plasma glucose <95 mg/dl had a 10.8% risk for future type 2 diabetes.
Figure 2.
Tree model based on 1-h plasma glucose (PG) concentration, presence or absence of the metabolic syndrome, and fasting plasma glucose concentration. The numbers in each nodule represent the number of subjects converting to diabetes/total number of subjects in each particular group and the incidence rate of conversion to diabetes over 8 years. 1-h PG, 1-h plasma glucose concentration during the OGTT; MS+, metabolic syndrome present; MS−, metabolic syndrome absent.
Because the waist circumference is rarely measured in clinical practice and is part of the ATP III definition of the metabolic syndrome, we also examined the predictive value of triglyceride–to–HDL cholesterol ratio >3.5 in place of the metabolic syndrome (Table 2). Although the metabolic syndrome was a better predictor compared with the triglycerides–to–HDL cholesterol ratio, a model based on 1-h plasma glucose concentration and triglyceride–to–HDL cholesterol ratio could classify subjects to three risk groups: low, intermediate, and high risk (Table 2).
CONCLUSIONS
The American Diabetes Association Consensus Statement has recommended metformin, in addition to diet and exercise, in individuals with IGT/IFG to reduce their risk for future diabetes (18). This recommendation for pharmacologic intervention underscores the need for models that reliably identify individuals at increased risk for future development of type 2 diabetes. The results of this study demonstrate that the plasma glucose concentration at 1 h during the OGTT is a useful tool that can be used to stratify the risk of future type 2 diabetes into three groups: low, intermediate, and high risk. In general, subjects with NGT have low risk for progression to type 2 diabetes (∼0.67% annual rate) (2). However, ∼40% of individuals who develop type 2 diabetes have NGT at baseline (2) and, in the present study, 16.7% of normal glucose-tolerant subjects with a 1-h plasma glucose concentration (OGTT) >155 mg/dl developed type 2 diabetes over a 7- to 8-year period. In this group of normal glucose-tolerant subjects, the annual risk for future type 2 diabetes was significantly greater (2.2% per year) compared with subjects whose 1-h plasma glucose concentration did not exceed 155 mg/dl (0.39% per year, P < 0.00001). Further, NGT subjects with a 1-h plasma glucose >155 mg/dl who fulfilled the ATP III criteria for the metabolic syndrome had a 4.3% annual risk for future type 2 diabetes. Thus, the group of normal glucose-tolerant subjects with 1-h PG >155 mg/dl plus the metabolic syndrome is at very high risk for the development of type 2 diabetes, their risk exceeds that of subjects with IFG or IGT, and their odds ratio for developing diabetes is double that of IGT subjects with a 1-h plasma glucose <155 mg/dl (Table 2). Consistent with the American Diabetes Association Consensus Conference Statement (18), this group of high-risk NGT individuals could benefit from an intervention program employing diet, exercise, and pharmacotherapy (metformin) to reduce future risk for diabetes.
Subjects with CGI have the greatest risk for future type 2 diabetes, with an annual risk >10% per year, while subjects with isolated IFG or IGT have an intermediate risk between CGI and NGT. However, within the IFG and IGT groups, the 1-h plasma glucose during the OGTT also stratifies the future diabetes risk into intermediate and high risk. Thus, IFG and IGT subjects with a 1-h plasma glucose <155 mg/dl have an annual risk of ∼1.5% compared with an annual risk of ∼5% for IGT and IFG subjects with a 1-h plasma glucose >155 mg/dl. It is noteworthy that every CGI subject had a 1-h plasma glucose concentration >155 mg/dl. Thus, the plasma glucose concentration at 1 h during the OGTT is a strong predictor for future type 2 diabetes, independent of the glucose tolerance status, and a 155 mg/dl cutoff point divides individuals with NGT, IFG, and IGT into low-, intermediate-, and high-risk groups.
A predictive model based on the plasma glucose concentration at 1 h during the OGTT and the presence or absence of the metabolic syndrome, independent of the 2-h plasma glucose concentration, performs equally well in stratifying subjects for future risk of type 2 diabetes compared with the model that includes the 2-h plasma glucose concentration. The earlier model had 0.82 sensitivity and 0.63 specificity compared with 0.82 and 0.67 sensitivity and specificity, respectively, for the model based on 1-h plasma glucose concentration. Moreover, the later model (individuals with 1-h plasma glucose >155 mg/dl plus the metabolic syndrome or FPG >95 mg/dl) reduces the number of subjects in the very-high-risk group (>6.5% incidence per year), who are candidates for pharmacological intervention, from 18% (based on the model that includes the 2-h plasma glucose concentration) to 14% of the total study population. Furthermore, the model with the 1-h plasma glucose concentration plus the metabolic syndrome performs better in predicting future diabetes than does the American Diabetes Association criteria of IGT or IFG. Most importantly, ∼17% of normal glucose-tolerant subjects, who have intermediate and high risk for future type 2 diabetes and who were identified with the 1-h plasma glucose plus metabolic syndrome, would have been missed with the American Diabetes Association criteria alone. These observations underscore the importance of obtaining the plasma glucose concentration at 1 h during the OGTT.
Substituting the metabolic syndrome with the triglyceride–to–HDL cholesterol ratio in the second model slightly reduces its predictability. However, the second model with the triglyceride–to–HDL cholesterol ratio is a good predictor for future risk of type 2 diabetes and classifies subjects into three risk groups. Because measurement of triglyceride and HDL cholesterol is part of the routine clinical practice, the second model could be used in routine clinical practice to assess the risk of nondiabetic subjects for future risk of type 2 diabetes.
Why is the 1-h plasma glucose concentration a better predictor for future type 2 diabetes than the 2-h plasma glucose? It could be argued that the high predictability for 1-h plasma glucose is due to its high correlation with the 2-h plasma glucose (r = 0.58, P < 0.0001). However, the 1-h plasma glucose stratifies subjects with NGT, as well as subjects with IGT, into two risk groups, high and low. Thus, it is unlikely that its predictability is secondary to its correlation with the 2-h plasma glucose. Subjects who are destined to develop type 2 diabetes manifest two major defects: 1) insulin resistance in liver and skeletal muscle and 2) impaired β-cell function (19). Previous studies have demonstrated that subjects with hepatic insulin resistance have an increased FPG concentration and impaired suppression of hepatic glucose production during the OGTT, resulting in an excessive rise in plasma glucose concentration at 30 and 60 min (20). In nondiabetic subjects, the decline in plasma glucose concentration at 30–60 min during the OGTT is dependent on insulin sensitivity in skeletal muscle and β-cell function (21,22). Thus, insulin resistance in liver and skeletal muscle, as well as impaired β-cell function, would result in an increase in 1-h plasma glucose concentration. This renders the 1-h plasma glucose a good indicator for the major metabolic abnormalities that lead to the development of type 2 diabetes. Consistent with this, we previously demonstrated that the plasma glucose concentration at 1 h during the OGTT has a stronger correlation with surrogate measures of hepatic and muscle insulin resistance and β-cell dysfunction compared with the 2-h plasma glucose value (9).
In summary, the plasma glucose concentration at 1 h during the OGTT is a strong predictor of future risk for type 2 diabetes. A cutoff point at 155 mg/dl plus the ATP III criteria for the metabolic syndrome can be used to stratify nondiabetic subjects into three risk groups—low, intermediate, and high risk—independent of the 2-h plasma glucose concentration.
Published ahead of print at http://care.diabetesjournals.org on 16 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Diabetes Prevention Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unwin N, Shaw J, Zimmet P, Alberti KGMM: Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabet Med 19:708–723, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Ghani MA, Williams K, DeFronzo R, Stern M: Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 29:1613–1618, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Stern MP, Williams K, Haffner SM: Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 136:575–581, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG, Lennon L, Morris RW: Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 165:2644–2650, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kanaya AM, Wassel Fyr CL, de Rekeneire N, Shorr RI, Schwartz AV, Goodpaster BH, Newman AB, Harris T, Barrett-Connor E: Predicting the development of diabetes in older adults: the derivation and validation of a prediction rule. Diabetes Care 28:404–408, 2005 [DOI] [PubMed] [Google Scholar]
- 7.McNeely MJ, Boyko EJ, Leonetti DL, Kahn SE, Fujimoto WY: Comparison of a clinical model, the oral glucose tolerance test, and fasting glucose for prediction of type 2 diabetes risk in Japanese Americans. Diabetes Care 26:758–763, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, the San Antonio Heart Study: The metabolic syndrome as predictor of type 2 diabetes: the San Antonio Heart Study. Diabetes Care 26:3153–3159, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M: What is the best predictor of future type 2 diabetes? Diabetes Care 30:1544–1548, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP: Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 54:333–339, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Stern MP, Rosenthal M, Haffner SM, Hazuda HP, Franco LJ: Sex difference in the effects of sociocultural status on diabetes and cardiovascular risk factors in Mexican Americans: the San Antonio Heart Study. Am J Epidemiol 120:834–851, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Stern MP, Patterson JK, Haffner SM, Hazuda HP, Mitchell BD: Lack of awareness and treatment of hyperlipidemia in type II diabetes in a community survey. JAMA 262:360–364, 1989 [PubMed] [Google Scholar]
- 13.Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP: Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med 159:1450–1456, 1999 [DOI] [PubMed] [Google Scholar]
- 14.WHO Study Group: Diabetes Mellitus. Geneva, World Health Org., 1985. (Tech. rep. ser., no. 727) [PubMed]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol (Adult Treatment Panel III). JAMA 285:2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Clark LA, Pregibon D: Tree model analysis. In Statistical Models. Chambers JM, Hastie TJ, Eds. Pacific Grove, CA, Wadsworth and Brooks, 1992
- 17.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B, the American Diabetes Association: Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30:753–759, 2007 [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA: Lilly lecture 1987: The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55:1430–1435, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA: Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30:89–94, 2007 [DOI] [PubMed] [Google Scholar]