Abstract
OBJECTIVE—The purpose of this study was to identify reproductive risk factors associated with dysglycemia (diabetes, impaired glucose tolerance, and impaired fasting glucose) in a contemporary multiethnic population.
RESEARCH DESIGN AND METHODS—We studied 14,661 women screened with an oral glucose tolerance test for the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial. Reproductive risk factors were compared in normoglycemic and dysglycemic women.
RESULTS—Dysglycemia was significantly associated with the number of children born (odds ratio 1.03 per child [95% CI 1.01–1.05]), age (1.05 per year [1.04–1.05]), non-European ancestry (1.09 [1.01–1.17]), preeclampsia/eclampsia (1.14 [1.02–1.27]), irregular periods (1.21 [1.07–1.36]), and gestational diabetes mellitus (GDM) (1.53 [1.35–1.74]). The relationship between GDM and dysglycemia did not differ across BMI tertiles (P = 0.84) nor did the relationships of other risk factors.
CONCLUSIONS—Reproductive factors, particularly GDM, are associated with dysglycemia in middle-aged women from many ethnicities. Reproductive factors can be used to counsel young women about their future risk of dysglycemia, whereas in middle age they may help screen for dysglycemia.
Gestational diabetes mellitus (GDM) is a well-known reproductive risk factor for subsequent type 2 diabetes (1). Other reproductive factors such as preeclampsia are associated with insulin resistance during pregnancy and may also increase the subsequent risk for diabetes. Furthermore, some (2–4) but not all (5) studies suggest that pregnancy itself is a risk factor for future type 2 diabetes. For example, a population-based study of 1,186 elderly women showed that, even after accounting for age, obesity, and family history of diabetes, parity was associated with an increased risk of type 2 diabetes, with an odds ratio (OR) of 1.16 per pregnancy (95% CI 1.04–1.20) (3). An even larger study comprising 2,310 women with type 2 diabetes reported that parity greater than six was associated with a relative risk (RR) of diabetes of 1.56 (95% CI 1.27–1.91); however, the estimate of the RR decreased to 1.19 (0.97–1.48) after adjustment for current age (2). The applicability of these results is limited by the homogeneity of the population (registered nurses with relatively high socioeconomic status and 98% Caucasian) and the use of the older fasting plasma glucose cutoff for diabetes of >7.8 mmol/l (>140 mg/dl) rather than the current, more sensitive value of 7.0 mmol/l (126 mg/dl) (6).
The prevalence of dysglycemia (type 2 diabetes, impaired glucose tolerance [IGT], and impaired fasting glucose [IFG]) is increasing; however, reproductive risk factors are often underrecognized. In particular, their association with the more recently recognized forms of glucose dysregulation, IGT and IFG, have not yet been well studied. The detection of dysglycemia could be improved if risk factors were better known. Moreover, if reproductive factors such as parity and preeclampsia are risk factors for dysglycemia, they could be used to refine screening approaches. The goal of this research was to identify reproductive risk factors for dysglycemia in a contemporary, multiethnic group of women.
RESEARCH DESIGN AND METHODS
This is a study of 14,661 women screened as possible participants in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial (7,8), a large, international, multicenter, randomized, double-blind, controlled diabetes prevention trial. Participants were volunteers recruited from 21 countries on five continents from a wide variety of sources including first-degree relatives of diabetic individuals in diabetes clinics, ads in newspapers, pharmacies, national diabetes associations, newsletters, clinics, community announcements, screening programs, and targeted mailings. Institutional research ethics boards at each site approved the DREAM trial.
Assessment
After an overnight (8–18 h) fast, participants consumed 75 g anhydrous glucose and provided fasting and 2-h blood samples for local measurement of plasma glucose. At the same time they completed a 12-page questionnaire regarding baseline characteristics, medications, and personal and family history, and women completed a 1-page reproductive questionnaire regarding regularity of menstrual cycles, fertility, how many children they had given birth to, and complications of pregnancy including GDM, preeclampsia, or eclampsia.
Definitions
Type 2 diabetes was defined as fasting plasma glucose ≥7.0 mmol/l (≥126 mg/dl) or plasma glucose ≥11.1 mmol/l (≥200 mg/dl) 2 h after a 75-g oral glucose load. IGT was defined as plasma glucose 7.8–11.0 mmol/l (140–199 mg/dl) 2 h after a 75-g oral glucose load. IFG was defined as fasting glucose of 6.1–6.9 mmol/l (110–124 mg/dl). Dysglycemia was defined as IFG, IGT, or type 2 diabetes. Parity was defined as the number of infants a woman had borne. Irregular menses was defined as six or fewer menstrual cycles per year between the ages of 18 and 45 years not including pregnancy and was used as a surrogate for polycystic ovary syndrome. Early menopause was defined as the permanent cessation of menstrual periods before age 45. Income range tables with five strata were developed specific to each country in which recruitment occurred; low socioeconomic status was defined as the lowest strata for that country. In Canada, for example, that included a household income ≤$29,999 and for the U.S. it was ≤$15,400. Non-European ancestry was defined as anyone indicating any ancestry other than European at the time of their clinic visit.
Statistical analysis
Women were classified as those with and without dysglycemia. Continuous variables were compared using a t test, and categorical variables were compared with a χ2 test. Logistic regression was used to calculate age-adjusted ORs and 95% CI for reproductive risk factors for dysglycemia. Factors that were statistically significant at P < 0.10 in the age-adjusted analysis were included in the multivariate logistic regression to determine their independent relationship with prevalent dysglycemia. This model was rerun for each tertile of BMI (and P values for heterogeneity were calculated) to determine whether the risk of dysglycemia for each risk factor varied with BMI. All P values are reported as two-tailed. All analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Table 1 presents the base- line characteristics of the women with (n = 6, 298) and without (n = 8, 363) dysglycemia, whose 2-h plasma glucose concentrations were 10.0 ± 3.1 versus 5.7 ± 1.1 mmol/l, respectively (P < 0.0001), with fasting values of 6.3 ± 1.4 versus 5.0 ± 1.1 mmol/l (P < 0.0001, respectively). Women with dysglycemia were significantly older than those without dysglycemia (55.1 vs. 50.0 years, respectively, P < 0.0001). After adjustment for age, most of the reproductive factors remained significantly associated with dysglycemia, including the number of children a woman gave birth to (OR 1.05 per child [95% CI 1.04–1.06]), a history of preeclampsia/eclampsia (1.19 [1.07–1.32]), irregular menses (1.2 [1.09–1.38]), GDM (1.58 [1.40–1.78]), low socioeconomic status (1.09 [1.01–1.17]), and non-European ancestry (1.10 [1.02–1.17]) (Table 1).
Table 1.
Baseline characteristics of participants and age-adjusted univariate analyses of reproductive risk factors and the risk of dysglycemia
| Dysglycemia | No dysglycemia | P value (unadjusted) | Age-adjusted OR (95% CI) of dysglycemia | |
|---|---|---|---|---|
| n | 6,298 | 8,363 | ||
| Age (years) | 55.1 ± 11.1 | 50.0 ± 10.0 | <0.0001 | NA |
| Number of children | 2.74 ± 1.6 | 2.53 ± 1.3 | <0.0001 | 1.05 (1.04–1.06) |
| Low socioeconomic states | 2,243 (37.7) | 2,636 (33.2) | <0.0001 | 1.09 (1.01–1.17) |
| Non-European ancestry | 3,339 (53.0) | 4,649 (55.6) | 0.002 | 1.10 (1.02–1.17) |
| Preeclampsia/eclampsia | 700 (11.0) | 853 (10.1) | 0.10 | 1.19 (1.07–1.32) |
| Irregular periods | 608 (9.6) | 734 (8.8) | 0.08 | 1.22 (1.09–1.38) |
| GDM | 588 (9.4) | 673 (8.1) | 0.01 | 1.58 (1.40–1.78) |
| Early menopause | 124 (2.0) | 217 (2.6) | 0.01 | 1.24 (0.99–1.56) |
| BMI (kg/m2) | 31.6 ± 6.0 | 29.4 ± 6.0 | <0.0001 | 1.06 (1.06–1.07) |
Data are means ± SD and n (%) unless otherwise indicated. Dysglycemia indicates IFG, IGT, or type 2 diabetes, irregular periods indicates ≤6 menstrual cycles per year between the ages of 18 and 45 years not including pregnancy, and early menopause indicates permanent cessation of menses <45 years of age. NA, not applicable.
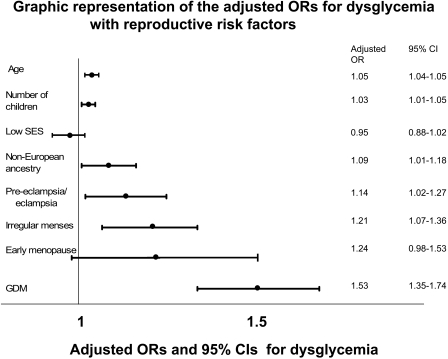
In a multivariate model that included age, ancestry, and fertility-related factors, dysglycemia was significantly associated with the number of children a woman gave birth to (OR 1.03 per child [95% CI 1.01–1.05]), age (1.05 per year [1.04–1.05]), non-European ancestry (1.09 [1.01–1.17]), a history of preeclampsia/eclampsia (1.14 [1.02–1.27]), irregular menses (1.21 [1.07–1.36]), and GDM (1.53 [1.35–1.74]) (Fig. 1). Early menopause (1.24 [0.98–1.53]) and low socioeconomic status (0.95 [0.88–1.02]) were no longer significantly associated with dysglycemia.
Figure 1.
Dysglycemia indicates IFG, IGT, or type 2 diabetes, CI represents the Wald CI, low SES indicates low socioeconomic status, and irregular menses indicates ≤6 menstrual cycles per year between the ages of 18 and 45 years not including pregnancy.
To determine whether there was an interaction between BMI and the reproductive risk factors, particularly GDM, we reran the multiple regression model for each tertile of BMI, with 27.1 and 32.2 defining lower, middle, and upper levels. The relationships between GDM and current dysglycemia did not differ significantly across tertiles of BMI (Pheterogeneity = 0.84), nor for any of the other risk factors (Pheterogeneity ≥0.10 for all). The ORs for GDM and current dysglycemia for each tertile of BMI (<27.1, 27.1–32.2, and >32.2) were 1.81 [95% CI 1.42–2.30], 1.44 [1.14–1.81], and 1.47 [1.20–1.81], respectively.
CONCLUSIONS
This large, multiethnic study of middle-aged women showed that a history of GDM is independently associated with prevalent dysglycemia, confirming that pregnancy is a “stress test for life” (9,10). This observation may be understood in light of the fact that the occurrence of GDM is clear evidence of an impaired ability to maintain normoglycemia under the metabolic stress of pregnancy and is consistent with previous reports (11–14) from smaller studies. Hence, in young women of child-bearing age, reproductive factors, particularly GDM, can be used to counsel patients about their future risk of dysglycemia regardless of future BMI, whereas in middle-aged women a history of reproductive risk factors may be useful as a screening tool for dysglycemia.
This study also showed that a history of a variety of reproductive risk factors, including irregular menses, parity, and preeclampsia, was independently associated with dysglycemia and was not explained by age, ethnicity, or socioeconomic status. One potential explanation is the association of many of these factors with insulin resistance, including preeclampsia (15,16), pregnancy (17), and, even in nonobese women, polycystic ovary syndrome (PCOS) (of which irregular menses is a key component) (18–21).
This is the only study, to our knowledge, examining reproductive risk factors for dysglycemia involving a broad population, allowing for wide applicability of results. Participation spanned all socioeconomic strata and involved 21 countries on five continents. Another strength is the large sample size (14, 661 women), by far the largest study in the literature on reproductive risk factors and dysglycemia, which allowed for control of multiple confounding factors. Another strength is the fact that reproductive risk factors that identified women at increased risk of dysglycemia were elicited with simple screening questions, which are part of a routine history, and do not require serology or imaging investigations such as pelvic ultrasound. Participants were asked, for instance, about irregular menses as a surrogate for PCOS, as PCOS remains undiagnosed in many patients or they are unfamiliar with the medical term. Although this approach has the potential for misclassifying some patients who had had fertility-related risk factors as being unaffected, it suggests that the associations between fertility-related risk factors and dysglycemia are probably even stronger than those observed.
Limitations of the study include the fact that the participants were asked to recall events, such as pregnancies, which in many instances occurred several decades earlier. However, by gathering this baseline information before the administration of the oral glucose tolerance test, recall bias was limited. We did not have access to the participants’ medical charts and relied on patient history, which may not always be reliable and may underestimate some of the above associations.
In summary, in this large, multiethnic study of middle-aged women without a previous diagnosis of diabetes, prevalent dysglycemia was independently associated with a history of several reproductive risk factors, particularly GDM. Moreover, the relationship between prior GDM and current dysglycemia persisted across BMI strata.
Acknowledgments
The DREAM Trial was funded by the Canadian Institutes of Health Research, sanofi-aventis, GlaxoSmithKline, and King Pharmaceuticals through the University Industry Grant Program. S.Y. holds a Heart and Stroke Foundation Chair in Cardiovascular Research. S.S.A. holds the Eli Lilly Canada–May Cohen Chair in Women's Health. H.C.G. holds the Population Health Institute Chair in Diabetes Research sponsored by aventis.
Published ahead of print at http://care.diabetesjournals.org on 5 May 2008.
The funding organizations had no part in the design of the study; the collection, analysis, or interpretation of the data; or the decision to approve publication of the finished manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.O'Sullivan JB: Diabetes mellitus after GDM. Diabetes 40 (Suppl. 2):131–135, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, Arky RA, Rosner B, Hennekens CH, Speizer FE: Parity and incidence of non-insulin-dependent diabetes mellitus. Am J Med 93:13–18, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Kritz-Silverstein D, Barrett-Connor E, Wingard DL: The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med 321:1214–1219, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Peters RK, Kjos SL, Xiang A, Buchanan TA: Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 347:227–230, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Collins VR, Dowse GK, Zimmet PZ: Evidence against association between parity and NIDDM from five population groups. Diabetes Care 14:975–981, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS: Comparison of diabetes diagnostic categories in the U.S. population according to the 1997 American Diabetes Association and 1980–1985 World Health Organization diagnostic criteria. Diabetes Care 20:1859–1862, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR: Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368:1096–1105, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR: Effect of ramipril on the incidence of diabetes. N Engl J Med 355:1551–1562, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Williams D: Pregnancy: a stress test for life. Curr Opin Obstet Gynecol. 15:465–471, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Sibai BM, el-Nazer A, Gonzalez-Ruiz A: Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol 155:1011–1016, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Pallardo LF, Herranz L, Martin-Vaquero P, Garcia-Ingelmo T, Grande C, Janez M: Impaired fasting glucose and impaired glucose tolerance in women with prior gestational diabetes are associated with a different cardiovascular profile. Diabetes Care 26:2318–2322, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Lauenborg J, Hansen T, Jensen DM, Vestergaard H, Molsted-Pedersen L, Hornnes P, Locht H, Pedersen O, Damm P: Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care 27:1194–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dalfra MG, Lapolla A, Masin M, Giglia G, Dalla BB, Toniato R, Fedele D: Antepartum and early postpartum predictors of type 2 diabetes development in women with gestational diabetes mellitus. Diabetes Metab 27:675–680, 2001 [PubMed] [Google Scholar]
- 14.Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK: Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes 47:1302–1310, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kaaja R, Laivuori H, Laakso M, Tikkanen MJ, Ylikorkala O: Evidence of a state of increased insulin resistance in preeclampsia. Metabolism 48:892–896, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Fuh MM, Yin CS, Pei D, Sheu WH, Jeng CY, Chen YI, Reaven GM: Resistance to insulin-mediated glucose uptake and hyperinsulinemia in women who had preeclampsia during pregnancy. Am J Hypertens 8:768–771, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Ciaraldi TP, Kettel M, el-Roeiy A, Madar Z, Reichart D, Yen SS, Olefsky JM: Mechanisms of cellular insulin resistance in human pregnancy. Am J Obstet Gynecol 170:635–641, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Amanti-Kandarakis E, Papavassiliou A: Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med 12:324–332, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Sawathiparnich P, Weerakulwattana L, Santiprabhob J, Likitmaskul S: Obese adolescent girls with polycystic ovary syndrome (PCOS) have more severe insulin resistance measured by HOMA-IR score than obese girls without PCOS. J Med Assoc Thai 88 (Suppl. 8):S33–S37, 2005 [PubMed] [Google Scholar]
- 20.Yilmaz M, Biri A, Karakoc A, Toruner F, Bingol B, Cakir N, Tiras B, Ayvaz G, Arslan M: The effects of rosiglitazone and metformin on insulin resistance and serum androgen levels in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest 28:1003–1008, 2005 [DOI] [PubMed] [Google Scholar]
- 21.DeUgarte CM, Bartolucci AA, Azziz R: Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 83:1454–1460, 2005 [DOI] [PubMed] [Google Scholar]