Abstract
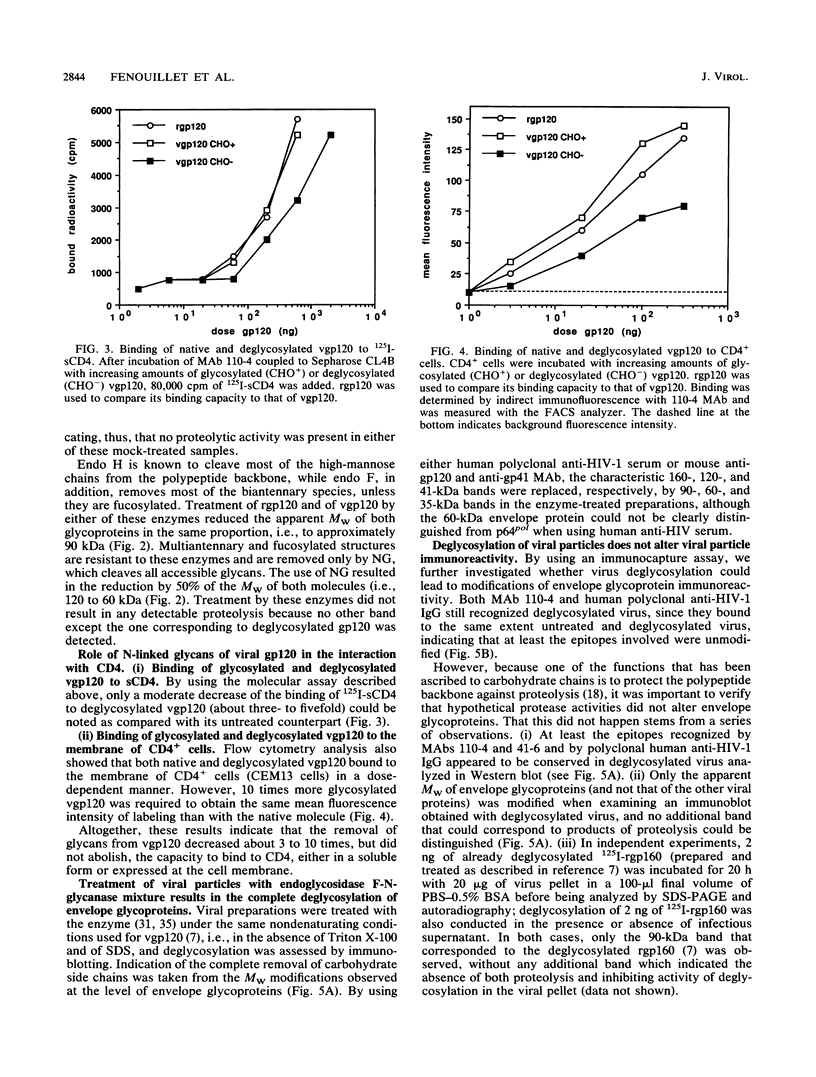
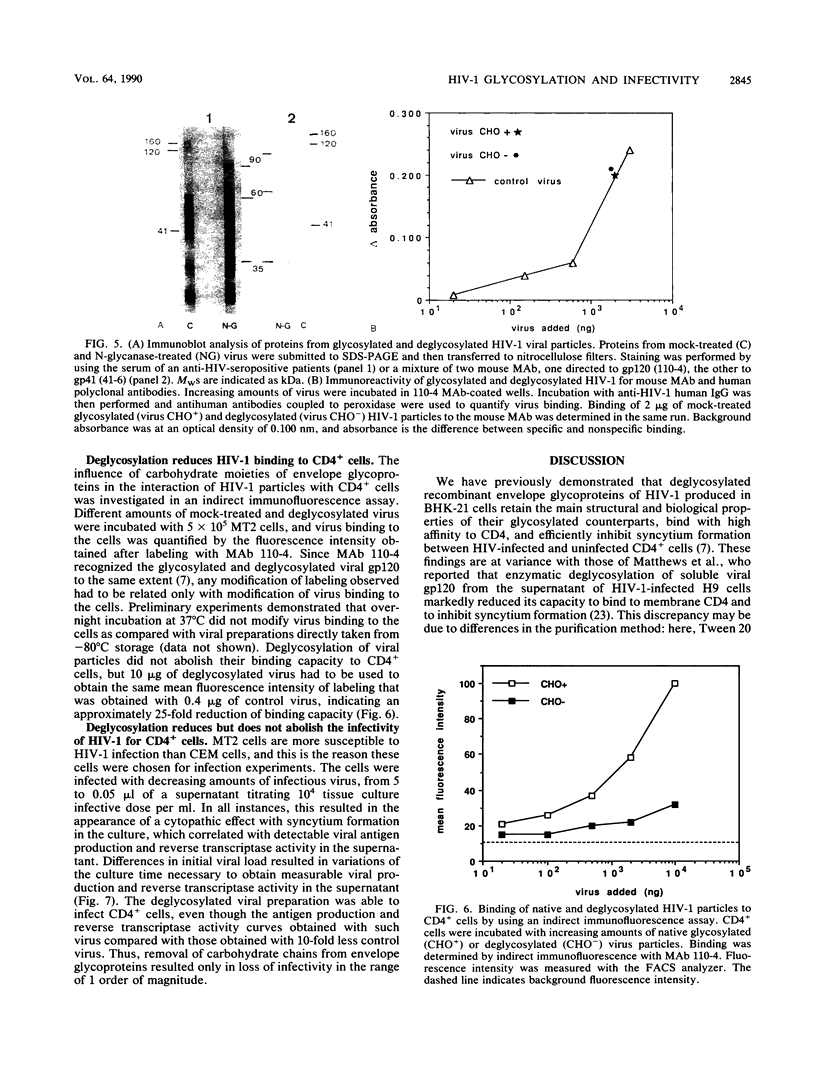
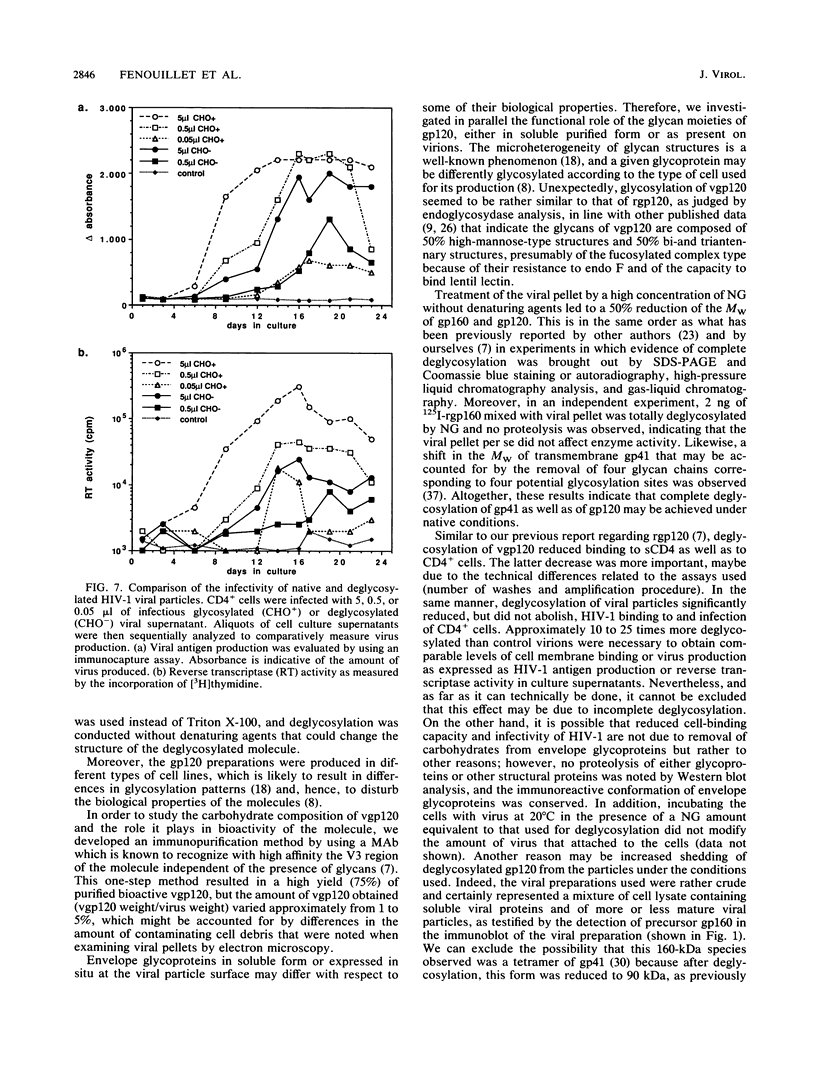
We have shown that enzymatic removal of N-linked glycans from human immunodeficiency virus type 1 (HIV-1) recombinant envelope glycoproteins gp160 and gp120 produced in BHK-21 cells did not significantly reduce their ability to bind to CD4, the cellular receptor for the virus. Because recombinant proteins may behave differently from proteins present on virions, we investigated whether such viral envelope glycoproteins either in a purified form or present on viral particles could be deglycosylated by treatment with an endoglycosidase F-N-glycanase mixture which cleaves all accessible glycan moieties. Endoglycosidase analysis of the carbohydrate composition of purified viral gp120 (vgp120) indicated a glycosylation pattern similar to that for recombinant gp120 (rgp120), and treatment with endoglycosidase F-N-glycanase resulted in comparable molecular weight (MW) reduction for both molecules. Similarly, after immunoblotting of the deglycosylated viral preparation, the characteristic 160- and 120-kilodalton (kDa) bands were replaced by 90- and 60-kDa bands, respectively. The apparent MW of gp41 shifted to 35 kDa. These results are consistent with complete deglycosylation. The immunoreactive conformation of envelope glycoproteins remained unaltered after deglycosylation: they were recognized to the same extent by specific human polyclonal or mouse monoclonal antibodies, and no proteolysis of viral proteins occurred during enzymatic treatment. Deglycosylation of vgp120 resulted in a less than 10-fold reduction of the ability to bind to CD4, presented either in a soluble form or at the cell membrane. In addition, deglycosylation significantly reduced, but did not abolish, HIV-1 binding to and infectivity of CD4+ cells as determined, respectively, by an indirect immunofluorescence assay and a quantitative dose-response infection assay. Taken together, these results indicate that removal of glycans present on mature envelope glycoproteins of HIV-1 diminishes but does not abolish either virus binding to CD4 or its capacity to infect CD4+ cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S., Elder J. H. Carbohydrate dramatically influences immune reactivity of antisera to viral glycoprotein antigens. Science. 1984 Dec 14;226(4680):1328–1330. doi: 10.1126/science.6505693. [DOI] [PubMed] [Google Scholar]
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Berman M. I., Thomas C. G., Jr, Manjunath P., Sairam M. R., Nayfeh S. N. The role of the carbohydrate moiety in thyrotropin action. Biochem Biophys Res Commun. 1985 Dec 17;133(2):680–687. doi: 10.1016/0006-291x(85)90958-1. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem. 1984;16(1):21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- Fennie C., Lasky L. A. Model for intracellular folding of the human immunodeficiency virus type 1 gp120. J Virol. 1989 Feb;63(2):639–646. doi: 10.1128/jvi.63.2.639-646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Clerget-Raslain B., Gluckman J. C., Guétard D., Montagnier L., Bahraoui E. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J Exp Med. 1989 Mar 1;169(3):807–822. doi: 10.1084/jem.169.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Fayet G., Hovsepian S., Bahraoui E. M., Ronin C. Immunochemical evidence for a role of complex carbohydrate chains in thyroglobulin antigenicity. J Biol Chem. 1986 Nov 15;261(32):15153–15158. [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Gibson R., Kornfeld S., Schlesinger S. The effect of oligosaccharide chains of different sizes on the maturation and physical properties of the G protein of vesicular stomatitis virus. J Biol Chem. 1981 Jan 10;256(1):456–462. [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Hongo S., Sugawara K., Homma M., Nakamura K. Effects of glycosylation on the conformation and antigenicity of influenza C viral glycoproteins. Vaccine. 1985 Sep;3(3 Suppl):223–226. doi: 10.1016/0264-410x(85)90111-2. [DOI] [PubMed] [Google Scholar]
- Jouault T., Chapuis F., Olivier R., Parravicini C., Bahraoui E., Gluckman J. C. HIV infection of monocytic cells: rôle of antibody-mediated virus binding to Fc-gamma receptors. AIDS. 1989 Mar;3(3):125–133. [PubMed] [Google Scholar]
- Karpas A., Fleet G. W., Dwek R. A., Petursson S., Namgoong S. K., Ramsden N. G., Jacob G. S., Rademacher T. W. Aminosugar derivatives as potential anti-human immunodeficiency virus agents. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9229–9233. doi: 10.1073/pnas.85.23.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kozarsky K., Penman M., Basiripour L., Haseltine W., Sodroski J., Krieger M. Glycosylation and processing of the human immunodeficiency virus type 1 envelope protein. J Acquir Immune Defic Syndr. 1989;2(2):163–169. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lifson J., Coutré S., Huang E., Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV) infectivity and virus-induced cell fusion. J Exp Med. 1986 Dec 1;164(6):2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Hamamoto Y., Yoshida T., Kido Y., Kobayashi S., Kobayashi N., Yamamoto N. Effect of culture supernatant of MT-2 cells on human immunodeficiency virus-producing cells, MOLT-4/HIVHTLV-IIIB cells. Jpn J Cancer Res. 1988 Feb;79(2):156–159. doi: 10.1111/j.1349-7006.1988.tb01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Mizuochi T., Spellman M. W., Larkin M., Solomon J., Basa L. J., Feizi T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem J. 1988 Sep 1;254(2):599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Mitchell W. M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Hoke G. M., Sarngadharan M. G. Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3384–3388. doi: 10.1073/pnas.86.9.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tilley S. A., Bona C., Zaghouani H., Gorny M. K., Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Evidence that mannosyl residues are involved in human immunodeficiency virus type 1 (HIV-1) pathogenesis. AIDS Res Hum Retroviruses. 1987 Fall;3(3):265–282. doi: 10.1089/aid.1987.3.265. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Kitame F., Nishimura H., Nakamura K. Operational and topological analyses of antigenic sites on influenza C virus glycoprotein and their dependence on glycosylation. J Gen Virol. 1988 Mar;69(Pt 3):537–547. doi: 10.1099/0022-1317-69-3-537. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Zvelebil M. J., Sternberg M. J., Cookson J., Coates A. R. Predictions of linear T-cell and B-cell epitopes in proteins encoded by HIV-1, HIV-2 and SIVMAC and the conservation of these sites between strains. FEBS Lett. 1988 Dec 19;242(1):9–21. doi: 10.1016/0014-5793(88)80976-1. [DOI] [PubMed] [Google Scholar]