Abstract
OBJECTIVE—1,5-anhydroglucitol (1,5-AG) is a short-term marker of metabolic control in diabetes. Its renal loss is stimulated in hyperglycemic conditions by glycosuria, which results in a lowered plasma concentration. As a low renal threshold for glucose has been described in hepatocyte nuclear factor-1α (HNF-1α) maturity-onset diabetes of the young (MODY), the 1,5-AG level may be altered in these patients. The purpose of this study was to assess the 1,5-AG levels in patients with HNF-1α MODY and in type 2 diabetic subjects with a similar degree of metabolic control. In addition, we aimed to evaluate this particle as a biomarker for HNF-1α MODY.
RESEARCH DESIGN AND METHODS—We included 33 diabetic patients from the Polish Nationwide Registry of MODY. In addition, we examined 43 type 2 diabetic patients and 47 nondiabetic control subjects. The 1,5-AG concentration was measured with an enzymatic assay (GlycoMark). Receiver operating characteristic (ROC) curve analysis was used to evaluate 1,5-AG as a screening marker for HNF-1α MODY.
RESULTS—The mean 1,5-AG plasma concentration in diabetic HNF-1α mutation carriers was 5.9 μg/ml, and it was lower than that in type 2 diabetic patients (11.0 μg/ml, P = 0.003) and in nondiabetic control subjects (23.9 μg/ml, P < 0.00005). The ROC curve analysis revealed 85.7% sensitivity and 80.0% specificity of 1,5-AG in screening for HNF-1α MODY at the criterion of <6.5 μg/ml in patients with an A1C level between 6.5 and 9.0%.
CONCLUSIONS—1,5-AG may be a useful biomarker for differential diagnosis of patients with HNF-1α MODY with a specific range of A1C, although this requires further investigation. However, the clinical use of this particle in diabetic HNF-1α mutation carriers for metabolic control has substantial limitations.
1,5-anhydroglucitol (1,5-AG) is a monosaccharide that shows a structural similarity to glucose. Its main source in humans is dietary ingestion, particularly meats and cereals (1). In addition, ∼10% of 1,5-AG is derived from endogenous synthesis. 1,5-AG is generally not metabolized, and in healthy subjects it achieves a stable plasma concentration that reflects a steady balance between ingestion and urinary excretion (1).
1,5-AG is reabsorbed in renal tubules by an AG/fructose/mannose common transport system that is distinct from the major glucose reabsorption system (2,3). In hyperglycemic conditions, the excess of glucose is reabsorbed owing to chemical similarity not only by its own specific active transporters, but also by the AG/fructose/mannose transporter; therefore, it competes with 1,5-AG. Subsequently, 1,5-AG urinary excretion is increased during hyperglycemia, and it results in a lowered plasma concentration (4). This explains its low plasma level in patients with poorly controlled diabetes. This particle was established in clinical practice as a short-term marker of metabolic control, and recently it has been investigated as a marker of postprandial hyperglycemia (5,6). In contrast to A1C, it reflects changes in glycemic control over a period of 1–2 weeks (5).
The 1,5-AG excretion rate depends on the renal threshold for glucose (7). Thus, its clinical usefulness in evaluation of some groups of patients, for example, pregnant women and subjects with end-stage renal disease, is obvious (7,8). Interestingly, the decreased renal threshold for glucose was observed in patients with hepatocyte nuclear factor-1α (HNF-1α) maturity-onset diabetes of the young (MODY), formerly classified as MODY3, and in nondiabetic mutation carriers of this gene (9,10). Diabetes that results from HNF-1α mutations is usually accompanied by extrapancreatic features. One of them is a tubulopathy that results in a low renal threshold for glucose. An animal model suggests that it is caused by decreased expression of sodium-glucose cotransporter 2, a low-affinity, high-capacity transporter in proximal renal tubules (11). Thus, hypothetically, the increased glucose load in renal tubules in these patients may cause stronger competition with 1,5-AG for reabsorption and, subsequently, increased urinary loss. Moreover, one cannot exclude the impact of HNF-1α mutations on the expression of the AG/fructose/mannose transporter. Therefore, in HNF-1α MODY, lower plasma concentrations of 1,5-AG compared with those in other types of diabetes can be expected at a similar A1C. In the scenario of a very low 1,5-AG level, this particle should also be considered as a candidate biochemical marker for this monogenic type of diabetes, which may have considerable clinical implications and enable screening for patients with HNF-1α MODY in large cohorts, potentially avoiding the expensive and laborious technique of direct gene sequencing. In this study, we compared 1,5-AG plasma concentrations in diabetic HNF-1α mutation carriers with those in patients with type 2 diabetes and in nondiabetic subjects.
RESEARCH DESIGN AND METHODS
The Nationwide Registry of MODY has been established at the Department of Metabolic Diseases, Jagiellonian University Medical College, Krakow, Poland. Details of the inclusion criteria and the examination were reported previously (12). So far, 57 HNF-1α mutation carriers have been identified. We contacted 47 adult subjects from this group, and 41 agreed to enter this study. The following exclusion criteria were used: pregnancy, liver cirrhosis, hypo- or hyperalimentation, steroid therapy, gastrectomy, and elevated serum creatinine level. Based on these criteria, we excluded four patients because of increased serum creatinine concentration (i.e., >97 μmol/l, the upper reference limit for the assay). Thus, the study group for examination consisted of 37 subjects. The number of patients with HNF-1α MODY, with diabetes diagnosed according to World Health Organization criteria, was 33, whereas 4 subjects were normoglycemic in the fasting condition and were classified as nondiabetic subjects, which is also supported by the fact that they all had A1C levels within the normal range (<6.0%). Two more groups were examined. The first group, 47 apparently healthy control subjects who were normoglycemic in the fasting condition, contained spouses of patients with MODY and volunteers from the medical personnel of the Department of Metabolic Diseases. The second group included 43 patients with type 2 diabetes diagnosed at age ≥35 years for whom no insulin therapy was used for at least 2 years after diagnosis, ascertained as previously described (13). The same exclusion criteria were used as for the MODY group. The study protocol and informed consent procedures were approved by the ethics committee of Jagiellonian University, Medical College and were concordant with the Declaration of Helsinki.
The blood samples were collected in fasting conditions for biochemical evaluation. Serum and EDTA-plasma were obtained by spinning at 3,500 rpm and stored at −80°C. The 1,5-AG concentration was measured in EDTA-plasma samples with an enzymatic, colorimetric method using GlycoMark chemicals (Tomen America) by Nippon Kayaku Tokyo Co. (Tokyo, Japan) (14). A1C was measured with high-performance liquid chromatography (Bio-Rad). Fasting glucose levels were determined by an enzymatic technique (automated analysis glucose GOD-PAP method; Boehringer Mannheim). Serum creatinine concentrations were measured with the Jaffe method.
Comparisons were made with the χ2 or Fisher's exact test, where applicable, for frequencies of qualitative traits. For quantitative traits, Student's t test or ANOVA was used with the Tukey post hoc test when data fit into a normal distribution. Otherwise, the Mann-Whitney or the Kruskal-Wallis test was used. The data for which the Kruskal-Wallis test was significant were further analyzed with a nonparametric multiple comparison Steel test. Normality was tested with the Kolmogorov-Smirnov test. Spearman's rank correlation was used to test the relationships between quantitative traits. Multiple linear regression was applied to determine independent predictors for the 1,5-AG level. These predictors were included as covariates in a general linear model analysis performed in all study groups and in the diabetic groups. Computations were performed with MiniTab 14.20 statistical software and the R Language and Environment, version 2.4.1. P < 0.05 was considered significant. In analysis of plasma 1,5-AG measurements as a biochemical test for HNF-1α MODY, the test parameters were calculated and receiver operating characteristic (ROC) curve analysis was performed with MedCalc 9.3.8.0 statistical software.
RESULTS
Clinical characteristics of the study groups are presented in Table 1. Both diabetic groups were similar for traditional measures of metabolic control such as fasting glucose and A1C. They varied, however, for age, BMI, and diabetes duration, which is in line with the way both groups were defined. Subjects from all groups also had an almost identical serum creatinine level.
Table 1.
Clinical characteristics of patients with type 2 diabetes and HNF-1α MODY and nondiabetic control subjects
| Type 2 diabetes | P value (post hoc)* | HNF-1α MODY | P value (post hoc)* | Control | P value | |
|---|---|---|---|---|---|---|
| n | 43 | NA | 33 | NA | 47 | NA |
| Sex (% female) | 65.1 | NA | 75.8 | NA | 61.7 | 0.41† |
| Age (years) | 56.2 ± 9.3 | <0.0005‡ | 42.9 ± 16.1 | 0.68‡ | 45.5 ± 15.3 | <0.0005§ |
| BMI (kg/m2) | 35.1 ± 7.9 | <0.0005‡ | 23.0 ± 3.4 | 0.13‡ | 25.5 ± 4.2 | <0.0005§ |
| Diabetes duration (years) | 8.8, 6.0, 6.0 | NA | 17.3, 16.0, 12.2 | NA | NA | 0.004‖ |
| Fasting glucose (mmol/l) | 7.6, 7.5, 1.7 | 0.62¶ | 7.9, 7.4, 1.9 | <0.00005¶ | 4.7, 4.8, 0.6 | <0.00005** |
| A1C (%) | 7.6, 7.1, 1.4 | NA | 7.6, 7.7, 1.1 | NA | NA | 0.77‖ |
| % OHA | 62.8 | NA | 45.5 | NA | NA | 0.13† |
| Creatinine (μmol/l) | 70.7 ± 12.7 | NA | 69.2 ± 12.6 | NA | 69.8 ± 10.6 | 0.86§ |
| 1,5-AG (μg/ml) | 11.0, 11.5, 6.7 | 0.003¶ | 5.9, 2.6, 4.3 | <0.00005¶ | 23.9, 24.5, 4.9 | <0.00005** |
Data are means ± SD or mean, median, quartile deviation. % OHA is the proportion of patients treated with oral hypoglycemic agents; the rest of the subjects were insulin treated in monotherapy or in combination with OHAs. Age, BMI, and serum creatinine concentration fit into normal distribution.
Non–normally distributed data, for which the Kruskal-Wallis test was significant, were further analyzed with Steel's nonparametric multiple comparison test.
χ2 test.
Tukey post hoc test.
One-way ANOVA.
Mann-Whitney test.
Steel test.
Kruskal-Wallis test. NA, not applicable.
The mean 1,5-AG plasma concentration in the diabetic HNF-1α mutation carriers group was 5.9 μg/ml (median 2.6 μg/ml, quartile deviation 4.3), whereas in the type 2 diabetic group it was 11.0 μg/ml (11.5 μg/ml, 6.7) and in the nondiabetic control group it was 23.9 μg/ml (24.5 μg/ml, 4.9). There was a highly significant difference between the MODY group and the control group (P < 0.00005) and between the MODY and type 2 diabetic groups (P = 0.003). To justify application of a parametric approach in further analysis, the data were reevaluated with ANOVA and the Tukey test, results of which were also significant (P < 0.00005 and P = 0.013, respectively). In four HNF-1α normoglycemic mutation carriers, the mean 1,5-AG level was 21.1 μg/ml (range 16.7–32.9 μg/ml) and was not different from the level in the control group (P = 0.32). The mean age of the four subjects was 24.7 years, mean BMI was 21.0 kg/m2, and mean A1C was 5.6%.
The 1,5-AG concentration was lower in women than in men in the control group (21.5 vs. 27.7 μg/ml, P = 0.003), whereas there were no significant differences between sexes in the MODY and type 2 diabetic groups (P = 0.71 and P = 0.91, respectively). In the MODY group, the 1,5-AG level significantly correlated only with the A1C level (ρ = −0.72, P < 0.0005), whereas in the type 2 diabetic group there was significant correlation of the 1,5-AG level with A1C (ρ = −0.72, P < 0.0005) and age of diabetes diagnosis (ρ = 0.36, P = 0.018). In multiple linear regression the A1C level remained the only significant predictor for 1,5-AG (P < 0.0005). In the nondiabetic control group, the 1,5-AG concentration significantly correlated with age (ρ = −0.52, P < 0.0005) and BMI (ρ = −0.34, P = 0.02). In the multiple regression model, the only significant predictor was age (P = 0.004).
The general linear model for 1,5-AG concentration as a dependent variable was applied in the three study groups combined (R2 = 52.1%). The differences in 1,5-AG level between the study groups remained significant (P < 0.0005), and age was a significant covariable (P = 0.044). In the diabetic study groups (R2 = 53.2%), the general linear model revealed a significant difference in 1,5-AG level between type 2 diabetes and HNF-1α MODY (P = 0.006), with A1C level as a significant covariable (P < 0.0005).
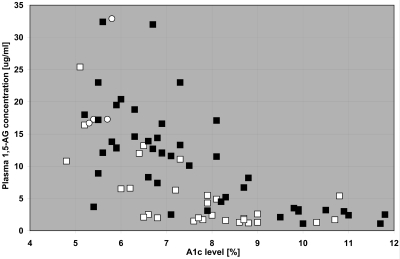
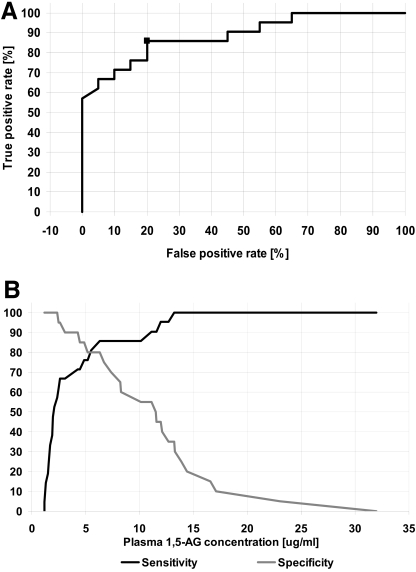
The ROC curve analysis was performed in diabetic patients: 33 with HNF-1α MODY and 43 with type 2 diabetes. Area under the ROC curve (AUC) was 0.732 (95% CI 0.618–0.827). The optimal sensitivity and specificity to test for MODY were 72.7% (54.5–86.7%) and 65.1% (49.1–79.0%), respectively, at a criterion of <6.6 μg/ml of plasma 1,5-AG. To further improve the parameters of the test, we performed additional analyses with a narrowed A1C range based on the plot showing relationships between A1C and 1,5-AG in diabetic groups (Fig. 1). Because type 2 diabetic patients with an A1C level >9.0% had a very low 1,5-AG level, similar to subjects with HNF-1α MODY, we first excluded all individuals with A1C above this value (5 patients with MODY and 10 with type 2 diabetes, respectively). This resulted in better ROC curve analysis outcomes, particularly the test specificity. The AUC was 0.823 (0.704–0.909), and optimal sensitivity and specificity at the criterion of <6.6 μg/ml 1,5-AG were 71.4% (51.3–86.7%) and 84.85% (68.1–94.8%), respectively. Additionally, we excluded 7 patients with MODY and 13 with type 2 diabetes who had A1C <6.5% because of a lack of correlation of 1,5-AG with A1C in both groups (P = 0.34 for MODY and P = 0.44 for type 2 diabetes). This exclusion improved the test specificity and resulted in the following parameters: AUC 0.887 (0.749–0.964) and optimal sensitivity at the criterion for MODY of <6.5 μg/ml 1,5-AG 85.7% (63.6–96.8%) and specificity 80.0% (56.3–94.1%). Figure 2 shows this ROC curve analysis in the group of patients with an A1C range between 6.5 and 9.0%.
Figure 1.
Individual value plot of 1,5-AG concentrations against A1C levels in HNF-1α MODY and type 2 diabetic groups. □, adult MODY patients; ▪, type 2 diabetic patients; ○, nondiabetic HNF-1α mutation carriers.
Figure 2.
ROC curve analysis for the diabetic patients with normal serum creatinine concentrations and A1C levels ≥6.5 and ≤9.0%. A: ROC curve. Marker represents optimal value. B: Sensitivity and specificity plotted against a cutoff value of plasma 1,5-AG concentration. Additional vertical gridlines represent the optimal cutoff value of 6.5 μg/ml.
CONCLUSIONS
Here we report for the first time the plasma 1,5-AG levels in patients with HNF-1α MODY. We observed that the levels of 1,5-AG in this group were significantly lower than those in type 2 diabetic subjects with similar glycemic control as assessed by A1C levels and fasting glucose level. We were prompted to perform this study by an earlier description of decreased renal threshold and increased glycosuria in HNF-1α mutation carriers (9,10). The major cause of low 1,5-AG levels in diabetic HNF-1α mutation carriers is, as in other diabetic patients, most likely its competition in the renal tubule with a large amount of glucose for the AG/fructose/mannose transporter (3). However, in HNF-1α mutation carriers, the threshold for glucosuria is lower, and therefore the excessive glucose in the renal tubule would be expected to result in greater decrements in 1,5-AG. One cannot rule out, however, that the additional mechanism associated with the impaired expression of this transporter is a direct result of a mutation in the HNF-1α gene encoding a transcription factor present in kidney. The confirmation of this later hypothesis will require expression gene experiments.
Our observation may have two important clinical implications. First, 1,5-AG measurements have limited usefulness in HNF-1α MODY as a marker of metabolic control. 1,5-AG is accepted by the U.S. Food and Drug Administration as a tool of monitoring glycemic control of diabetic patients. There were already some groups of patients described for whom 1,5-AG should not be recommended as a diabetes control measure owing to a low renal threshold for glucose, such as pregnant women and patients with end-stage renal disease (7,8), although some promising attempts of its use in gestational diabetes were described (15). In addition, there were some efforts to use 1,5-AG as a screening tool in diabetes (16,17). It should be emphasized, however, that no scientific diabetes organization recommended it for this purpose. If, however, 1,5-AG is accepted as a screening tool for diabetes at any time in the future, this should not include situations in which the renal threshold for glucose is altered, such as HNF-1α MODY.
At the same time, our observation points to 1,5-AG as a potential differential diagnosis biomarker for MODY associated with HNF-1α mutations. The measurement of plasma 1,5-AG level seems to have high sensitivity and specificity to differentiate between HNF-1α MODY and type 2 diabetes as long as A1C values are between 6.5 and 9.0%. It should be noted that most of the patients seen in everyday clinical practice fall into this range. Our study should be perhaps criticized for dissecting the data to improve the ROC curve and for choosing an arbitrary range of A1C for this analysis. As shown in Fig. 1, all individuals, MODY patients, and type 2 diabetic subjects with A1C >9.0% had very low 1,5-AG levels, probably due to a massive overload of renal tubules by glucose, and thus the marker is not specific in this range of A1C. The exclusion of subjects with low A1C values is justified by the fact that some degree of hyperglycemia is necessary to cause glycosuria and a subsequent fall in 1,5-AG level. The renal threshold in patients with HNF-1α MODY was measured at a mean whole blood glucose level of 6.5 mmol/l (the equivalent of 7.4 mmol/l in plasma) (9,10). In another study, during an oral glucose tolerance test, glycosuria was detected only in nondiabetic subjects with HNF-1α mutations and with impaired glucose tolerance (10). An additional argument constitutes a lack of correlation between A1C and 1,5-AG levels among subjects with well-controlled diabetes in our study and in an earlier report (18). Nevertheless, the specific range of A1C for which 1,5-AG is a useful biomarker for differential diagnosis will require further confirmation. Interestingly, all nondiabetic HNF-1α mutation carriers had levels of marker in the range of the 10th–90th percentile of the control subjects, indicating that 1,5-AG measurement is not useful for predictive testing.
Lack of postprandial hyperglycemia data in the subjects examined is a limitation of our study. As a consequence, we cannot entirely rule out the possibility that a higher degree of postprandial hyperglycemia in HNF-1α MODY patients might have partially contributed to the observed large, almost twofold difference in 1,5-AG level between both diabetic groups. There are, however, important indirect arguments on the key effect of renal tubulopathy. First, it was shown in two other clinical groups, pregnant women and patients with end-stage renal disease, that the renal threshold for glucose, which is substantially different from normal, seriously influenced the circulating 1,5-AG level (7,8). Because renal tubulopathy in HNF-1α MODY has been well documented previously (9,10), its influence on 1,5-AG seems inevitable. Second, our diabetic study groups were not only identical for A1C but also very similar for fasting glucose. Because the A1C level is influenced by both postprandial and fasting plasma glucose (19), it is very unlikely that putative significantly higher postprandial hyperglycemia had a substantial, if any, contribution to this huge difference in the 1,5-AG level. HNF-1α gene mutation carriers were previously reported as having higher postchallenge hyperglycemia compared with glucokinase mutation carriers (MODY2) (20). The latter group, however, is very specific and unusual, with its own very low postchallenge glucose increase (2–3 mmol at 2 h). No published data support a notion that HNF-1α MODY is characterized by greater postprandial hyperglycemia than type 2 diabetes. Previously, a particularly high effect of postprandial hyperglycemia (as assessed by a continuous glucose-monitoring system) on 1,5-AG level was described in a group of diabetic patients with moderately controlled diabetes (with A1C <8%); most of these patients had type 1 diabetes, which is characterized by absolute insulin deficiency (6). The phenomenon of a greater contribution of postprandial hyperglycemia to overall glycemic control in such patients with moderately controlled diabetes was described earlier (19). The comparison done between our diabetic groups with respect to 1,5-AG level included patients with a variable degree of glycemic control and also those with A1C values >8% (>30% of MODY subjects), in whom the impact of fasting plasma glucose is larger. Our study should also perhaps be criticized for choosing type 2 diabetic patients for the comparison, as their clinical characteristics were different from those of patients with HNF-1α mutations and, thus, some variables could have an impact on individual glucose profiles and therefore contribute to lower 1,5-AG despite seemingly equal A1C values. Two more groups of patients should be considered. The first group is subjects with other forms of MODY. Among those, the carriers of HNF-4α (MODY1) seem to be the most appropriate for the comparison because of its clinical similarity to HNF-1α MODY, relatively high frequency, and the lack of data on the altered glucose renal threshold (21,22). However, the families from The Polish Nationwide Registry of MODY have not been systematically tested for mutations in this gene, and we do not have a cohort of an appropriate size in this ethnic group. A second group that might be considered for the comparison of 1,5-AG with the diabetic HNF-1α mutation carriers is type 1 diabetic patients. There are, however, already biomarkers available to differentiate between MODY and type 1 diabetes, such as islet-specific antibodies and C-peptide.
In addition, it is difficult to provide positive and negative predictive values for the test based on serum 1,5-AG measurements. It would require a different study design in which testing is prospective and would not, as in our study, involve testing two discrete groups. Thus, we propose this as a future study. We believe that an application for 1,5-AG as a marker for differentiation between other MODY subtypes or in young type 2 diabetic subjects will be found eventually. The relative prevalence of HNF-1α MODY may constitute from one-third to two-thirds of families with early-onset autosomal dominant diabetes (23,24) and about 8% of young patients with type 2 diabetes (25). This should give a 1,5-AG–based test appropriate positive and negative predictive values. Finally, we should acknowledge that a relatively small number of HNF-1α MODY patients were examined.
Some general observations should also be emphasized. First, as expected, the 1,5-AG concentration was strongly inversely correlated with the A1C level in diabetic patients from both groups. The influence of sex and age on 1,5-AG levels was observed previously in control subjects (14,18) but, as with our data, not in the diabetic study groups, as reported from other populations (18). The creatinine level did not influence plasma 1,5-AG concentration in these study groups: all three groups had normal creatinine levels. The mean level of 1,5-AG in the control group was slightly higher than that in an American reference population (14), which may be caused by different nutritional habits in the Polish population, especially higher consumption of cereal food.
In summary, we conclude that 1,5-AG may be a useful biomarker for the differential diagnosis of patients with HNF-1α MODY with a specific range of A1C, although it requires further investigation in larger sets of patients and other diabetic groups, as well as direct assessment of the relative contribution of the low renal threshold for glucose and postprandial hyperglycemia. However, the clinical use of this particle in diabetic HNF-1α mutation carriers for metabolic control has substantial limitations.
Acknowledgments
This study was supported by the Polish Ministry of Education and Science (Grant 2 P05B 070 28) and by the Polish Diabetes Association. M.T.M. is supported by the EU Seventh Framework Programme CEED3 grant.
Published ahead of print at http://care.diabetesjournals.org on 20 May 2008.
E.B. is the president and managing director of BioMarker Group, which distributes 1,5-anhydroglucitol in the U.S.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Yamanouchi T, Tachibana Y, Akanuma H: Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am J Physiol 263:E268–E273, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Yamanouchi T, Shinohara T, Ogata N, Tachibana Y, Akaoka I, Miyashita H: Common reabsorption system of 1,5-anhydro-d-glucitol, fructose, and mannose in rat renal tubule. Biochim Biophys Acta 1291:89–95, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Ohtomo T, Inui K: Na+-dependent uptake of 1,5-anhydro-d-glucitol via the transport systems for d-glucose and d-mannose in the kidney epithelial cell line, LLC-PK1. Nippon Jinzo Gakkai Shi 38:435–440, 1996 [PubMed] [Google Scholar]
- 4.Stickle D, Turk J: A kinetic mass balance model for 1,5-anhydroglucitol: applications to monitoring of glycemic control. Am J Physiol 273:E821–E830, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, Akaoka L, Miyashita H: Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet 347:1514–1518, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S: 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 29:1214–1219, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick ES, Keevilt BG, Richmond KL, Newland P, Addison GM: Plasma 1,5-anhydroglucitol concentrations are influenced by variations in the renal threshold for glucose. Diabet Med 16:496–499, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Emoto M, Tabata T, Inoue T, Nishizawa Y, Morii H: Plasma 1,5-anhydroglucitol concentration in patients with end-stage renal disease with and without diabetes mellitus. Nephron 61:181–186, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Menzel R, Kaisaki PJ, Rjasanowski I, Heinke P, Kerner W, Menzel S: A low renal threshold for glucose in diabetic patients with a mutation in the hepatocyte nuclear factor-1α (HNF-1α) gene. Diabet Med 15:816–820, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Stride A, Ellard S, Clark P, Shakespeare L, Salzmann M, Shepherd M, Hattersley AT: β-Cell dysfunction, insulin sensitivity, and glycosuria precede diabetes in hepatocyte nuclear factor-1α mutation carriers. Diabetes Care 28:1751–1756, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Pontoglio M, Prie D, Cheret C, Doyen A, Leroy C, Froguel P, Velho G, Yaniv M, Friedlander G: HNF1α controls renal glucose reabsorption in mouse and man. EMBO Rep 1:359–365, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malecki MT, Skupien J, Gorczynska-Kosiorz S, Klupa T, Nazim J, Moczulski DK, Sieradzki J: Renal malformations may be linked to mutations in the hepatocyte nuclear factor-1α (MODY3) gene. Diabetes Care 28:2774–2776, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Bochenski J, Placha G, Wanic K, Malecki M, Sieradzki J, Warram JH, Krolewski AS: New polymorphism of ENPP1 (PC-1) is associated with increased risk of type 2 diabetes among obese individuals. Diabetes 55:2626–2630, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Nowatzke W, Sarnob MJ, Birchc NC, Sticklec DF, Edend T, Cole TG: Evaluation of an assay for serum 1,5-anhydroglucitol (GlycoMark) and determination of reference intervals on the Hitachi 917 analyzer. Clin Chim Acta 350:201–209, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Dworacka M, Wender-Ozegowska E, Winiarska H, Borowska M, Zawiejska A, Pietryga M, Brazert J, Szczawinska K, Bobkiewicz-Kozłowska T: Plasma anhydro-d-glucitol (1,5-AG) as an indicator of hyperglycaemic excursions in pregnant women with diabetes. Diabet Med 23:171–175, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Yamanouchi T, Akanuma Y, Toyota T, Kuzuya T, Kawai T, Kawazu S, Yoshioka S, Kanazawa Y, Ohta M, Baba S: Comparison of 1,5-anhydroglucitol, A1C, and fructosamine for detection of diabetes mellitus. Diabetes 40:52–57, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Robertson DA, Alberti KG, Dowse GK, Zimmet P, Tuomilehto J, Gareeboo H: Is serum anhydroglucitol an alternative to the oral glucose tolerance test for diabetes screening? The Mauritius Noncommunicable Diseases Study Group. Diabet Med 10:56–60, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Phillipou G, James SK, Frith RG, Farrant RK, Phillips PJ: Enzymatic quantification of 1,5-anhydro-d-glucitol: evaluation and clinical application. Clin Chem 40:1322–1326, 1994 [PubMed] [Google Scholar]
- 19.Monnier L, Lapinski H, Colette C: Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of A1C. Diabetes Care 26:881–885, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Stride A, Vaxillaire M, Tuomi T, Barbetti F, Njølstad PR, Hansen T, Costa A, Conget I, Pedersen O, Søvik O, Lorini R, Groop L, Froguel P, Hattersley AT: The genetic abnormality in the β cell determines the response to an oral glucose load. Diabetologia 45:427–435, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Stride A, Hattersley AT: Different genes, different diabetes: lessons from maturity-onset diabetes of the young. Ann Med 34:207–216, 2002 [PubMed] [Google Scholar]
- 22.Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, Wierzbicki AS, Clark PM, Lebl J, Pedersen O, Ellard S, Hansen T, Hattersley AT: Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4α mutations in a large European collection. Diabetologia 48:878–885, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kaisaki PJ, Menzel S, Lindner T, Oda N, Rjasanowski I, Sahm J, Meincke G, Schulze J, Schmechel H, Petzold C, Ledermann HM, Sachse G, Boriraj VV, Menzel R, Kerner W, Turner RC, Yamagata K, Bell GI: Mutations in the hepatocyte nuclear factor-1α gene in MODY and early-onset NIDDM: evidence for a mutational hotspot in exon 4. Diabetes 46:528–535, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Pearson ER, Velho G, Clark P, Stride A, Shepherd M, Frayling TM, Bulman MP, Ellard S, Froguel P, Hattersley AT: β-Cell genes and diabetes: quantitative and qualitative differences in the pathophysiology of hepatic nuclear factor-1α and glucokinase mutations. Diabetes 50(Suppl. 1):S101–S107, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki N, Oda N, Ogata M, Hara M, Hinokio Y, Oda Y, Yamagata K, Kanematsu S, Ohgawara H, Omori Y, Bell GI: Mutations in the hepatocyte nuclear factor-1α/MODY3 gene in Japanese subjects with early- and late-onset NIDDM. Diabetes 46:1504–1508, 1997 [DOI] [PubMed] [Google Scholar]