Abstract
OBJECTIVE—Heme oxygenase (HO) leads to the generation of free iron, carbon monoxide, and bilirubin. A length polymorphism of GT repeats in the promoter of human HO-1 gene has been shown to modulate gene transcription. The aim of this study was to assess the association of the length of (GT)n repeats in the HO-1 gene promoter with serum bilirubin, markers of iron status, and the development of coronary artery disease (CAD).
RESEARCH DESIGN AND METHODS—We screened the allelic frequencies of (GT)n repeats in the HO-1 gene promoter in 986 unrelated individuals who underwent coronary angiography. Serum bilirubin and markers of iron status were evaluated.
RESULTS—The distribution of numbers of (GT)n repeats was divided into two subclasses: class S included shorter (<27) repeats, and class L included longer (≥27) repeats. Among those with diabetes, subjects with the L/L genotype had significantly lower bilirubin levels than those with S/S and S/L genotypes (0.70 ± 0.22 vs. 0.81 ± 0.24 mg/dl, P = 0.001) and higher serum ferritin values (4.76 ± 0.72 vs. 4.28 ± 1.05 μg/l for log ferritin, P = 0.001). Compared with those carrying the S allele, diabetic subjects with the L/L genotype had an almost threefold increase in CAD risk after controlling for conventional risk factors (odds ratio 2.81, [95% CI 1.22–6.47], P = 0.015). With adjustment for both serum bilirubin and ferritin, the effect of HO-1 promoter polymorphism on susceptibility to CAD disappeared.
CONCLUSIONS—Length polymorphism in the HO-1 gene promoter is correlated with susceptibility to CAD in diabetic patients, and this effect might be conveyed through its influence on serum bilirubin and ferritin.
Heme oxidase (HO) is a rate-limiting enzyme in heme degradation that leads to the generation of free iron, biliverdin, and carbon monoxide. Biliverdin is subsequently converted to bilirubin via the action of biliverdin reductase, and free iron is promptly sequestered into ferritin. There are two genetically distinct isozymes of HO: the inducible HO-1 and a constitutively expressed HO-2. HO-1 is a cytoprotective enzyme upregulated in mammals mostly dependent on transcriptional activation of the HO-1 gene to diverse cellular stress.
The relationship of HO to atherosclerotic vascular disease was suggested initially in 1994 by an observational study reporting that low serum concentrations of bilirubin are associated with increased risk of coronary artery disease (CAD) (1). The human HO-1 gene has been mapped to chromosome 22q12, and a (GT)n dinucleotide repeat has been identified in the proximal promoter region (2). The (GT)n repeat is highly polymorphic and modulates gene transcription by oxidant challenge (3). We and others have demonstrated that a longer (GT)n repeat exhibits lower transcriptional activity and is associated with susceptibility to CAD in high-risk patients (4,5).
Bilirubin, a natural product of heme catabolism by HO, has been recognized to be an antioxidant and can inhibit lipid peroxidation (6). There is accumulating evidence that individuals with high-normal or just greater than normal plasma bilirubin levels have a lesser incidence of CAD and carotid plaque formation (7,8). HO-1 is also of critical contribution to iron homeostasis. The association between body iron status and the risk of cardiovascular disease was first postulated by Sullivan in the early 1980s (9) and thereafter by a number of epidemiological studies (10). Because HO-1 promoter polymorphism can conceivably affect the development of CAD, in the present study, the associations of the HO-1 promoter polymorphism with bilirubin levels, markers of iron status, and the development of CAD were examined.
RESEARCH DESIGN AND METHODS
The study population consisted of 986 unrelated adult patients who consecutively underwent coronary angiography in the Cardiology Division at Taipei Veterans General Hospital from August 1999 to October 2000. CAD was documented by angiographic evidence of ≥75% stenosis of at least one major coronary artery or a history of prior angioplasty, coronary artery bypass surgery, or myocardial infarction by history validated by electrocardiographic changes. The non-CAD group consisted of subjects who had normal coronary arteries as documented by angiography (<20% intraluminal obstruction) and had neither a history of atherosclerosis nor clinical or laboratory evidence of atherosclerosis in other vascular beds. This study protocol was approved by the review committee of Taipei Veterans General Hospital, and all participants gave their written informed consent.
Analysis of length variability of (GT)n repeats in HO-1 gene promoter
Genomic DNA was extracted from leukocytes by the conventional procedure. The 5′-flanking region containing (GT)n repeats of the HO-1 gene was amplified by PCR with a FAM-labeled sense primer, 5′-AGAGCCTGCAGCTTCTCAGA-3′, and an antisense primer, 5′-ACAAAGTCTGGCCATAGGAC-3′, as described previously (4). The PCR products were mixed together with a GenoType TAMRA DNA ladder (size range 50–500 bp; GibcoBRL) and analyzed with an automated DNA sequencer (ABI Prism 377). Each size of the (GT)n repeat was calculated using GeneScan Analysis software (PE Applied Biosystems).
Baseline measurements
Hypertension was defined as measured systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg. Diabetes was diagnosed on the basis of the World Health Organization criteria. Patients with hypercholesterolemia were defined as those having a total cholesterol level of >240 mg/dl or those who were receiving lipid-lowering therapy. Laboratory measurements were made on 12-h fasting venous blood samples. The biochemical indicators of iron status in this study included the serum iron concentration, the serum ferritin levels, the serum total iron-binding capacity (TIBC), and the serum transferrin saturation. Serum iron was measured with a colorimetric assay. Serum ferritin and TIBC values were assessed with an immunometric assay (Boehringer Mannheim). Transferrin saturation was calculated as the ratio of serum iron to TIBC.
Statistical analysis
All statistical analyses were conducted using the SPSS statistical package (version 10.0; SPSS, Chicago, IL). Distributions of continuous variables in groups were expressed as means ± SD and compared by t test for two groups or ANOVA using the least significant difference method for post hoc multivariate comparison of the means for more than three groups. Values of serum ferritin were log transformed because of their skewed distributions. Categorical variables were analyzed by χ2 test or Fisher's exact test. The association of CAD status with the allele frequency was assessed with consideration of confounding effects by known coronary risk factors, such as age, sex, diabetes, hypercholesterolemia, hypertension, and smoking habits. After preliminary bivariate analysis using the t test and χ2 test, multiple logistic regression analysis with forward stepwise selection was performed to evaluate the effect of genotype on CAD after controlling for other established risk factors of CAD. Significance was accepted at P < 0.05. All of the study participants were Chinese from northern Taiwan and had similar ethnic backgrounds.
RESULTS
The allele frequencies of (GT)n microsatellite polymorphism in the HO-1 promoter region were highly polymorphic, ranging from 16 to 38 (4). Because the proportion of allele frequencies of either <27 or >27 GT repeats was ∼50%, we classified the alleles into two subgroups: the lower component, with repeat number <27, was designated as “class S,” and the upper component, with ≥27 GT repeats, was designated as “class L.” These patients were then classified as having an S/S, S/L, or L/L genotype according to each of their HO-1 alleles.
Table 1 of the online appendix (available at http://dx.doi.org/10.2337/dc07-2126) shows the distribution of HO-1 promoter genotypes in all subjects and in those with hypertension (n = 639), diabetes (n = 263), or hypercholesterolemia (n = 179) and those who currently smoked (n = 260) stratified by the status of CAD. No significant difference in genotypic frequencies between the two groups (CAD vs. non-CAD) in the whole study population was observed. However, diabetes was found to have a significant interaction with genotypes: the proportions of S/S, S/L, and L/L genotypes were 36.5, 47.6, and 15.9%, respectively, in diabetic subjects without CAD and 18.5, 51.5, and 30.0%, respectively, in diabetic subjects with CAD.
Table 1.
General characteristics of the study population stratified by HO-1 promoter genotypes
| Genotype
|
P in all subjects | P in diabetic subjects | ||||||
|---|---|---|---|---|---|---|---|---|
| S/S
|
S/L
|
L/L
|
||||||
| All subjects | With diabetes | All subjects | With diabetes | All subjects | With diabetes | |||
| n | 221 | 60 | 489 | 133 | 276 | 79 | ||
| Age (years) | 70 ± 8 | 69 ± 8 | 68 ± 10 | 68 ± 9 | 69 ± 9 | 68 ± 9 | NS | NS |
| Male sex (%) | 90 | 87 | 89 | 85 | 88 | 80 | NS | NS |
| Hypertension (%) | 68 | 78 | 65 | 66 | 64 | 73 | NS | NS |
| Current smoker (%) | 34 | 28 | 28 | 26 | 29 | 29 | NS | NS |
| Fasting blood glucose (mg/dl) | 109 ± 36 | 148 ± 45 | 112 ± 46 | 155 ± 65 | 110 ± 44 | 151 ± 62 | NS | NS |
| Total cholesterol (mg/dl) | 192 ± 42 | 187 ± 43 | 187 ± 35 | 180 ± 35 | 189 ± 36 | 188 ± 40 | NS | NS |
| LDL cholesterol (mg/dl) | 124 ± 35 | 120 ± 31 | 119 ± 30 | 111 ± 33 | 122 ± 28 | 116 ± 32 | NS | NS |
| HDL cholesterol (mg/dl) | 40 ± 11 | 37 ± 11 | 39 ± 11 | 36 ± 9 | 40 ± 11 | 40 ± 15 | NS | NS |
| Serum triglycerides (mg/dl) | 144 ± 91 | 171 ± 109 | 146 ± 97 | 168 ± 109 | 159 ± 150 | 176 ± 104 | NS | NS |
| Bilirubin (mg/dl)l | 0.85 ± 0.27 | 0.83 ± 0.21 | 0.84 ± 0.30 | 0.81 ± 0.25 | 0.79 ± 0.25 | 0.70 ± 0.22 | 0.021 | 0.006 |
| Serum iron (μg/dl) | 80 ± 42 | 82 ± 90 | 78 ± 40 | 73 ± 84 | 73 ± 39 | 112 ± 17 | NS | NS |
| Serum ferritin (μg/l) | 99 ± 105 | 82 ± 63 | 121 ± 107 | 123 ± 105 | 127 ± 99 | 148 ± 104 | 0.031 | 0.009 |
| Log ferritin | 4.19 ± 0.95 | 4.10 ± 0.87 | 4.40 ± 0.98 | 4.36 ± 1.12 | 4.54 ± 0.88 | 4.76 ± 0.72 | 0.003 | 0.008 |
| TIBC (μg/dl) | 272 ± 102 | 279 ± 92 | 297 ± 160 | 296 ± 114 | 278 ± 124 | 287 ± 127 | NS | NS |
| Transferrin saturation (%) | 27 ± 17 | 27 ± 17 | 27 ± 13 | 23 ± 16 | 25 ± 15 | 24 ± 17 | NS | NS |
Data are expressed as means ± SD unless indicated otherwise.
The characteristics of the whole study population and subjects with diabetes stratified by HO-1 genotype are presented in Table 1. Across the three genotypes, only serum bilirubin and ferritin concentrations were significantly different in both the whole study population and subjects with diabetes. There were no significant differences in age, sex, percentages of risk factors, levels of serum cholesterol, triglycerides, fasting blood glucose, or markers of iron status including serum iron, TIBC, and transferrin saturation values.
The mean serum bilirubin level was higher in carriers of the S allele (0.85 ± 0.32 mg/dl) than in those with the L/L genotype (0.79 ± 0.25 mg/dl) (P = 0.013) in the whole study population, and the difference was more pronounced (0.81 ± 0.24 vs. 0.70 ± 0.22 mg/dl, P = 0.001) in subjects with diabetes. Serum ferritin levels were highest in subjects with the L/L genotype, intermediate in those with the S/L genotype, and lowest in those with the S/S genotype in the whole study population and in subjects with diabetes. When subjects with the L/L genotype and those carrying the S allele were compared, the ferritin level was significantly higher in subjects with the L/L genotype (127 ± 99 or 4.54 ± 0.88 μg/l for log ferritin) than in carriers of the S allele (114 ± 107 or 4.33 ± 0.98 μg/l for log ferritin) (P = 0.008 for log ferritin) in the whole study population. Among subjects with diabetes, this difference was again much greater (148 ± 104 vs. 111 ± 96 μg/l for ferritin, P = 0.031 or 4.76 ± 0.72 vs. 4.28 ± 1.05 for log ferritin, P = 0.001).
The baseline characteristics of the whole study population and subjects with diabetes stratified by the status of CAD are summarized in Table 2. When all subjects were considered, patients with CAD were older and had a higher percentage of male sex, higher fasting blood glucose and triglyceride levels, and a lower HDL value compared with those without CAD, as expected. Serum bilirubin levels were significantly lower (0.81 ± 0.30 vs. 0.87 ± 0.32 mg/dl, P = 0.006) and a trend toward a higher serum ferritin level was observed in patients with CAD (126 ± 124 vs. 110 ± 95 μg/l, P = 0.061). There was no difference in serum iron value, TIBC, or transferrin saturation between subjects with versus without CAD. On the other hand, with respect to demographic characteristics, the two groups of diabetic patients with versus without CAD only differed in percentages of male sex. Diabetic patients with CAD had significantly lower serum bilirubin levels (0.76 ± 0.23 vs. 0.86 ± 0.32 mg/dl, P = 0.040) and higher serum ferritin levels (141 ± 139 vs. 104 ± 102 μg/l or 4.54 ± 1.01 vs. 4.16 ± 1.10 μg/l for log ferritin, P = 0.024 for log ferritin).
Table 2.
Characteristics of the study population
| Subjects without CAD
|
Patients with CAD
|
P
|
||||
|---|---|---|---|---|---|---|
| All subjects | With diabetes | All subjects | With diabetes | CAD vs. non-CAD in all subjects | CAD vs. non-CAD in diabetes | |
| n | 322 | 63 | 664 | 200 | ||
| Age (years) | 67 ± 10 | 68 ± 9 | 69 ± 9 | 68 ± 9 | 0.002 | NS |
| Male sex (%) | 82 | 71 | 92 | 88 | <0.001 | 0.009 |
| Hypertension (%) | 63 | 78 | 66 | 68 | NS | NS |
| Current smoker (%) | 26 | 25 | 31 | 29 | NS | NS |
| Fasting blood glucose (mg/dl) | 104 ± 34 | 147 ± 47 | 114 ± 47 | 154 ± 63 | <0.001 | NS |
| Total cholesterol (mg/dl) | 187 ± 35 | 184 ± 33 | 190 ± 38 | 184 ± 40 | NS | NS |
| LDL cholesterol (mg/dl) | 118 ± 26 | 111 ± 26 | 122 ± 33 | 115 ± 34 | NS | NS |
| HDL cholesterol (mg/dl) | 42 ± 11 | 38 ± 12 | 39 ± 11 | 37 ± 11 | 0.001 | NS |
| Serum triglycerides (mg/dl) | 134 ± 75 | 164 ± 94 | 157 ± 128 | 173 ± 111 | 0.001 | NS |
| Bilirubin (mg/dl)l | 0.87 ± 0.32 | 0.86 ± 0.32 | 0.81 ± 0.30 | 0.76 ± 0.23 | 0.006 | 0.04 |
| Serum iron (μg/dl) | 80 ± 43 | 70 ± 32 | 75 ± 37 | 77 ± 42 | NS | NS |
| Serum ferritin (μg/l) | 110 ± 95 | 104 ± 102 | 126 ± 124 | 141 ± 139 | NS | NS |
| Log ferritin | 4.31 ± 0.98 | 4.16 ± 1.10 | 4.42 ± 0.99 | 4.54 ± 1.01 | NS | 0.024 |
| TIBC (μg/dl) | 285 ± 129 | 312 ± 197 | 286 ± 144 | 299 ± 158 | NS | NS |
| Transferrin saturation (%) | 30 ± 14 | 26 ± 12 | 28 ± 12 | 28 ± 12 | NS | NS |
Data are expressed as means ± SD; n = 986.
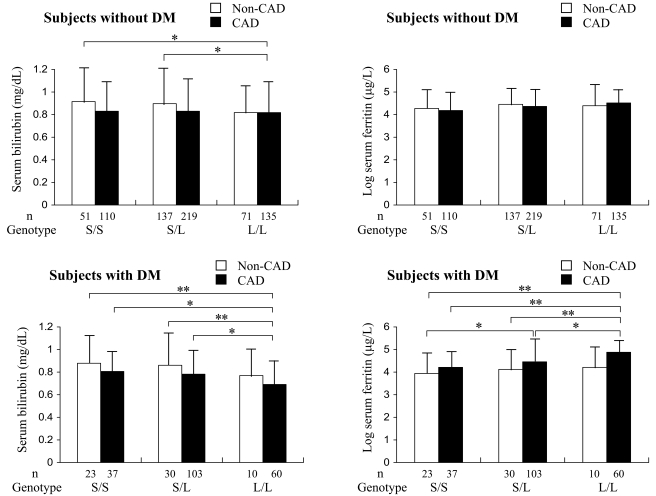
The relations between serum bilirubin and ferritin levels, HO-1 genotypes, and CAD are shown in Fig. 1. Among subjects with diabetes, serum bilirubin levels in patients with CAD who had the L/L genotype were significantly lower than those in carriers of the S allele, regardless of their CAD status, whereas differences in serum bilirubin levels between carriers of the L/L genotype with and without CAD were not statistically significant. On the other hand, log ferritin values in patients with CAD who had the L/L genotype were significantly higher than those in carriers of the S allele with or without CAD. Differences in log ferritin values between patients with and without CAD who had the L/L genotype, although substantial, did not reach statistical significance. Differences in both serum bilirubin and ferritin levels in nondiabetic subjects were much less prominent than those in subjects with diabetes.
Figure 1.
Serum bilirubin and log ferritin levels according to three genotypes and CAD status. Data are means ± SD. *P < 0.05; **P < 0.01. DM, diabetes.
We then performed multivariate analyses to further examine the links between serum bilirubin and ferritin levels, HO-1 genotypes, and CAD in diabetic patients. After we controlled for conventional risk factors, carriers of the L/L genotype showed significantly enhanced susceptibility to CAD compared with those carrying the S allele, resulting in an odds ratio (OR) of 2.81 (95% CI 1.22–6.47, P = 0.015) (Table 3). As a next step, we investigated the association of serum bilirubin and ferritin levels with CAD separately. When the HO-1 genotype was not included in the model, a 0.1 mg/dl increase in bilirubin levels decreased CAD risk by 16% and 1 log unit elevation in ferritin values increased CAD risk by 41%. After we included both the HO-1 promoter genotype and bilirubin levels in the logistic regression model, the OR of HO-1 effect fell to 2.65 and became less significant (95% CI 1.05–6.69, P = 0.040). When both the HO-1 promoter genotype and ferritin values were included, the OR of HO-1 effect decreased to 2.31 and was of borderline significance (95% CI 0.97–5.49, P = 0.058). With adjustment of both serum bilirubin and ferritin values, the OR of HO-1 effect was reduced further to 1.71 and became nonsignificant (95% CI 0.75–3.90, P = 0.203) (Table 3).
Table 3.
Association of HO-1 promoter genotypes with the risk of CAD among diabetic patients
| OR (95% CI)
|
|||
|---|---|---|---|
| L/L vs. L/S + S/S | Bilirubin (per 0.1 mg/dl) | Log ferritin (per 1 unit) | |
| Adjusted for traditional risk factors* | 2.81 (1.22–6.47), P = 0.015 | 0.84 (0.73–0.97), P = 0.016 | 1.41 (1.04–1.89), P = 0.025 |
| Additionally adjusted for bilirubin | 2.65 (1.05–6.69), P = 0.040 | ||
| Additionally adjusted for ferritin | 2.31 (0.97–5.49), P = 0.058 | ||
| Additionally adjusted for both bilirubin and ferritin | 1.71 (0.75–3.90), P = 0.203 | ||
Adjusted for age, sex, history of hypertension, hypercholesterolemia, and smoking status.
CONCLUSIONS
Decreased HO-1 expression has been shown in humans and experimental animals with diabetes (11,12), and an inverse relationship between the HO-1 activity and vascular complications associated with diabetes was demonstrated (13). In line with these findings, our previous study (4) revealed that the length polymorphism in the HO-1 gene promoter is correlated with susceptibility to CAD in diabetic patients. In the present study, we further demonstrated that this effect might be conveyed through its influence on bilirubin and ferritin.
The concept that HO-1 may be causally related to cardiovascular diseases in humans has been suggested by studies assessing the (GT)n dinucleotide length polymorphism in the 5′-flanking sequence of the human HO-1 gene. By using HO-1 promoter/luciferase reporter genes carrying different lengths of (GT)n repeats, we demonstrated previously that the more (GT)n repeats in the promoter region, the less transcriptional activity of the HO-1 gene in rat aortic smooth muscle cells (4); a similar result was also shown earlier in Hep3B cells (3).
Bilirubin is a natural product of heme catabolism by HO. Here we demonstrated that there is an association between HO-1 promoter polymorphism and serum bilirubin levels, which are correlated with the development of CAD. The mean serum bilirubin level was significantly higher in carriers of the S allele than in those with the L/L genotype. In a previous case-control study of individuals with early familial CAD, higher serum bilirubin concentrations within the normal range were associated with a significant and marked reduction in CAD risk (7). In the prospective Framingham Offspring Study, higher serum bilirubin concentrations were associated with a decreased incidence of ischemic heart disease (8). Considering the antioxidant and antiatherogenic properties of bilirubin, the beneficial influence on serum bilirubin in carriers of the S allele might exert a protective effect against the development of CAD.
HO releases free ferrous (Fe2+) iron from heme. The toxic effect of free iron has been linked to oxidative stress through the Fenton reaction, in which Fe2+ oxidizes H2O2, leading to the generation of hydroxyl radicals (14), which in turn initiate lipid peroxidation. The amount of free ferrous iron is normally maintained at a very low level in humans. Of all the iron in the body (4 g), approximately two-thirds is found in association with hemoglobin in the ferrous form, and the majority of the remainder is stored as ferritin. In 1981, Sullivan (9) suggested that a state of iron depletion was potentially protective against coronary heart disease. Although the majority of animal research and the in vitro human studies support a role of iron in the pathogenesis of atherosclerosis, prospective human studies have provided inconsistent results in terms of clinical cardiovascular outcomes (10). Some investigators have hypothesized that iron may be primarily involved in the early stage of atherosclerosis, and focusing on cardiovascular morbidity and mortality (reflecting later stages of the disease) may not give insight into the potential mechanistic role of iron (15). Likewise, one recent study demonstrated that reduction of body iron stores by phlebotomy in patients with peripheral arterial disease produced a significant improvement in cardiovascular outcomes in patients aged <60 years but not in those at an older age (and thus with more advanced atherosclerosis) (16).
In the present study, for the first time, we demonstrated that there is an association between HO-1 promoter polymorphism and serum ferritin concentrations, a measure of the body's iron stores, and an association between ferritin concentrations and the development of CAD in diabetic subjects. The mechanisms by which HO-1 polymorphism confers the variance in ferritin values remain to be elucidated. Nevertheless, a few animal studies and clinical data provided some indirect clues. A mouse model deficient in mammalian HO-1 (Hmox1) developed pathological accumulation of tissue iron stores associated with an increase in serum ferritin levels (17). HO-1 deficiency is very rare in humans. The first autopsy case of HO-1 deficiency was a 6-year-old boy who presented with growth retardation, anemia, elevated serum levels of ferritin and heme, low serum bilirubin concentrations, and hyperlipidemia. Fatty streaks and fibrous plaques were noted in his aorta (18). Moreover, treatment of healthy volunteers, patients with primary biliary cirrhosis, and patients with idiopathic hemochromatosis substantially with HO inhibitors increased serum ferritin concentrations (19). We hence postulated that the lower expression level of HO-1 imposed by the L allele under higher oxidative stress, as in the setting of diabetes, increases iron load in the vascular system, which may contribute to the development of atherosclerosis in such a virulent way.
The present study has strengths and limitations. Strengths include the large number of patients and the fact that all subjects had coronary arteriography and measures of bilirubin and ferritin. Furthermore, the homogeneous ethnic background possibly reduces variability in measurements. Among the study limitations is that the sample is primarily Chinese, making generalization to the other ethic groups uncertain. Furthermore, the present study design was cross-sectional, and we cannot infer causality.
In summary, we have demonstrated that the microsatellite polymorphism in the promoter of HO-1 gene imposes modulation on serum bilirubin and ferritin levels, which might be associated with the development of CAD among diabetic subjects.
Supplementary Material
Published ahead of print at http://care.diabetesjournals.org on 28 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Schwertner HA, Jackson WG, Tolan G: Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 40:18–23, 1994 [PubMed] [Google Scholar]
- 2.Lavrovsky Y, Schwartzman MC, Levere RD, Kappas A, Abraham NG: Identification of binding sites for transcription factors NF-κB and AP-2 in the promoter region of the human heme oxygenase-1 gene. Proc Natl Acad Sci USA 91:5987–5991, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H: Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 66:187–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY: Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111:1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, Aizawa T, Ishizaka N, Nagai R: Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol 22:1680–1685, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN: Bilirubin is an antioxidant of possible physiological importance. Science 235:1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR: Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 16:250–255, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Djousse L, Levy D, Cupples LA, Evans JC, D'Agostino RB, Ellison RC: Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol 87:1196–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JL: Iron and the sex difference in heart disease risk. Lancet 1:1293–1294, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Danesh J, Appleby P: Coronary heart disease and iron status: meta-analysis of prospective studies. Circulation 99:852–854, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Bruce CR, Carey AL, Hawley JA, Febbraio MA: Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52:2338–345, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D'Amico M: Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes 54:803–810, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Abraham NG, Rezzani R, Rodella L, Kruger A, Taller D, Li Volti G, Goodman AI, Kappas A: Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am J Physiol Heart Circ Physiol 287:H2468–H2477, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Heinecke JW, Rosen H, Chait A: Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest 74:1890–1894, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakrishna G, Rooke TW, Cooper LT: Iron and peripheral arterial disease: revisiting the iron hypothesis in a different light. Vasc Med 8:203–210, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW: Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA 297:603–610, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Poss KD, Tonegawa S: Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94:10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawashima A, Oda Y, Yachi A, Koizumi S, Nakanishi I: Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol 33:125–130, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Berglund L, Galbraith RA, Emtestam L, Drummond GS, Angelin B, Kappas A: Heme oxygenase inhibitors transiently increase serum ferritin concentrations without altering other acute-phase reactants in man. Pharmacology 59:51–56, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.