Abstract
OBJECTIVE—To determine whether increased daily physical activity improves mitochondrial function and/or lipid oxidation in type 2 diabetes.
RESEARCH DESIGN AND METHODS—Volunteers with (n = 10) and without (n = 10) type 2 diabetes were matched for habitual physical activity, age, sex, and weight. Basal and maximal mitochondrial activity, physical activity, and resting substrate oxidation were measured at baseline and after 2 and 8 weeks of increased physical activity.
RESULTS—Baseline physical activity (6,450 ± 851 vs. 7,638 ± 741 steps/day), basal ATP use (12 ± 1 vs. 12 ± 1 μmol · ml−1 · min−1), phosphocreatine recovery from exercise (31 ± 5 vs. 29 ± 3 s), and basal lipid oxidation (0.57 ± 0.07 vs. 0.65 ± 0.06 mg · kg body wt−1 · min−1) were similar in people with and without type 2 diabetes. There was a significant increase in physical activity after 8 weeks (12,322 ± 1,979 vs. 9,187 ± 1,159 steps/day, respectively). Following increased physical activity, there were no changes in basal ATP use or phosphocreatine recovery after exercise in either group. Basal lipid oxidation increased after 8 weeks of increased physical activity in people with type 2 diabetes (0.79 ± 0.08 mg · kg−1 · min−1) but not people without (0.68 ± 0.13 mg · kg body wt−1 · min−1).
CONCLUSIONS—Resting and maximal ATP turnover are not impaired in people with well-controlled type 2 diabetes compared with control subjects matched for physical activity as well as age and weight. Increased unsupervised daily physical activity is sustainable and improves lipid oxidation independent of change in mitochondrial activity in people with type 2 diabetes.
The potential role of the mitochondria in the development of insulin resistance and type 2 diabetes has recently attracted much interest. Muscle biopsies taken from people with type 2 diabetes demonstrate smaller mitochondria and lower activities of oxidative enzymes compared with those of lean individuals without diabetes (1). Insulin-resistant people with a family history of diabetes have reduced basal mitochondrial activity in skeletal muscle compared with insulin-sensitive individuals (2). These observations, in combination with others (3–6), raise the possibility that mitochondrial defects could underlie type 2 diabetes. Defects in oxidative function could possibly help explain the impaired fatty acid oxidation (7) and elevated intramyocellular lipid (IMCL) (8) characteristic of impaired insulin action and type 2 diabetes. The elevated intramuscular lipid may affect insulin signaling in skeletal muscle (5), exacerbating insulin resistance.
However, other studies have not observed abnormalities in basal mitochondrial activity in skeletal muscle of people with type 2 diabetes (9). Recent biopsy work has also shown that differences in oxidative enzymes between people with and without type 2 diabetes disappear when corrected for mitochondrial density (10). These data raise the possibility that type 2 diabetes is associated with normal mitochondrial function but that the mitochondrial capacity is reduced. This is an important differentiation, as it holds implications for the therapeutic approach to type 2 diabetes.
People with type 2 diabetes are more sedentary than those without diabetes (11). It is clear that reversing this sedentary lifestyle with physical activity and/or exercise can produce significant improvements in long-term glucose control (12). These benefits could be mediated, at least in part, by changes in mitochondrial function (13). In people with type 2 diabetes, moderate-intensity exercise combined with moderate weight loss produced a significant improvement in insulin sensitivity and mitochondrial density (14). However, such moderate intensity exercise programs are difficult to implement and usually require close supervision. In contrast, unsupervised walking has been shown to produce significant improvements in long-term glucose control and is a sustainable behavior over long periods of time (2 years) (15). Little is known about how low-intensity physical activity interventions such as walking influence muscle metabolism in people with type 2 diabetes.
This study was designed to 1) determine whether there are differences in basal and stimulated mitochondrial activity in people with type 2 diabetes compared with physical activity–matched control subjects and 2) establish whether an increase in daily physical activity is associated with changes in mitochondrial ATP turnover and changes in lipid oxidation.
RESEARCH DESIGN AND METHODS
Subject information and initial testing
Sedentary people with type 2 diabetes (>2 years duration, A1C <7.5%, stable control on either diet or diet plus sulfonylurea and/or metformin) (n = 10) and age-, weight-, and physical activity–matched people without type 2 diabetes (n = 10) were recruited. Volunteers with heart, liver, kidney, or diabetic foot disease or those undertaking a physical activity program were excluded. Participants were assessed before and after 2 and 8 weeks of increased physical activity. At each time point, physical activity, resting substrate oxidation, fasting plasma glucose, and A1C were assessed. Basal and maximal ATP use and IMCL were quantitated using magnetic resonance techniques. For all metabolic evaluations, participants were transported to the magnetic resonance facility by taxi and data were collected in the fasted state. Participants provided informed consent to join the study, and the study was approved by the local research ethics committee.
Magnetic resonance acquisition
Magnetic resonance data were acquired using a 3T Achieva scanner (Philips, Best, the Netherlands) with an in-built body coil used for imaging, a 14-cm diameter surface coil for phosphorus spectroscopy, and a 10-cm diameter pair of flexible coils (Philips) for proton spectroscopy.
Resting ATP flux
This technique has previously been described in nontechnical terms (16). In brief, a saturation transfer sequence was used to measure transfer of magnetization between γ-ATP and inorganic phosphate (Pi) (17). The steady-state magnetization of Pi was measured during selective irradiation of γ-ATP (Mz) and compared with the equilibrium Pi magnetization with the irradiation placed symmetrically downfield from the Pi frequency (TR = 25 s, bandwidth = 3,000 Hz, 2,048 points, 16 averages) (Mo). The fractional reduction of Pi magnetization upon saturation of γ-ATP, (Mo − Mz)/Mo, was used to calculate the pseudo–first order rate constant using the Forsen-Hoffman equation (18). T1* was measured using an inversion recovery experiment (τ1 − 180° − τ2 − 90° − acquire, TR = 25 s, four averages), while saturation of γ-ATP was performed during the delay times τ1 and τ2. Broadband proton decoupling was used. Eight variable τ2 time delays were used ranging from 635 to 9,035 ms. The intraday variability of the method is 6.5% and interday variation 8.0%.
Maximal ATP generation
Plantar flexion exercise at 30% of the maximum voluntary contraction was performed in the magnetic resonance imaging scanner on a custom-built device. The study protocol consisted of 3 min of plantar flexion at 2 Hz and 3 min of rest, changing pH levels as little as possible (19). Phosphorus spectra were collected at 10-s intervals throughout exercise (NS = 2, bandwidth = 3,000 Hz, 2,048 points, broadband decoupling, and NOE).
IMCL
Proton magnetic resonance spectroscopy was acquired using a localized PRESS sequence (voxel size 15 × 15 × 20 mm) in soleus with water suppression (TR/TE/NSA = 3,000 ms/37 ms/32,2048 samples, bandwidth 2,000 Hz). Sixteen unsuppressed averages were collected for reference.
Quantitation of spectra
Analysis of all spectra was performed with jMRUI (version 3.0) (20) using AMARES (21), with custom prior knowledge. Phosphcreatine (PCr) concentrations were calculated by measuring PCr relative to β-ATP, correcting for magnetic saturation and assuming a resting (ATP) of 8.2 mmol/l (19). Maximal ATP production was assessed from postexercise PCr kinetics (19). Proton spectra were analyzed for IMCL and expressed relative to the water reference peak.
Physical activity
Physical activity was assessed over 3 days using a validated multisensor armband (22) (SenseWear; Bodymedia, Pittsburgh, PA). Physical activity goals were set, with participants targeting 45 min of extra walking per day, and the benefits to glucose control (diabetes group) and long-term well-being (control group) discussed. Participants were also provided with a pedometer, they recorded the pedometer reading, and they received periodic phone calls from the research team.
Indirect calorimetery
Expired gases were collected from a constant-flow hood calorimeter (Deltatrac; Datex Ohmeda, Hertfordsire, U.K.) over 30 min. Substrate oxidation rates and energy expenditure were calculated from oxygen consumption and carbon dioxide production values using stoichiometric equations (23).
Whole-blood glucose and plasma insulin
Whole-blood glucose was measured (YSI glucose analyzer; YSI, Yellow Springs, OH). Plasma insulin was measured using an enzyme-linked immunosorbent assay kit (DAKO, Ely, U.K.). A1C was measured using high-performance liquid chromatography (TOSOH, Tokyo, Japan).
Statistical analysis
Statistical calculations were performed using SPSS version 11 (SPSS, Chicago, IL). Two-way ANOVA (time and treatment) was used to assess metabolic and physiological differences between groups. Statistical significance was accepted at P < 0.05. Data are presented as means ± SE of the mean, unless otherwise stated.
RESULTS
Baseline group description
The characteristics of the type 2 diabetes and control groups are given in Table 1. Groups were matched for age, sex, and weight. The diabetic group was shorter than the control group, resulting in a higher BMI. Habitual physical activity was similarly low in both groups. Participants with type 2 diabetes had good glucose control, demonstrated by a mean A1C of 6.7 ± 0.3% and a fasting whole-blood glucose concentration of 7.1 ± 0.4 mmol/l.
Table 1.
Baseline physical and metabolic characteristics of subjects
| Type 2 diabetic subjects | Control subjects | |
|---|---|---|
| Age (years) | 59 ± 2 | 56 ± 2 |
| Weight (kg) | 91 ± 4 | 88 ± 4 |
| BMI | 33 ± 1* | 30 ± 1 |
| Energy expenditure (kcal/day) | 2,319 ± 112 | 2,460 ± 146 |
| Total steps/day | 6,450 ± 851 | 7,638 ± 741 |
| Systolic blood pressure (mmHg) | 143 ± 2* | 134 ± 5 |
| Diastolic blood pressure (mmHg) | 87 ± 2 | 85 ± 3 |
| A1C (%) | 6.7 ± 0.3† | 5.7 ± 0.1 |
| Fasting glucose (mmol/l) | 7.1 ± 0.4† | 5.5 ± 0.2 |
| Insulin (mμ/l) | 13.9 ± 3.5† | 7.6 ± 1.5 |
Data are means ± SE.
Significantly different from control subjects (P < 0.05).
Significantly different from control subjects (P < 0.01).
Physical activity
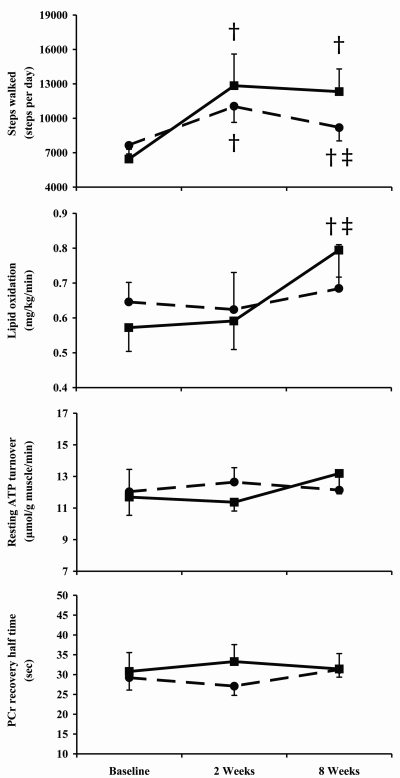
The physical activity monitors showed that both groups had low baseline activity levels. There was no significant difference in the number of steps taken per day between the diabetes and control groups at baseline (Fig. 1). Following counseling, both groups demonstrated a sustained increase in the number of steps taken per day (P < 0.05, Fig. 1). At 8 weeks, both groups undertook more steps than baseline; however, the control group had reduced the number of steps from the 2-week point (P < 0.05) (Fig. 1).
Figure 1.
Number of steps walked, basal ATP flux, and basal lipid oxidation by people with (▪) and without (•) type 2 diabetes at baseline and after 2 and 8 weeks of increased physical activity. †Significantly different from baseline. ‡Significantly different from 2 weeks (P < 0.05).
Resting skeletal muscle metabolites
Skeletal muscle metabolites were similar between the two groups (Table 2). Resting skeletal muscle ADP concentrations were similar between people with or without diabetes and did not change after 2 or 8 weeks of increased physical activity (Table 2). There was no difference in resting skeletal muscle pH between people with and without diabetes, and this was not influenced by 2 and 8 weeks of increased physical activity (Table 2). Similarly, the ratio of IMCL to water was comparable between people with and without diabetes at baseline and also did not change after 2 and 8 weeks of increased physical activity (Table 2).
Table 2.
Magnetic resonance spectroscopy measurements on muscle in people with and without type 2 diabetes before and after 2 and 8 weeks of increased physical activity
| Resting data | Type 2 diabetic subjects
|
Control subjects
|
||||
|---|---|---|---|---|---|---|
| Baseline | 2 weeks | 8 weeks | Baseline | 2 weeks | 8 weeks | |
| ADP (μmol/l) | 9.54 ± 0.17 | 9.75 ± 0.13 | 9.47 ± 0.12 | 9.38 ± 0.18 | 9.43 ± 0.13 | 9.33 ± 0.12 |
| pH | 7.04 ± 0.01 | 7.05 ± 0.01 | 7.04 ± 0.01 | 7.03 ± 0.01 | 7.03 ± 0.01 | 7.03 ± 0.01 |
| IMCL (relative to H2O) | 0.032 ± 0.006 | 0.035 ± 0.009 | 0.029 ± 0.003 | 0.037 ± 0.005 | 0.037 ± 0.004 | 0.038 ± 0.005 |
| End-exercise data | ||||||
| End-exercise ADP (μmol/l) | 33.3 ± 5.1 | 33.4 ± 4.9 | 36.8 ± 4.8 | 34.8 ± 3.7 | 33.9 ± 4.7 | 38.9 ± 3.8 |
| PCr use during exercise (% resting) | 25 ± 4 | 27 ± 5 | 29 ± 4 | 27 ± 3 | 25 ± 3 | 29 ± 3 |
| Glucose control | ||||||
| A1C (%) | 6.7 ± 0.3* | 6.7 ± 0.3* | 6.6 ± 0.2* | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.6 ± 0.1 |
| Fasting glucose (mmol/l) | 7.1 ± 0.4* | 7.0 ± 0.4* | 6.5 ± 0.5 | 5.5 ± 0.2 | 5.7 ± 0.2 | 5.5 ± 0.2 |
| Insulin (mu/l) | 13.9 ± 3.5 | 13.1 ± 2.2 | 11.7 ± 2.3 | 8.1 ± 1.4 | 10.2 ± 3.0 | 7.6 ± 1.5 |
| Homeostasis model assessment | 4.4 ± 1.1* | 4.2 ± 0.8* | 3.4 ± 0.8* | 2.0 ± 0.4 | 2.1 ± 0.4 | 1.9 ± 0.4 |
Data are means ± SE.
Significantly different from people without diabetes (P < 0.05).
Mitochondrial activity
The type 2 diabetic and control groups showed similar basal and maximal ATP turnover rates (Table 3). There was no relationship between basal and maximal ATP turnover (R2 = 0.194, P > 0.05). Both basal and maximal ATP turnover remained constant following 2 or 8 weeks of increased physical activity in either the type 2 diabetes or control group (Table 3). End-exercise ADP and PCr use during exercise were similar (Table 3), ensuring that the recovery modeling of phosphocreatine was completed from similar metabolic starting points.
Indirect calorimetery
People with and without type 2 diabetes showed similar levels of carbohydrate (2.82 ± 0.19 vs. 2.61 ± 0.17 mg · kg body wt−1 · min−1) and lipid oxidation (Fig. 1). There were no changes in basal energy expenditure at any time point. Following 8 weeks of increased physical activity, the diabetes group showed an increase in rate of lipid oxidation (Fig. 1) and a decrease in rate of carbohydrate oxidation (to 2.26 ± 0.022 mg · kg body wt−1 · min−1).
Plasma glucose, insulin, and A1C
People with type 2 diabetes had higher A1C (P < 0.01, Table 2) and fasting plasma glucose levels (P < 0.01) (Table 2). Insulin sensitivity, as assessed using homeostasis model assessment, was lower in people with type 2 diabetes than in control subjects (P < 0.05) (Table 2). In the type 2 diabetes group, there was a nonsignificant trend toward lower fasting plasma glucose (P = 0.08) (Table 2).
Anthropometric measurements
There were no differences in weight between people with and without type 2 diabetes (Table 1). There was no change in weight in the type 2 diabetes group after 2 weeks, although after 8 weeks there was a significant decrease relative to both baseline and 2 weeks (P < 0.05) (Table 2). Control subjects showed no change in weight after 2 or 8 weeks of increased physical activity (Table 2).
CONCLUSIONS
We observed no abnormality in mitochondrial function in people with well-controlled type 2 diabetes compared with physical activity–, age-, and weight-matched control subjects. There was no relationship between basal (fasted) and maximal (recovery from exercise) ATP synthesis, suggesting that the factors influencing basal and maximal ATP synthesis are different. The physical activity intervention markedly increased the number of steps taken per day during the 8-week intervention. Fasting lipid oxidation was increased, but there was no change in ATP turnover or maximal ATP production.
The observation of no abnormality in basal ATP flux contrasts with recent studies. The seminal study (2) indicating the possible importance of mitochondrial function in development of type 2 diabetes observed basal ATP flux in extreme phenotypes of insulin sensitivity. From 150 screened subjects, the most and least insulin-sensitive people underwent assessment of basal ATP flux and IMCL using magnetic resonance techniques. The 14 least insulin-sensitive subjects (with a family history of diabetes) had lower basal ATP flux and higher intramuscular lipid levels than the 10 most insulin-sensitive people. It was suggested that impaired mitochondrial function reduces the ability to oxidize lipids, with accumulation of intramuscular lipid impeding insulin signaling (5), forming a pathway from impaired mitochondrial function to the development of type 2 diabetes. A further study (3) demonstrated impaired ATP flux in people with type 2 diabetes, but the control subjects were unmatched for habitual physical activity. The present data agree with a recent study (9) reporting no difference in basal ATP use between age-, weight-, and physical activity–matched subjects with and without type 2 diabetes using saturation transfer magnetic resonance. This study was only able to demonstrate differences in ATP flux in people with type 2 diabetes under insulin-stimulated conditions. The observation of no abnormality in basal ATP flux in diabetes implies that abnormal basal mitochondrial function is unlikely to be a primary causative factor in type 2 diabetes.
In contrast to basal ATP synthesis rates, which are primarily influenced by steady-state energy demand, the recovery of PCr from exercise is a robust measure of maximal oxidative ATP turnover (19). This also reflects the recovery from muscular activity that happens frequently throughout the waking day, and any abnormality could bring about marked differences in muscle metabolism that may be associated with type 2 diabetes. The present data show no differences in the recovery of PCr from exercise between people with and without type 2 diabetes when controlled for habitual physical activity, weight, and age. To our knowledge, the present study is the first report to include both measures of basal and maximal ATP turnover in people with or without diabetes. No correlation was found between the basal and maximal ATP turnover rates. The lack of relationship between basal and maximal ATP turnover supports the concept that ATP turnover in these tests is determined by different factors. In the fasted, nonexercise state, the level of insulin-stimulated glucose uptake is likely to dominate requirement for ATP synthesis. This suggestion is based on studies that show a robust relationship between ATP synthesis and insulin-stimulated glucose uptake in skeletal muscle (9). In the postexercise stimulated state, the ability to supply and use oxygen is likely to dominate the rate of ATP turnover. The lack of correlation between the basal and maximal measures of ATP synthesis highlights the importance of examining separately these differing states.
A previous study (3) reported maximal ATP turnover to be reduced in people with type 2 diabetes compared with a BMI-matched control group. However, this study did not objectively control for differences in habitual physical activity, a factor that can influence mitochondrial function (13). People with type 2 diabetes tend to be less physically active than people without diabetes (11). Indeed, the reported PCr recovery data postexercise of people with type 2 diabetes (3) are comparable with both the type 2 diabetes and control groups in the present study, raising the likelihood that the differences in maximal ATP turnover may lay in differences in habitual physical activity. Recent support has also been given to the idea that type 2 diabetes is not necessarily associated with impaired mitochondrial function but may reflect differences in mitochondrial volume. Direct analyses of mitochondria from biopsies taken from people with type 2 diabetes show that any apparent defect in mitochondrial ATP production disappears when corrected for mitochondrial density (10). The reduced oxidative capacity accompanying type 2 diabetes may be the result of a deconditioning phenomenon (24).
The present study shows that walking an extra 45 min per day over an 8-week period is an insufficient stimulus to induce detectable mitochondrial biogenesis. The physical activity was deliberately chosen to be of low intensity, as walking has been shown to be achievable and sustainable by people with type 2 diabetes (15,25). More intensive and prolonged physical activity and diet does change mitochondrial density and aerobic capacity (14). These changes correlate well with improvements in long-term glucose control and fasting insulin sensitivity. Other biopsy data suggest that the beneficial effect of exercise and moderate weight loss upon mitochondrial density is modest (26). However, the in vitro function of mitochondria is improved, with a disproportionate increase in electron transfer chain activity following intervention. The improvements of mitochondrial function accompanying exercise are not replicated by weight loss alone (27), stressing the importance of exercise in modifying oxidative capacity and maintaining metabolic flexibility. Further work is required to define the long-term effects of practically sustainable physical activity on mitochondrial function in muscle.
In type 2 diabetes changes in mitochondrial capacity are intertwined with changes in lipid oxidation (7). The present data demonstrate physical activity–induced enhancement of resting lipid oxidation, independent of intramuscular lipid levels. Type 2 diabetes is characterized by both abnormal lipid storage and oxidation, with glucose control commonly reported to be negatively related to intramuscular lipid content (2,8,28) via an effect on insulin action (5). Walking for an extra 45 min each day increases skeletal muscle mRNA expression of genes implicated in glucose and lipid metabolism (25). The cumulative effect of a sustained increase in lipid oxidation and decrease in IMCL would be expected to improve blood glucose control (15). The effects of increased physical activity upon circulating triglyceride turnover, and the consequential influence upon insulin action, remain to be determined.
An important aspect of this study is that people with type 2 diabetes were able to sustain a more physically active lifestyle without supervision, and this would be expected to influence metabolic risk and glucose control (29). The study was not powered to detect changes in glucose control in the basal state, as this was not a primary objective. However, measures of glucose control were lower following increased physical activity. Other dynamic testing methods may have been more sensitive to changes in insulin sensitivity than homeostasis model assessment. These data are in line with larger, better powered studies of walking interventions (12). No change in serum triglycerides occurred in either group (data not shown). It is also possible that the various motivations for taking part in this study produced differences in the physical activity behavior. It is notable that the diabetic individuals sustained the level of physical activity better than the nondiabetic control group. The challenge ahead is to better understand how we can engage people with type 2 diabetes in reducing sedentary periods as well as to define how the underlying physiological mechanisms produce these benefits.
The magnetic resonance methods applied here, and previously (2,3,9,17), are not without limitation. As the mitochondria are not isolated, ATP production may be limited by external factors, such as the supply of oxygen or ATP demand. However, with these limitations noted, it is clear that these noninvasive techniques provide a patient-friendly methodology that can be applied serially and complements more detailed in vitro techniques.
In summary, resting and maximal ATP turnover are not impaired in people with well-controlled type 2 diabetes, when compared with control subjects matched for physical activity as well as age and weight. Increased daily physical activity in the form of walking is sustainable and improves lipid oxidation independent of mitochondrial activity in people with type 2 diabetes.
Acknowledgments
The study was funded by the Wellcome Trust (grant no. 073561). M.I.T. is supported by a Diabetes U.K. RD Lawrence Fellowship.
The authors are most grateful to the volunteers, Sister Jean Gerrard, Louise Morris, and Carol Smith.
Published ahead of print at http://care.diabetesjournals.org on 16 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Kelley DE, He J, Menshikova EV, Ritov VB: Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Petersen KF, Dufour S, Befroy D, Garcia BA, Shulman GI: Impaired mitochondrial activity in the insulin resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P: Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50:113–120, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Vondra K, Rath R, Bass A, Slabochová Z, Teisinger J, Vitek V: Enzyme activities in quadriceps femoris muscle of obese diabetic male patients. Diabetologia 13:527–529, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI: Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115:3587–3593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI: Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56:1376–1381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley DE, Simoneau JA: Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitu. J Clin Invest 94:2349–2345, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L: Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M: Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLos Med 4:e154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F: Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW: Physical activity in U.S. adults with diabetes and at risk for developing diabetes. Diabetes Care 30:203–209, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Thomas DE, Elliott EJ, Naughton GA: Exercise for type 2 diabetes mellitus. Coch Data System Rev CD002968, 2006 [DOI] [PMC free article] [PubMed]
- 13.Holloszy JO: Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242:2278–2282, 1967 [PubMed] [Google Scholar]
- 14.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE: Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56:2142–2147, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Di Loreto C, Fanelli C, Lucidi P, Murdolo G, De Cicco A, Parlanti N, Ranchelli A, Fatone C, Taglioni C, Santeusanio F, De Feo P: Make your diabetic patients walk: long-term impact of different amounts of physical activity on type 2 diabetes. Diabetes Care 28:1295–1302, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Taylor R: Causation of type 2 diabetes: the Gordian Knot unravels. N Engl J Med 350:639–641, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, Slezak LA, Cline GW, Rothman DL, Shulman GI: Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest 108:733–737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsen S, Hoffman R: Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys 39:2892–2901, 1963 [Google Scholar]
- 19.Kemp GJ, Radda GK: Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Mag Res Quart 10:43–63, 1994 [PubMed] [Google Scholar]
- 20.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D: Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 31:269–286, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D: Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Mag Res 140:120–130, 1999 [DOI] [PubMed] [Google Scholar]
- 22.St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R: Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr 85:742–749, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Frayn K: Calculation of substrate oxidation rates in vivo from gaseous exchange. J App Physiol 55:628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 24.Hawley J, Lessard S: Mitochondrial function: use it or lose it. Diabetologia 50:699–702, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Fritz T, Krämer DK, Karlsson HK, Galuska D, Engfeldt P, Zierath JR, Krook A: Low-intensity exercise increases skeletal muscle protein expression of PPARdelta and UCP3 in type 2 diabetic patients. Diabetes Metab Res Rev 22:492–498, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Menshikova EV, Ritov VB, Toledo FGS, Ferrell RE, Goodpaster BH, Kelley DE: Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 288:E818–E825, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Toledo FG, Menshikova EV, Azuma K, Radiková Z, Kelley CA, Ritov VB, Kelley DE: Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57:987–994, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI: Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N: Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 31:369–371, 2008 [DOI] [PubMed] [Google Scholar]