Abstract
OBJECTIVE—Hyperglycemia is a risk factor for microvascular complications and may increase the risk of cardiovascular disease in patients with type 2 diabetes. This study tested the LDL cholesterol–lowering agent colesevelam HCl (colesevelam) as a potential novel treatment for improving glycemic control in patients with type 2 diabetes on sulfonylurea-based therapy.
RESEARCH DESIGN AND METHODS—A 26-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter study was carried out between August 2004 and August 2006 to evaluate the efficacy and safety of colesevelam for reducing A1C in adults with type 2 diabetes whose glycemic control was inadequate (A1C 7.5–9.5%) with existing sulfonylurea monotherapy or sulfonylurea in combination with additional oral antidiabetes agents. In total, 461 patients were randomized (230 given colesevelam 3.75 g/day and 231 given placebo). The primary efficacy measurement was mean placebo-corrected change in A1C from baseline to week 26 in the intent-to-treat population (last observation carried forward).
RESULTS—The least squares (LS) mean change in A1C from baseline to week 26 was −0.32% in the colesevelam group and +0.23% in the placebo group, resulting in a treatment difference of −0.54% (P < 0.001). The LS mean percent change in LDL cholesterol from baseline to week 26 was −16.1% in the colesevelam group and +0.6% in the placebo group, resulting in a treatment difference of −16.7% (P < 0.001). Furthermore, significant reductions in fasting plasma glucose, fructosamine, total cholesterol, non–HDL cholesterol, and apolipoprotein B were demonstrated in the colesevelam relative to placebo group at week 26.
CONCLUSIONS—Colesevelam improved glycemic control and reduced LDL cholesterol levels in patients with type 2 diabetes receiving sulfonylurea-based therapy.
Hyperglycemia is a risk factor for microvascular complications in patients with type 2 diabetes (1), and landmark clinical studies have documented that improved glycemic control results in decreased development and progression of the microvascular complications of type 2 diabetes (2–5). The American Diabetes Association (ADA) recommends an A1C target of <7.0% (6), the level at which clinical trials have demonstrated fewer long-term microvascular complications (7). Although the impact of hyperglycemia on macrovascular complications is unknown, individuals with type 2 diabetes have a two- to fourfold higher risk for initial coronary events, and more importantly, those developing coronary heart disease have a relatively poor prognosis for recurrent coronary heart disease events and coronary death (8,9). In addition to hyperglycemia, dyslipidemia and hypertension also contribute to the risk of complications in patients with type 2 diabetes. Therefore, treatment regimens for type 2 diabetes should aim to address multiple clinical features of this disease.
Effective lipid management reduces macrovascular disease and mortality in individuals with type 2 diabetes, particularly in those who have had prior cardiovascular events (10–12). However, in a study by Kennedy et al. (13), the ADA goal of LDL cholesterol <100 mg/dl (2.6 mmol/l) was achieved by only 49% of patients with type 2 diabetes, and only 16% achieved LDL cholesterol <70 mg/dl (1.8 mmol/l), the optional goal for very-high-risk individuals. Individuals with type 2 diabetes may exhibit a characteristic dyslipidemia that includes elevated triglyceride levels, decreased HDL cholesterol levels, and small dense LDL particles, which increases the risk of complications.
Preliminary evidence suggests that altering bile acid metabolism with a bile acid sequestrant in patients with type 2 diabetes has a beneficial effect on glucose control. Colesevelam HCl (Welchol [colesevelam]; Daiichi Sankyo), a specifically engineered bile acid sequestrant that significantly lowers LDL cholesterol levels in patients with primary hypercholesterolemia, improved glycemic control in adults with type 2 diabetes based on post hoc analysis of data from a 6-month primary lipid trial. A short-term, double-blind, placebo-controlled pilot study in subjects with type 2 diabetes inadequately controlled with metformin and/or sulfonylurea therapy was conducted; after 12 weeks, colesevelam reduced A1C by 0.50% in the total population (P = 0.007 vs. placebo) and by 1.0% in those with a baseline A1C ≥8.0% (P = 0.002 vs. placebo) (14). A subsequent study in which colesevelam was added to insulin-based therapy showed that the addition of colesevelam reduced A1C by 0.5% relative to placebo after 16 weeks (15). The present study was designed to evaluate the longer-term efficacy of colesevelam for improving glycemic control and the lipid profile in patients with type 2 diabetes not adequately controlled on a stable sulfonylurea-based antidiabetes regimen.
RESEARCH DESIGN AND METHODS
This 26-week, randomized, double-blind, placebo-controlled, parallel-group study was conducted at 49 sites in the U.S. and 2 in Mexico. The study protocol was conducted in compliance with institutional review board regulations, good clinical practice guidelines, and the 4th amendment of the Declaration of Helsinki. All individuals provided written informed consent.
This study enrolled adults with type 2 diabetes that was inadequately controlled (A1C 7.5–9.5%, inclusive) on a stable dose of sulfonylurea alone or in combination with additional oral antidiabetes agents for ≥90 days. All subjects were advised to follow ADA dietary recommendations.
Subjects were excluded for the following reasons: LDL cholesterol <60 mg/dl (1.6 mmol/l); triglycerides >500 mg/dl (5.7 mmol/l); BMI >45 kg/m2; uncontrolled hypertension (blood pressure >160/95 mmHg); history of type 1 diabetes, ketoacidosis, dysphagia, swallowing disorders, or intestinal motility disorders; any serious medical/psychiatric disorder; drug/alcohol abuse within 2 years; hospitalization within 14 days; treatment with colesevelam within 8 weeks; chronic use or recent initiation of insulin; participation in a weight loss program with ongoing weight loss; starting an intensive exercise program within 4 weeks; use of any investigational drug within 30 days of the first dose of study medication; or any condition or therapy that may pose a risk or make participation not in the best interest of the subject.
Subjects taking oral corticosteroids, cholestyramine, and colestipol were excluded. Subjects on oral contraceptives, hormone replacement therapy, thyroid replacement therapy, and lipid-altering drugs (HMG-CoA reductase inhibitors, fibrates, niacin, fish oils, and cholesterol absorption inhibitors) were included provided a stable dose had been maintained for ≥30 days before the initiation of the study and dosage changes were not anticipated.
Subjects were discontinued for hyperglycemia if A1C increased to ≥10.0% or fasting plasma glucose (FPG) increased to >260 mg/dl (14.4 mmol/l) and was confirmed within 3 days. The management, reporting, and actions taken in response to hypoglycemia (FPG <60 mg/dl) were left to the judgement of the investigators. Discontinuation was considered only after repeated measurements of hypoglycemia.
Subjects underwent a 1-week screening period and then entered a 2-week single-blind placebo run-in period, during which subjects took six placebo tablets per day. Subjects chose their preferred dosing regimen: three tablets with the noon and evening meals or six tablets with the evening meal. The dosing regimen selected was to be maintained throughout the double-blind treatment period for an individual subject. Following the placebo run-in period, subjects were randomized 1:1 to colesevelam 3.75 g/day (six tablets: 625 mg/tablet) or matching placebo for 26 weeks of double-blind treatment. Subjects continued taking their prestudy oral antidiabetes medication(s) at the same dose and time as before the start of the study.
The primary efficacy parameter was mean change in A1C from baseline to week 26 in the intent-to-treat (ITT) population with the last observation carried forward (LOCF) analysis. Secondary efficacy parameters included mean change in FPG, fructosamine, and C-peptide; mean change in A1C for the sulfonylurea monotherapy and sulfonylurea combination therapy cohorts; percentage of subjects achieving a reduction in FPG ≥30 mg/dl or A1C ≥0.7%; mean change and mean percent change in lipids, lipoproteins, and lipid and lipoprotein ratios; and median change and median percent change in high-sensitivity C-reactive protein (hsCRP) and triglycerides. For all secondary efficacy parameters, the change from baseline to week 26 was calculated using both LOCF and non-LOCF analyses.
All blood samples were obtained under fasting conditions. Tests were performed by a certified laboratory (Medical Research Laboratories International, Highland Heights, KY). Total cholesterol and triglycerides were measured by enzyme assay. HDL cholesterol was measured by cholesterol oxidase assay of the supernatant from the precipitate of non-HDL lipoproteins with heparin and manganese chloride. Apolipoprotein (apo)B, apoA-I, and hsCRP were quantitated by immunonephelometry. The method used to calculate LDL cholesterol was based on triglyceride concentration at screening (Friedewald equation for subjects with triglycerides ≤400 mg/dl [4.5 mmol/l] and Lipid Research Clinic β-quantification method for subjects with triglycerides >400 mg/dl [4.5 mmol/l] and ≤500 mg/dl [5.7 mmol/l]). The method of LDL cholesterol determination used at screening was used throughout the study for an individual subject, regardless of changes in triglycerides.
Safety assessments included treatment-emergent adverse events (AEs), clinical laboratory tests, changes in vital signs, and physical examinations. Compliance with the medication regimen was evaluated by counting unused tablets.
Statistical method
The ITT population was the primary analysis population for all efficacy parameters and included all randomized subjects who took at least one dose of the randomized study medication and had a baseline and at least one postbaseline A1C measurement. Analyses of the mean change in A1C from baseline were conducted for two mutually exclusive protocol-defined cohorts: subjects on background sulfonylurea monotherapy and those on background sulfonylurea combination therapy. The safety population included all randomized subjects who took at least one dose of study medication.
This study required 400 randomized subjects and had 86–95% power to detect a difference of a 0.50–0.80% reduction in mean A1C from baseline between colesevelam and placebo (with a two-sided type I error at 0.05), assuming a common SD of ≤1.5% and a maximum dropout rate of 15%.
Comparisons between the treatment groups in age, weight, height, BMI, A1C, and FPG at baseline were evaluated using a one-way ANOVA model with treatment as a factor. Sex and race were tested using a Fisher's exact/Fisher-Freeman-Halton test. An ANCOVA model with treatment and concomitant antidiabetes medication status as fixed effects and baseline A1C as a covariate was used to evaluate the treatment effect. The normality assumption of the efficacy data was examined before conducting the ANCOVA. When a significant departure from normality was observed, a nonparametric equivalent of ANCOVA (rank ANCOVA) was applied.
The treatment effect in A1C change from baseline with LOCF was estimated and presented as least-squares (LS) mean, SEM, corresponding two-tailed 95% CI, and two-sided P value. Secondary efficacy parameters were compared with the same statistical methodology unless otherwise noted.
Median change and median percent change in hsCRP and triglycerides were analyzed using a nonparametric ANCOVA model. The treatment difference was estimated by the Hodges-Lehmann estimator, and a two-tailed 95% CI for the treatment difference was obtained using the method of Moses. The glycemic-control response rate was tabulated and compared using Pearson's χ2 test.
RESULTS
Subject disposition and baseline characteristics
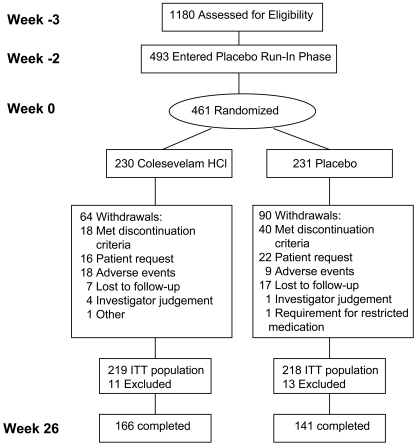
The study was conducted between August 2004 and August 2006. A total of 1,180 subjects were screened, and 493 entered the placebo run-in period. In total, 461 subjects were randomized (230 to the colesevelam and 231 to the placebo groups), with 307 subjects completing the 26-week study (Fig. 1). Baseline demographic characteristics did not differ between the colesevelam and placebo groups at randomization (Table 1).
Figure 1.
Subject disposition.
Table 1.
Demographic characteristics (randomized population)
| Colesevelam HCl | Placebo | P | |
|---|---|---|---|
| n | 230 | 231 | |
| Age (years) | 56.6 ± 10.3 | 57.0 ± 10.3 | 0.670 |
| Sex | 0.575 | ||
| Male | 128 (55.7) | 122 (52.8) | |
| Female | 102 (44.3) | 109 (47.2) | |
| Race/ethnicity | 0.438 | ||
| Caucasian | 135 (58.7) | 128 (55.4) | |
| Hispanic | 66 (28.7) | 59 (25.5) | |
| African-American | 23 (10.0) | 34 (14.7) | |
| Asian | 4 (1.7) | 7 (3.0) | |
| Other | 2 (0.9) | 3 (1.3) | |
| Weight (kg) | 95.0 ± 22.6 | 92.5 ± 20.2 | 0.197 |
| BMI (kg/m2) | 33.1 ± 5.95 | 32.5 ± 5.64 | 0.225 |
| A1C (%) | 8.2 ± 0.68 | 8.3 ± 0.72 | 0.054 |
| FPG (mg/dl) | 176.6 ± 46.5 | 181.0 ± 50.4 | 0.323 |
| Concomitant antidiabetes medication status | |||
| Sulfonylurea monotherapy | 75 (32.6) | 81 (35.1) | |
| Glibenclamide | 39 (52.0) | 44 (54.3) | |
| Glipizide | 24 (32.0) | 25 (30.9) | |
| Glimepiride | 10 (13.3) | 12 (14.8) | |
| Tolbutamide | 1 (1.3) | 0 | |
| Gliclazide | 1 (1.3) | 0 | |
| Sulfonylurea combination therapy | 154 (67.0) | 150 (64.9) | |
| Concomitant antidiabetes medications in the sulfonylurea combination therapy group* | |||
| Sulfonamides, urea derivatives | 120 (77.9) | 122 (81.3) | |
| Glipizide | 62 (40.3) | 50 (33.3) | |
| Glibenclamide | 37 (24.0) | 50 (33.3) | |
| Glimepiride | 21 (13.6) | 21 (14.0) | |
| Tolazamide | 1 (0.6) | 2 (1.3) | |
| Biguanides | 104 (67.5) | 105 (70.0) | |
| Thiazolidinediones | 40 (26.0) | 40 (26.7) | |
| Biguanide/sulfonamide fixed-dose combinations | 33 (21.4) | 27 (18.0) | |
| α-glucosidase inhibitors | 0 | 1 (0.7) | |
| Other antidiabetes agents† | 13 (8.4) | 10 (6.7) |
Data are means ± SD and n (%) unless otherwise indicated.
Some subjects took more than one oral antidiabetes agent in combination with background sulfonylurea therapy, and thus the total number of concomitant oral antidiabetes agents in the columns exceed the n values in the colesevelam HCl and placebo columns.
Other antidiabetes agents includes fixed-dose rosiglitazone/metformin, fixed-dose glipizide/metformin, nateglinide, and repaglinide.
Sixty-four subjects in the colesevelam group withdrew before study completion, relative to 90 subjects in the placebo group. Twenty-two subjects in the colesevelam group discontinued due to glycemia-related events, relative to 46 subjects in the placebo group. Sixteen subjects in the colesevelam group and 39 in the placebo group discontinued due to protocol-specified discontinuation criteria (FPG >260 mg/dl [14.4 mmol/l], A1C ≥10.0%, or change in antidiabetes regimen). Mean compliance was similar during the double-blind treatment period (92.7% of the colesevelam group and 90.8% of the placebo group).
Efficacy: glycemic parameters
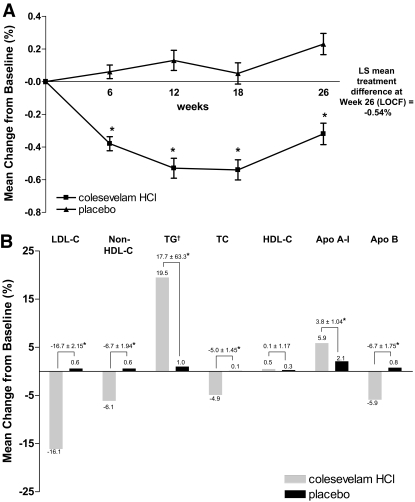
Colesevelam reduced A1C by 0.32 ± 0.066%, whereas placebo increased A1C by 0.23 ± 0.065%, resulting in a significant LS mean treatment difference of −0.54 ± 0.090% at week 26 by LOCF (P < 0.001; Fig. 2A and online appendix Table 1 [available at http://dx.doi.org/10.2337]). Similar treatment effects were observed in the sulfonylurea monotherapy (−0.79 ± 0.154%, P < 0.001) and sulfonylurea combination therapy (−0.42 ± 0.110%, P < 0.001) cohorts.
Figure 2.
A: Mean change in A1C (%) from baseline to weeks 6, 12, 18, and 26 (LOCF) in the ITT population. *P < 0.001 vs. placebo. B: Mean percent change in lipid and apo parameters from baseline to week 26 (LOCF) in the ITT population. *P < 0.001 vs. placebo. †Triglycerides (TGs) reported as median rather than mean.
A1C was also evaluated by stratifying the total population into two subgroups according to baseline A1C to evaluate the effect of baseline A1C on the response to the addition of colesevelam. In the subgroup with A1C ≤8.0% at baseline, the treatment effect for A1C was −0.48 ± 0.124% (P = 0.0002). A greater A1C treatment effect was observed in the subgroup with A1C >8.0% at baseline (−0.58 ± 0.128%, P < 0.0001).
A significant LS mean treatment difference in FPG was observed by week 26 by LOCF (−13.5 ± 5.14 mg/dl [0.75 ± 0.29 mmol/l], P = 0.009; online appendix Table 1), with a significant treatment difference observed as early as 6 weeks (−13.7 ± 3.98 mg/dl [0.76 ± 0.22 mmol/l], P < 0.001).
A significantly greater percentage of subjects in the colesevelam group achieved an A1C reduction ≥0.7% compared with those in the placebo group (35.2 vs. 16.5%, respectively, P < 0.001; online appendix Table 2). In addition, there was a significantly greater number of individuals in the colesevelam group relative to the placebo group who achieved either a reduction in A1C ≥0.7% or a reduction in FPG ≥30 mg/dl by study end (104 [47.5%] vs. 70 [32.1%], respectively, P = 0.001; online appendix Table 2). A significant LS mean treatment difference in fructosamine was reported at week 26 by LOCF (−21.4 ± 4.59 μmol/l, P < 0.001). There was no significant LS mean treatment difference in C-peptide at week 26 by LOCF (−0.17 ± 0.101 ng/ml, P = 0.102).
Lipid parameters
Significant LS mean percent treatment differences in LDL cholesterol, non–HDL cholesterol, triglycerides, total cholesterol, apoA-I, and apoB were observed after 26 weeks of treatment with colesevelam relative to placebo (P < 0.001 for all; Fig. 2B). The LS mean percent change in LDL cholesterol from baseline to week 26 (LOCF) was −16.1% in the colesevelam group and +0.6% in the placebo group, resulting in a treatment difference of −16.7% (P < 0.001). In addition, a significant mean percent reduction in non-HDL cholesterol, total cholesterol, and apoB concentrations occurred in the colesevelam group relative to the placebo group at week 26 (LOCF), while a significant mean percent increase in apoA-I concentration occurred with colesevelam relative to placebo. Although mean HDL cholesterol increased from baseline by week 26 in both the colesevelam and placebo groups (0.5 vs. 0.3%, respectively), there was no significant treatment difference by week 26 (LOCF; +0.1%, P = 0.916). Median triglyceride levels increased with colesevelam relative to placebo treatment (19.5 vs. 1.0%), resulting in a significant LS mean percent treatment difference at week 26 (LOCF; +17.7%, P < 0.001).
Significant LS mean treatment differences between the colesevelam and placebo groups were reported in the ratios of total cholesterol to HDL cholesterol (−0.24 ± 0.08), LDL cholesterol to HDL cholesterol (−0.43 ± 0.05), non–HDL cholesterol to HDL cholesterol (−0.24 ± 0.08), and apoB to apoA-I (−0.08 ± 0.01) at week 26 (LOCF; P ≤ 0.003 for all).
Inflammatory markers
There was an LS median treatment difference of marginal significance in hsCRP by week 26 (LOCF; −0.40 ± 4.50 mg/l, P = 0.063).
Safety
Colesevelam was generally safe and well tolerated in subjects with type 2 diabetes when added to sulfonylurea-based therapy. In total, 145 subjects in the colesevelam group and 126 in the placebo group experienced an AE during this study (online appendix Table 3). The most frequently reported AEs with colesevelam were constipation, upper respiratory tract infection, and urinary tract infection, and upper respiratory tract infection, headache, and nasopharyngitis were more common with placebo. Forty-seven subjects in the colesevelam group and 21 in the placebo group experienced a drug-related AE, with constipation being the most common drug-related AE in both groups (6.1 vs. 2.6%, respectively). Most AEs were mild to moderate in severity, although nine subjects in the colesevelam group and seven in the placebo group experienced a severe AE. Three severe AEs were judged to be drug-related (two in the colesevelam group and one in the placebo group). Eighteen subjects in the colesevelam group and nine in the placebo group withdrew due to an AE, mostly gastrointestinal in nature. Twelve subjects in the colesevelam and four in the placebo group withdrew due to a drug-related AE. There were 8 serious AEs in the colesevelam group and 11 in the placebo group; however, none was drug-related.
Six subjects in the colesevelam group experienced hypoglycemia relative to two subjects in the placebo group. None of these hypoglycemic episodes was considered severe, and no subject in either group discontinued due to hypoglycemia. Mean changes in safety laboratory parameters and vital signs were similar between the groups during the randomized treatment period. No weight gain was noted in either group; mean weight change was −0.01 kg in the colesevelam group and −0.4 kg in the placebo group.
CONCLUSIONS
This study investigated the glucose-lowering effect of colesevelam in patients with type 2 diabetes when added to an existing regimen of sulfonylurea, alone or in combination with additional oral antidiabetes agents. Colesevelam resulted in a significant mean A1C reduction (0.54%) by week 26 in the total population, with the sulfonylurea monotherapy and sulfonylurea combination therapy cohorts reporting a significant reduction at week 26 as well (0.79 and 0.42%, respectively). While the magnitude of A1C reduction may appear modest, it is similar to the observed effect from another study in which a new antidiabetes agent was combined with a sulfonylurea in patients with advanced type 2 diabetes (16). Importantly, individuals with declining β-cell function would be expected to be heavily represented in this population (17).
The lower dropout rate with colesevelam (27.8%) compared with placebo (39.0%) was likely related to treatment failure, as many patients discontinued in the placebo group due to meeting protocol-specified discontinuation criteria, attesting to the efficacy of colesevelam. Furthermore, colesevelam produced significant reductions in LDL cholesterol, total cholesterol, non-HDL cholesterol, and apoB by week 26, supporting its use as a novel treatment for improving glycemic control with added lipid benefits for patients with type 2 diabetes.
There was a rise in triglyceride levels in patients receiving colesevelam in this study (17.7%, P < 0.001 vs. placebo). This is a known and expected phenomenon with bile acid sequestrants. Although hypertriglyceridemia is a cardiovascular disease risk factor, the effect of elevated triglyceride levels in patients on existing statin therapy, or in the context of a reduction in LDL cholesterol, remains to be determined. Current studies are addressing the contribution of triglyceride levels to cardiovascular outcomes (18,19). Importantly, the rise in triglyceride with colesevelam was accompanied by a reduction in LDL cholesterol (16.7%) and apoB (6.7%) and an increase in apoA-I concentration (3.8%, P < 0.001 vs. placebo for LDL cholesterol, apoB, and apoA-I). Hence, the overall effect of colesevelam on circulating lipid levels may be interpreted as reassuring.
The exact mechanism(s) through which colesevelam demonstrates its effect on glycemic control is unknown. Potential explanations include the following: 1) bile acid sequestrants act in the gastrointestinal tract, reducing the amount of glucose absorbed and/or altering the time course of glucose absorption (20); and/or 2) bile acid sequestrants bind bile acids, thus disrupting the enterohepatic pathway of bile metabolism, which has indirect effects on glucose metabolism (21). It is known that bile acids are endogenous ligands of the farnesoid X receptor (FXR), a member of the nuclear receptor superfamily of ligand-activated transcription factors (22) that play an important role in bile acid, cholesterol, and glucose metabolism. There is a complex interplay between FXR and additional nuclear receptors, including the liver X receptor, hepatocyte nuclear factor-4α receptor, and the fibroblast growth factor-19 receptor. Little is currently known about how bile acids affect these pathways, particularly in patients with type 2 diabetes whose glucose regulation is impaired. Further research is needed to determine the mechanism underlying the glucose-lowering effect of colesevelam.
It is increasingly recognized that controlling hyperglycemia and cholesterol levels may afford better outcomes in patients with type 2 diabetes (23). In spite of this, approximately two-thirds of individuals with type 2 diabetes fail to achieve the ADA-recommended goal of A1C <7.0%, and almost 75% do not achieve the LDL cholesterol goal of <100 mg/dl. Thus, new treatment regimens that can improve both glycemic control and lipid management in individuals with type 2 diabetes would be clinically beneficial. This study showed that colesevelam significantly reduced A1C and LDL cholesterol concentration in patients with type 2 diabetes when added to a sulfonylurea-based therapy. Colesevelam therapy was safe and well tolerated in this study. No patient reported a severe episode of hypoglycemia, and none discontinued due to hypoglycemia. Furthermore, colesevelam did not result in weight gain.
The positive effects of colesevelam in patients with type 2 diabetes reported in this study suggest that the bile acid sequestrant colesevelam may represent a novel therapeutic add-on strategy for improving multiple metabolic parameters in patients with type 2 diabetes.
Supplementary Material
Acknowledgments
Financial support for the preparation of this manuscript was provided by Daiichi Sankyo, Inc.
Editorial assistance was provided by Karen Stauffer, PhD.
Published ahead of print at http://care.diabetesjournals.org on 5 May 2008. Clinical trial reg. no. NCT00147758, clinicaltrials.gov.
V.F. has received research support from GlaxoSmithKline, Novartis, Novo-Nordisk, Takeda, Astra-Zeneca, Pfizer, sanofi-aventis, Eli Lilly, Daiichi-Sankyo, the National Institutes of Health, and the American Diabetes Association and honoraria for consulting fees and lectures from GlaxoSmithKline, Novartis, Takeda, Pfizer, sanofi-aventis, and Eli Lilly.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Klein R: Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 18:258–268, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28:103–117, 1995 [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853, 1998 [PubMed] [Google Scholar]
- 5.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865, 1998 [PubMed] [Google Scholar]
- 6.American Diabetes Association: Standards of medical care in diabetes—2007. Diabetes Care 30(Suppl. 1):S4–S41, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannel WB, McGee DL: Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 2:120–126, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Stamler J, Vaccaro O, Neaton JD, Wentworth D: Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16:434–444, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Collins R, Armitage J, Parish S, Sleigh P, Peto R: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361:2005–2016, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G: Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 20:614–620, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 339:1349–1357, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Kennedy AG, MacLean CD, Littenberg B, Ades PA, Pinckney RG: The challenge of achieving national cholesterol goals in patients with diabetes. Diabetes Care 28:1029–1034, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL: Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther 29:74–83, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RB, Fonseca VA, Truitt KE, Jones MR: Efficacy and safety of colesevelam hydrochloride in patients with type 2 diabetes mellitus with inadequate glycemic control on insulin-based therapy. Arch Intern Med, in press [DOI] [PubMed]
- 16.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P: Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 9:733–745, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, and Matthews DR: Coefficient of failure: a methodology for examining longitudinal beta-cell function in type 2 diabetes. Diabet Med 19:465–469, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg HN, Bonds DE, Lovato LC, Crouse JR, Elam MB, Linz PE, O'Connor PJ, Leiter LA, Weiss D, Lipkin E, Fleg JL: Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 99:56i–67i, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG: Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370:1687–1697, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Garg A, and Grundy SM: Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus: a short-term, double-blind, crossover trial. Ann Intern Med 121:416–422, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Guzelian P, and Boyer JL: Glucose reabsorption from bile: evidence for a biliohepatic circulation. J Clin Invest 53:526–535, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang JY, Kimmel R, Weinberger C, Stroup D: Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem 275:10918–10924, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Saydah SH, Fradkin J, and Cowie CC: Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 291:335–342, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.